Back to Journals » Clinical Ophthalmology » Volume 16
Real-World Experience with Intracapsular Administration of Dexamethasone Intraocular Suspension 9% for Control of Postoperative Inflammation
Authors McCabe C, Desai P, Nijm L, Osher R, Weinstock R
Received 8 February 2022
Accepted for publication 7 June 2022
Published 20 June 2022 Volume 2022:16 Pages 1985—1992
DOI https://doi.org/10.2147/OPTH.S361146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video of "DEXYCU 9% for Control of Postoperative Inflammation" [ID 361146].
Views: 506
Cathleen McCabe,1 Priya Desai,2 Lisa Nijm,3 Robert Osher,4 Robert Weinstock5
1The Eye Associates; Eye Health America, Sarasota Memorial Hospital, Sarasota, FL, USA; 2Matossian Eye Associates, Pennington, NJ, USA; 3Warrenville Eye Care and LASIK Center, Northwestern Medicine, Warrenville, IL, USA; 4Cincinnati Eye Institute, University of Cincinnati, Cincinnati, OH, USA; 5The Eye Institute of West Florida, Weinstock Laser Eye Center, Largo, FL, USA
Correspondence: Cathleen McCabe, The Eye Associates; Eye Health America, Sarasota Memorial Hospital, Sarasota, FL, USA, Tel +1 941 792-2020, Email [email protected]
Abstract: Corticosteroids and non-steroidal anti-inflammatory drugs are commonly used prophylactically to control inflammation after ocular surgery. When prescribed as eye drops, as has been longstanding tradition, anti-inflammatory success is out of surgeons’ hands, dependent on patient compliance and proper instillation technique. Sustained-release, intraoperatively administered anti-inflammatory drugs are emerging as another option. DEXYCU (dexamethasone intraocular suspension) 9% is the first and only intraocular corticosteroid FDA-approved for postoperative inflammation, whose sustained-release formulation provides a high initial release of drug, followed by a gradual tapering. Administration of the drug directly into the capsular bag following cataract surgery enables reliable placement proximal to target tissues, ensuring surgeon control and visibility of delivery, safety, and efficiency. This technique also minimizes contact with metabolically active tissues such as the corneal endothelium, iris, and ciliary body. In this paper, we review the available literature on dexamethasone intraocular suspension and summarize surgeons’ consensus on best practices for intracapsular administration.
Keywords: cataract, corticosteroids, inflammation, technique
Plain Language Summary
Sustained-release, intraoperatively administered anti-inflammatory drugs, such as DEXYCU (dexamethasone intraocular suspension 9%), are emerging as alternatives to topical drops as a strategy for minimizing inflammation following ocular surgery. In this article, the authors summarize available literature on intraocular dexamethasone and describe the evolution of their administration technique.
Introduction
While advances in surgical devices and techniques have helped optimize cataract procedures, postoperative inflammation continues to adversely affect patient recovery and visual outcomes.1 Surgeons have traditionally prescribed a combination of topical ophthalmic corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) to control and prevent postoperative inflammation and pain. This approach relies on patient compliance and dexterity, while adding cost and stress to patients’ postoperative course.2 Topical drops also introduce risk for contamination and ocular surface trauma with improper dropper handling or imperfect instillation technique; and bioavailability of topical agents is limited.2,3 Furthermore, complicated postoperative drop regimens are burdensome to the office and clinical staff, who spend considerable time and energy counseling patients regarding filling prescriptions, dosing, and administration, and who must attend to logistical questions around prescription fulfillment and inter-pharmacy pricing variability, which frequently arise.
Despite significant liabilities and challenges, topical drops for postoperative inflammation control have remained the standard of care for decades. Indeed, development in the field of pharmacology for cataract surgery has until recently been largely dormant, in marked contrast to logarithmic advances that have taken place in other areas, including phacoemulsification, IOL technologies, and drug delivery to the posterior segment. Many of us have been eager for a sustained drug delivery method that could safely and reliably replace drops.
