Back to Journals » Patient Preference and Adherence » Volume 17
Real-World Experience of People with Hemophilia A Receiving Turoctocog Alfa Pegol (N8-GP): Results from a Patient Experience Survey
Authors Eichler H , Nagao A, Waller J, Stuber A
Received 26 July 2023
Accepted for publication 8 November 2023
Published 17 November 2023 Volume 2023:17 Pages 3001—3014
DOI https://doi.org/10.2147/PPA.S394216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Hermann Eichler,1 Azusa Nagao,2 John Waller,3 Alexander Stuber4
1Institute of Clinical Hemostaseology and Transfusion Medicine, Saarland University, Homburg, Germany; 2Department of Blood Coagulation, Ogikubo Hospital, Tokyo, Japan; 3Novo Nordisk, Zurich, Switzerland; 4Patient Author, Braunwald, Switzerland
Correspondence: Hermann Eichler, Institute of Clinical Hemostaseology and Transfusion Medicine, Saarland University, Ringstrasse 52, Geb. 1, Homburg, 66421, Germany, Tel +49 6841 1622530, Email [email protected]
Purpose: Turoctocog alfa pegol (N8-GP) is an extended half-life recombinant factor VIII molecule used for the treatment of hemophilia A (HA). The purpose of this study was to investigate real-world experiences of patients with HA treated with N8-GP.
Patients and Methods: A 25-minute online survey was completed by adults (≥ 18 years) and caregivers of adolescents (12– 16 years) with HA receiving N8-GP across six countries (Germany, Italy, Portugal, Spain, UK and US). Patients were recruited using a multichannel approach through recruitment panels, referrals from healthcare professionals and patient associations. The survey comprised a questionnaire with metrics including satisfaction and preferences for N8-GP, quality of life (QoL) and long-term impact.
Results: A total of 62 participants (98% male [n=61], mean age 29 years) comprising 46 patients and 16 caregivers completed the survey. Patients (60% non-severe [n=37] and 40% severe [25]) were on N8-GP for a mean period of 1.4 years. Patients expressed satisfaction (95% vs 42%, p< 0.001) and preference (91% vs 9%, p< 0.001) for N8-GP vs their previous treatments. Most patients with severe HA (87%, p=0.038) and patients on prophylaxis (84%, p< 0.001) stated lower frequency of injections as their main reason for satisfaction, while improved QoL drove satisfaction for non-severe patients (81%, p=0.053). Overall, patients perceived that QoL score improved (74.8 vs 65.9, p=0.01) with N8-GP treatment compared with previous treatments. Flexibility to store at room temperature was one of the key convenience factors driving satisfaction. Patients believed that N8-GP can offer a long-term impact in areas such as ability to perform day-to-day activities (68%), independence to live like a person without hemophilia (63%), ability to travel (60%) with a feeling of optimism and hopefulness (82%).
Conclusion: Lower frequency of injections, storage flexibility and improved QoL drove satisfaction and preference for N8-GP over previous treatments among patients with HA.
Plain Language Summary: Hemophilia A is a rare bleeding disorder that often runs in families. Although there is no cure, several therapeutic options are available to help control bleeding in people with hemophilia A. However, most treatments require intravenous (directly into a vein) or subcutaneous (directly under the skin) injections, which is a significant burden for all patients. In this study, adults and caregivers of adolescents, with hemophilia A answered a survey about their treatment experience with a medicine called N8-GP (turoctocog alfa pegol) compared with previous treatments.
This is the first real-world evidence study focused on satisfaction with and preference for N8-GP compared with previous treatments. Survey results showed that adults and adolescents with hemophilia A were very satisfied with N8-GP compared with previous medicines. The main reasons for satisfaction included less frequent injections, the flexibility to store at room temperature, and improved quality-of-life.
In addition, many patients expressed hopes for the future while taking N8-GP, such as confidence in ability to undertake physical activities and ability to plan and go on a holiday. Compared with previous treatments, patients were feeling optimistic and hopeful about N8-GP and expressed that they have started to think less about the disease.
Despite the unique advantages of taking N8-GP, patients overall still perceive their quality-of-life to be less than an average person’s, presenting an opportunity for future advancements. Overall, this study sheds light on the unique experiences of people with hemophilia A taking N8-GP and further opens up the scope for addressing the unmet needs.