That changed in 2018 when DEXYCU (dexamethasone intraocular suspension) 9% (intraocular DXM) became the first FDA-approved intraocular sustained-release corticosteroid indicated for postoperative inflammation.4 Designed for posterior chamber placement at the end of ocular surgery, intraocular DXM uses Verisome technology to provide a high initial release of drug, followed by a gradual tapering. When injected into an aqueous medium, the intraocular DXM 0.005mL suspension (517mcg) forms a 2mm spherule consisting of active drug suspended in a delivery vehicle of acetyl triethyl citrate.4,5
One of us (CM) first used intraocular DXM as an investigator in clinical trials; and all of us were among the early adopters of intraocular DXM, incorporating it into routine and complicated cataract surgeries within a year following approval. We were excited to have an alternative to drops to offer patients, relieving them of that added expense, confusion, stress, and burden and relieving ourselves of worries around lack of medication compliance and its sequelae. We were also highly motivated by the opportunity to now place medication where it had the potential to do the most good—directly at the site of inflammation.
The efficacy and safety of intraocular DXM in the treatment of postoperative inflammation are well documented.6–11 In a prospective, randomized Phase 3 clinical study, intraocular DXM was shown to be significantly superior to placebo (vehicle injection) for controlling cataract surgery-related inflammation.6 A separate prospective, randomized, open-label, multi-center phase 3 trial demonstrated similar safety outcomes among cataract surgery patients treated with intraocular DXM vs a 3-week regimen of topical prednisolone acetate 1%.7 Though not powered to study efficacy, rates of anterior chamber cell and flare clearing were similar between the two treatment groups through 90 days postoperatively.7 A post-hoc analysis of pooled phase 3 trial data revealed that, relative to topical prednisolone, intraocular DXM was not associated with a statistically significant increased risk for elevated intraocular pressure (IOP).6–8,11 In a placebo-controlled clinical trial, 65% of intraocular DXM-treated eyes showed anterior chamber cell (ACC) clearing on postoperative day 8 in contrast with 26% of placebo-treated eyes (P<0.05).6 A separate prospective, controlled clinical trial showed similar mean postoperative day 7 and day 14 Summed Ocular Inflammatory Scores (SOIS) among eyes treated with intraocular DXM, intracameral moxifloxacin, and once-daily topical bromfenac compared with a topical-only regimen of moxifloxacin, prednisolone acetate, and bromfenac.9
In a retrospective case-matched study comparing outcomes among patients treated with single dose intraocular DXM vs a month of topical prednisolone acetate following vitreoretinal surgery (N = 27 eyes of 27 patients in each treatment group), researchers found superior postoperative day 7 anterior chamber cell clearance rates among intraocular DXM-treated patients (67% vs 37%, respectively; P=0.029).10 Additionally, treatment with intraocular DXM resulted in significantly decreased optical coherence tomography macular thickness (OCT MT) at 4 weeks (−19 microns) and 8 weeks (−22 microns) post vitrectomy compared with increased OCT MT at the same timepoints (+36 and +44 microns, respectively) with topical prednisolone treatment.10
These results are consistent with our clinical experience—eyes treated with intraocular DXM are often completely quiet during the postoperative period, starting as early as postoperative day 1. Some of us have observed that the anti-inflammatory effectiveness of intraocular DXM is similar to topical agents; others of us judge it as a superior approach.
Several of us (CM, PD) have observed that intraocular DXM is more effective than drops in complicated cases where excess inflammation is expected or would be particularly risky, eg, dense cataracts, posterior synechiae, miotic pupil, diabetic macular edema, epiretinal membrane, history of cystoid macular edema (CME), or comorbid autoimmunity. Having anti-inflammatory medication in the eye at the time of surgery, as close as possible to affected tissues interrupts the inflammatory cascade early and protects vulnerable ocular tissues.
For those of us using intracameral antibiotics as well, intraocular DXM allows us to achieve an entirely or near “dropless” surgical approach, which is increasingly attractive to patients who desire state-of-the-art care. A simpler postoperative course afforded by intraoperative placement of intraocular DXM also benefits our staff, who have less dosing and other drop-related phone inquiries to answer.
Intraocular DXM Administration—Injection into Ciliary Sulcus
Intraocular DXM is designed to be administered as a single 0.005 mL spherule placed into the posterior chamber of the eye by injection at the end of surgery.4 The delivery method used in the clinical trials and originally taught to surgeons involved injecting the drug immediately behind the iris inferiorly into the ciliary sulcus. While this generally works well, many of us have been dissatisfied with the technique’s downsides: First, the sulcus delivery method is essentially a blind approach, offering the surgeon no visual or tactile feedback to confirm proper placement and volume of the drug depot.12 Given the small volume of drug delivered and the need to confirm its size, a method of administration enabling visualization by the surgeon would be ideal. This would allow confirmation of placement and volume and afford the surgeon an opportunity to correct any errors before withdrawing the cannula. For example, if the spherule is over 2mm in diameter, excess drug may be removed by aspiration.4
The sulcus delivery technique also requires touching the iris, which carries a slight risk of iatrogenic trauma. Additionally, following the fluid dynamics within the anterior segment, the spherule of drug may migrate from its original position in the sulcus into the anterior chamber, where it may contact metabolically active tissues.12 Migration to the anterior chamber is not uncommon; risk is highest with a widely dilated pupil, as the iris lip is smaller and less able to retain the Verisome. In the anterior chamber, the Verisome may contact the delicate corneal endothelium; although usually well tolerated, transient, focal corneal edema may result. Intraocular DXM placement or migration adjacent to the iris has been associated with iris atrophy and pupillary distortion.13
Finally, any technique that involves administration of intraocular DXM adjacent to the intraocular lens risks the drug depot moving to or being accidentally deposited onto the lens optic, resulting in the potential for transient effect on postoperative vision.
A Modified Surgical Technique
Genesis of the Capsular Bag Technique
Two of us independently (RO and RW), with a suggestion from Jay Duker, MD, developed the technique of injecting intraocular DXM into the capsular bag. Because of the difficulties cited above, surgeons became interested in an alternative to the sulcus delivery technique. We found that administration into the capsular bag would enable more accurate and reliable placement, better visualization, and greater stability.12
When injected into the capsular bag, the cohesive Verisome spherule adheres to the edge of the IOL optic or haptic, which reduces the likelihood of drug egress, keeping it in place and remote from the ciliary body, iris, trabecular meshwork, and corneal endothelium. Moreover, the location and size of the spherule are directly visible when injecting into the bag, enabling repositioning or aspiration of drug as needed (Figure 1). In addition, the capsular bag technique is easy to perform, as the hand movements used to enter and exit the capsular bag are second nature to cataract surgeons. Most of us target the inferonasal quadrant and agree that that the technique was straightforward to master with only a slight learning curve.
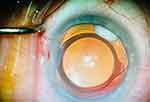 |
Figure 1 Intraocular DXM situated in the capsular bag at the edge of the intraocular lens. Image courtesy from Priya Desai, MD. |
Performing the Capsular Bag Technique for Intraocular DXM Administration
Preparation and Injection
Intraocular DXM preparation may be coordinated within the surgical team and timed so the drug is ready for injection at the conclusion of surgery. As with any new technology in the operating room, the comfort of the surgical staff is essential to successful administration of intraocular DXM. As the surgeon is irrigating and aspirating toward the end of the cataract removal or earlier, the 0.5 cc vial of intraocular DXM is placed in the vortex mixing device provided by the manufacturer in order to fully distribute the drug into suspension. The product has been demonstrated to remain in suspension for 10 minutes following thorough mixing with the vortex device. Intraocular DXM is then drawn up and cannula attached, using either the 1 cc syringe and 25-gauge cannula provided in the kit or a 1 cc tuberculin syringe and a 27-gauge cannula. One of us (PD) prefers the kit-supplied 25-gauge cannula because less manual force is required to dispense the medication; others of us (RW, CM, RO, LN) have come to prefer a smaller 27-gauge cannula for ease of control. It is important to ensure that any air bubbles are removed from the cannula prior to administration.
Following IOL implantation within the capsular bag and removal of the viscoelastic, the eye is filled with balanced salt solution (BSS), and any intracameral medications, such as moxifloxacin, are instilled. Following this step, the incisions are thoroughly hydrated, ensuring a deep chamber and secure wounds. It is important that both the main incision and the paracentesis are well sealed prior to injecting intraocular DXM. Following final pressure checks (pressure should be normal or slightly high), the prepared syringe of intraocular DXM should be handed to the surgeon.
After wiping the cannula tip of excess drug and avoiding intraocular DXM on the corneal surface, the cannula is inserted through a paracentesis port (the preferable route), depending on location and surgeon preference. The dose of intraocular DXM is injected inside the inferonasal quadrant of the capsular bag, either just distal to the optic or at the haptic-optic junction, using the edge of the IOL optic to wipe the droplet from the cannula. Size and placement are then quickly confirmed by direct visualization, and the cannula is withdrawn. If necessary, the anterior chamber and incisions may then be gently rehydrated with BSS, taking care not to disrupt the spherule.
At this point, the surgeon may elect to inject intracameral antibiotic (if not administered prior to intraocular DXM) and/or Miochol-E (Bausch & Lomb, Inc.), avoiding turbulence by directing the stream away from the spherule of drug, preferably into the anterior chamber.
Tips for Preparing the Eye
- A well-dilated pupil enables visualization of the haptic-optic junction and injection of the drug inside the capsular bag. (If the pupil is too small, the surgeon may consider using the ciliary sulcus technique.)
- Consider the orientation of the haptic-optic junction when placing the IOL; for example, some surgeons find success with orienting the haptic-optic junction in the inferior position in order to make maximum use of gravity for keeping the spherule in place. Others may prefer to align the haptic with the plane of the paracentesis so that the intraocular DXM cannula can be easily navigated to the haptic-optic junction.
- Intraocular DXM should be the final step before reinflating the eye and finishing the case. Prior to intraocular DXM injection, incisions should be perfectly sealed and surgery should be complete. Toric or multifocal lenses should be centered and oriented exactly as desired, so there is no further need to manipulate the IOL, which could dislodge the spherule.
- Having a slightly elevated intraocular pressure provides a buffer should BSS escape during intraocular DXM placement. Further, the ability to hyperpressurize the eye is a good indicator that wounds are indeed tight.
Capsular Bag Technique Pearls
- Mixing intraocular DXM thoroughly with the vortex mixer makes it easier to draw up and ensures the expected efficacy.
- The vial contains more intraocular DXM than is needed for a single dose. Aspirating only 0.2 cc into the syringe leaves enough in the bottle to repeat the draw if there is any need to do so.
- Choice of cannula (straight or curved) and site of entrance is based on the surgeon’s handedness, technique, and preference. Some right-handed surgeons use a curved cannula to deliver intraocular DXM to the inferonasal quadrant of the left eye, starting at the inferotemporal incision; and a straight cannula for the right eye (since the path from the supratemporal quadrant to the inferonasal quadrant is straight). This strategy would be reversed for left-handed surgeons, who may choose to use a curved cannula for the right eye. Other surgeons prefer to enter via a superior paracentesis (which, depending on eye/handedness, may need to be created specifically for intraocular DXM administration).
- Intraocular DXM placement peripheral to the optic, at the haptic/optic junction, or just peripheral to the haptic minimizes risk for displacement. At the end of forward pressure on the plunger, a gentle sweeping motion can help attach the spherule to the IOL.
Troubleshooting Tips
- Keep the cornea clear to avoid intraocular DXM falling and adhering to it.
- Keep the chamber stable
- If Dexycu (DXM) adheres to the IOL, it can easily be dislodged or moved with a gentle stream of fluid.
- If intraocular DXM comes out of solution and/or clogs the cannula, longer shaking in the vortex mixer may be needed.
- If the intraocular DXM spherule is prematurely injected or not placed where intended, the spherule may be aspirated back into the cannula and reinjected in the desired location.
- If small satellites of intraocular DXM are visible around the main bolus, they may either be reaspirated or left alone.
- The supplied injection kit includes a spacer for quantifying drug volume and other features to assist surgeons in administering the correct dose. However, if excess intraocular DXM is instilled (ie, if the spherule appears too large), it may be partially or completely aspirated back into the syringe. On the other hand, if too little intraocular DXM is injected, more can be added.
- If for any reason it becomes necessary to start over, for example if an air bubble interfered with the formation of the Verisome, the drug may be aspirated back into the syringe or gently “pressure washed” out of the anterior chamber and the process repeated. Typically, there is enough intraocular DXM left in the vial for a second attempt.
- If intraocular DXM injection causes the chamber to collapse due to torsion on the wound, reinflate the eye gently, preferably through a paracentesis.
- Make sure the spherule has released from the cannula before withdrawing. If intraocular DXM sticks to the cannula and migrates into the anterior chamber as the cannula is withdrawn, it is acceptable to leave undisturbed. Though we aim to keep the Verisome out of the angle, in our experience, when it happens most patients’ postoperative course is not adversely affected.
Ideal Candidates for Administering Intraocular DXM via the Capsular Bag Technique
Intraocular DXM administration by the capsular bag technique is appropriate for most routine and complicated cataract surgeries, including in conjunction with minimally invasive glaucoma surgery (MIGS) procedures.13 Patients with glaucoma were excluded from intraocular DXM clinical trials; however, 66 patients with glaucoma (N = 80 eyes) were among 527 patients included in a recent large, multi-center retrospective analysis of real-world intraocular DXM use following cataract surgery. Fifteen of 80 (19%) glaucomatous eyes had MIGS procedure at the time of cataract surgery. The study found that anterior chamber cell clearing (grade 0) and mean IOP on postoperative days 1, 8, 14, and 30 were similar in eyes with glaucoma treated with intraocular DXM compared with the overall intraocular DXM-treated cohort.14
In addition to routine cataract cases, patients with a history of uveitis, cystoid macular edema, or other conditions that may predispose them to excess postoperative inflammation are excellent candidates for intraocular DXM, either alone or with supplemental low-potency topical corticosteroid (the “belt and suspenders” approach).
There are a few instances in which the capsular bag technique is not advisable, for example, when there is a lack of capsular bag integrity, such as after a posterior capsular tear.
Anything that reduces the likelihood of maintaining a deep and stable chamber—such as a poor incision or leak—may compromise the success of the technique. Injection in the capsular bag may be difficult if the patient has tight lids, a proptotic eye, or other condition that causes positive pressure. The technique may not be advisable following an anterior vitrectomy or in an eye at risk for vitreous prolapse. If there is increased posterior vitreous pressure and an unstable eye, all elective injections, including intraocular DXM, are best avoided.
In a prospective study of 20 eyes treated with intraocular DXM using the capsular bag technique, 19 injections were successful and one was aborted after two attempts due to positive pressure. In 16 of the 19 eyes in which intraocular DXM was successfully injected, the drug depot remained in the bag; in three it was noted to have migrated into the anterior chamber by the first postoperative day or during the first week (Osher, personal communication).12
IOL Material
The intracapsular administration technique takes advantage of the fact that intraocular DXM adheres to the IOL, which helps it to stay in place, and it appears to work well with the range of available IOL types and materials, including hydrophilic acrylic, hydrophobic acrylic, silicone, single-piece, three-piece, toric, and multifocal lenses.
One of us (RW) has observed that intraocular DXM seems to have the greatest affinity for hydrophilic acrylic lenses, slightly less for hydrophobic acrylic lenses, and least for silicone lenses. None of us has tried it, but we can speculate that intracapsular administration of intraocular DXM might be more challenging in conjunction with a Crystalens (Bausch + Lomb) accommodating IOL (or other lens in which a larger capsulorrhexis is preferred).
Concomitant Intraocular Medications
We have observed no interactions or issues with concurrent use of other intraocular medications (eg, cefuroxime, moxifloxacin, Miochol, OMIDRIA [phenylephrine and ketorolac injection 1%/0.3%]) with intraocular DXM (RW, LN). This is consistent with findings from the retrospective analysis of intraocular DXM use cited above (N = 641 eyes in 527 patients) in which concomitant use of intraocular DXM with OMIDRIA (23%) and/or intracameral antibiotic (14%) was not associated with complications.15
Follow Up and Patient Satisfaction
Research has shown that nearly all patients prefer regimens with minimal drop burden following cataract surgery.9 A prospective study in which patients had one eye treated with intraocular DXM plus intracameral antibiotic and the other eye treated with a standard topical regimen demonstrated significantly higher satisfaction and lower out-of-pocket costs with the intraocular DXM-containing regimen.9 The study also showed comparable inflammation scores and a trend toward less pain experienced in the intraocular DXM-treated eye.
In our experience as well, patients treated with intraocular DXM administered by the capsular bag technique report high levels of satisfaction. Prior to surgery, most patients in our clinics are delighted to find out that they will not require a complex regimen of multiple daily drops following their surgery, particularly those who had personal experience with the standard drops regimen following a prior cataract surgery. Following surgery, patients are comfortable and report no other visual symptoms. We have been delighted that the inflammation seems controlled with rapid disappearance of cells. In our collective experience, intraocular DXM-treated patients have not reported visual symptoms or required topical corticosteroids for breakthrough inflammation. Our nursing and clinic staff also prefer the simpler postoperative course following intraocular DXM.
It is useful to assess intraocular DXM location on follow-up examination. If the drug depot has remained in place in the capsular bag, it can be seen on oblique view slit lamp examination through a dilated pupil by directing the patient’s gaze toward the side of placement, usually nasally, and rotating the slit lamp temporally. If intraocular DXM has migrated to the anterior chamber, which is less common with the capsular bag technique vs the sulcus technique, a small white dot will be visible on gross inspection.
Following a series of 20 patients treated using the capsular bag technique, gonioscopic examination revealed that intraocular DXM remains stable within the capsular bag for up to 3 weeks (Osher, personal communication).12 Intraocular DXM is typically visible for 2 to 3 weeks, whether located in the bag or the angle, which is consistent with the drug’s pharmacodynamics. In the series described, no patient experienced elevated IOP, corneal edema, or any unexpected problems.
Video Cases
Video 1 (Courtesy Robert Osher, MD)
Following viscoelastic removal and deepening the chamber, both incisions are hydrated until they are watertight. The 27-gauge cannula is then introduced into the bag, and intraocular DXM is injected, with excess withdrawn back into the cannula as needed. The wound continues to be watertight, and no further manipulation of the lens or disruption of the spherule occurs with the stream of BSS or miotic agent. On the first postoperative day, the cornea is clear and the eye is quiet.
Conclusions
Sustained drug delivery is the way of the future in ophthalmology—offering more reliable and consistent drug levels, greater surgeon control over the postoperative course, and a generally better experience for patients. A single-dose, sustained-release formulation of dexamethasone designed for placement at the end of surgery, intraocular DXM enables anti-inflammatory action directly at the site of greatest inflammation and effectively transfers the responsibility of drug delivery out of the hands of patients and into the hands of surgeons.
The original administration technique into the ciliary sulcus, immediately behind the iris, is associated with several challenges, including lack of visual confirmation of placement and significant risk for migration into the anterior chamber, where the drug may make direct contact with the iris, ciliary body, trabecular meshwork, and corneal endothelium. Some surgeons now favor an alternative technique of injecting intraocular DXM into the capsular bag, which enables better visualization and stability. A growing body of evidence shows that the capsular bag technique is effective, safe, reproducible, and simple to perform.12
Administration of intraocular DXM by the capsular bag technique is appropriate for most patients undergoing cataract surgery and may be used with common IOL materials. Keys to success include having a well-dilated pupil, well-sealed incisions, a deep and stable chamber, and a well-pressurized eye with an intact capsular bag. The capsular bag technique is associated with excellent clinical outcomes and high patient and surgeon satisfaction.
Abbreviations
BSS, balanced salt solution; CME, cystoid macular edema; DXM, dexamethasone intraocular suspension 9%; IFIS, intraoperative floppy iris syndrome; IOP, intraocular pressure; MIGS, minimally invasive glaucoma surgeries; NSAIDs, non-steroidal anti-inflammatory drugs; OCT MT, optical coherence tomography macular thickness; SOIS, Summed Ocular Inflammatory Scores.
Acknowledgments
Noelle Lake, MD, provided medical writing support.
Funding
Supported by EyePoint Pharmaceuticals.
Disclosure
Cathleen McCabe, MD, is a consultant to EyePoint Pharmaceuticals, Alcon, Novartis, Bausch + Lomb, Ocular Therapeutix, Kala, Omeros, Johnson and Johnson, and Lensar. Priya Desai, MD, is a consultant to EyePoint Pharmaceuticals. Lisa Nijm, MD, is a consultant to EyePoint Pharmaceuticals and Omeros. Robert Osher, MD, has been a consultant to EyePoint Pharmaceuticals. Robert Weinstock, MD, is a consultant to Alcon, Bausch + Lomb, Johnson and Johnson, Lensar, Omeros, and EyePoint Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000;11:3–6. doi:10.1097/00055735-200002000-00002
2. Shorstein NH, Myers WG. Drop-free approaches for cataract surgery. Curr Opin Ophthalmol. 2020;31:67–73. doi:10.1097/ICU.0000000000000625
3. Salinger CL, Gaynes BI, Rajpal RK. Innovations in topical ocular corticosteroid therapy for the management of postoperative ocular inflammation and pain. Am J Manag Care. 2019;25(12 Suppl):S215–S226.
4. DEXYCU® (dexamethasone intraocular suspension) 9% full US. Prescribing information; 2020.
5. Wong VG, White WS, Hu MW, Huang GT, Karasina F. Use of sustained release dexamethasone in post-cataract surgery inflammation. US patent application 14/893,381. 2016 May 5.
6. Donnenfeld E, Holland E. Dexamethasone intracameral drug-delivery suspension for inflammation associated with cataract surgery: a randomized, placebo-controlled, Phase III trial. Ophthalmology. 2018;125:799–806. doi:10.1016/j.ophtha.2017.12.029
7. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: phase 3 multicenter study. J Cataract Refract Surg. 2018;44:1236–1246. doi:10.1016/j.jcrs.2018.07.015
8. Matossian C, Hovanesian J, Bacharach J, et al. Impact of dexamethasone intraocular suspension 9% on intraocular pressure after routine cataract surgery: post hoc analysis. J Cataract Refract Surg. 2021;47(1):53–64. doi:10.1097/j.jcrs.0000000000000363
9. Hovanesian J, Donnenfeld E. The D3 Study: drug delivery vs drops—a prospective clinical study evaluating Dexycu vs prednisolone acetate 1% in controlling post-operative pain and inflammation in patients undergoing sequential cataract surgery.
10. Kiernan DF. Dexamethasone intracameral drug-delivery suspension for inflammation associated with vitreoretinal surgery. BMJ Open Ophthalmol. 2020;5(1):e000491. doi:10.1136/bmjophth-2020-000491
11. Weinstock RJ. Dexamethasone intraocular suspension 9% after cataract surgery: data from a retrospective study.
12. Osher RH, Weinstock RJ. Dexamethasone intraocular suspension 9% in the capsular bag. J Cataract Refract Surg. 2021;47(9):1239. doi:10.1097/j.jcrs.0000000000000458
13. Tan NE, Radcliffe N. Outcomes of dexamethasone intraocular suspension during cataract surgery and concomitant cataract-micro-invasive glaucoma surgery (MIGS).
14. Hanson P, Tan N, Tracer N, Radcliffe NM. Use of dexamethasone intraocular suspension 9% in patients with glaucoma: results from a retrospective study. Invest Ophthalmol Vis Sci. 2021;62(8):3433.
15. Matossian C. Outcomes with dexamethasone intraocular suspension 9% and concomitant postoperative antiinflammatory medications.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