Keywords: hemophilia A, turoctocog alfa pegol, patient preference, real-world evidence, patient satisfaction, quality of life
Introduction
Hemophilia A (HA) is a congenital X-linked recessive bleeding disorder that occurs due to insufficiency or absence of coagulation factor VIII (FVIII). The clinical hallmark of HA is repeated and prolonged bleeding into muscles and joints that results in pain, limitations to physical functioning, and impact on wider health-related quality of life (HRQoL).1,2 The current standard of care for patients with HA includes prophylactic treatment with clotting factor or non-factor replacement products.3 While standard half-life (SHL) factor products have proved to be effective in the management of HA, their short half-life (8–12 hours) and requirement for frequent intravenous infusions make treatment burdensome.4,5 Extended half-life (EHL) factor products offer improved pharmacokinetic characteristics and quality of life (QoL) when compared with SHL products due to their longer half-life (13–20 hours) and need for fewer injections.6,7 Turoctocog alfa pegol (N8-GP, Novo Nordisk, Bagsvaerd, Denmark) is an EHL recombinant FVIII molecule used for both prophylaxis and on-demand treatment of HA.8
The safety and efficacy of N8-GP has been demonstrated in the pathfinder clinical trials program where the prolonged half-life (~1.6 fold) and increased stability at room temperature (RT, 30 to 40°C) with N8-GP resulted in reduced injection frequency, increased storage flexibility, and reduced annualized bleeding rates (ABRs). This in turn improved patients’ performance in their day-to-day activities.8,9 Despite accumulating evidence on the benefits of N8-GP in clinical trials, it is important to understand if real world evidence (RWE) studies also support the existing literature through assessment of patients’ experiences with the treatment.10,11 These studies can provide patients’ perspectives regarding benefits, adverse events, unmet needs, and overall experience. Therefore, by complementing traditional clinical data, RWE studies provide additional insights into the broader impact of a drug on patients’ lives.10,11 In this online survey, we aimed to understand the real-world experiences of patients with HA receiving N8-GP.
Materials and Methods
Study Design and Participants
This was an observational, 25-minute online survey for patients and caregivers of adolescents with HA receiving N8-GP across six countries (Germany, Italy, Portugal, Spain, UK, US), conducted between 04 January 2022 and 28 July 2022. The data were collected by a third-party vendor (Lumanity). Patients (adults and adolescents) aged ≥12 years diagnosed with HA without inhibitors who had been using N8-GP for >3 months (Portugal) and >6 months (other countries) were included in the study. The caregivers of adolescents were required to be relatives, spouses, friends, or unpaid carers living in the same household as the patient or spending ≥25 hours a week caring for the patient. Patients and members of their household employed by the pharmaceutical industry were excluded from the study. All participants signed an electronic written informed consent form through a survey link and were remunerated for their time. The participants consent for publication was obtained prior to the commencement of the survey and data were anonymized. All data and information relating to the patients and caregivers were protected to ensure adherence to appropriate quality and ethics guidelines.
Patient recruitment was performed via a multichannel approach which used recruitment panels, referrals from healthcare professionals treating patients with HA, private social media campaigns, and patient associations (Appendix 1). The study was approved by BRANY, an independent review board in the United States, with the study number 21-10-531-961.
Lumanity and their partnering agencies (Gillian Kenny Associates, MGCRRC, IR Berlin, Guru Research, Nueva Investigacion, Indago Health, Seed Planning and Field and Fact, UGAM, and GlobaLexicon) abided by market research guidelines from the British Healthcare Business Intelligence Association and European Pharmaceutical Market Research Association. Those involved in data collection and processing were required to sign confidentiality non-disclosure forms to secure patients’ and caregivers’ information anonymously. The survey was executed in compliance with European General Data Protection Regulations.
Patient Reported Outcomes and Questionnaires
Data regarding patients’ current health status and their perceptions about previous and current treatments were collected using structured questionnaires in English, French, German, Italian, Portuguese, and Spanish (please refer to Appendix 2 for all the questionnaires). The current health status according to patients and caregivers was assessed using the EQ-5D-5L instrument previously described.12 In addition, in order to record the overall quantitative measure of current health status on a vertical visual analogue scale, the EuroQol-visual analogue scales (EQ-VAS) questionnaire was used with endpoints ranging from worst to best health on a scale of 0 (worst health) to 100 (best health). Data on the perceptions of patients and caregivers on current and previous treatments included several metrics such as treatment satisfaction and the convenience of factor administration, effect on joint health, impact on QoL, and activities of daily living.
Data Processing and Statistical Analysis
The data were cleaned and responses to open-ended questions were back coded. The final data were labelled appropriately along with key quality data such as serial number, date, time, and length of interview. Responses to open-ended questions (verbatim) were translated and coded manually according to the code frame (a list of themes and groups of themes that are assigned a code number, and then used to code and quantify answers to open-ended questions), which was quality controlled and approved by a member of the core project team at Lumanity. These aspects, together with key criteria such as age, time since diagnosis, duration of use with N8-GP, etc were used as cross-breaks to produce a complete set of tables for the data (refer to Supplementary Materials).
The data were summarized descriptively and p-values of <0.05 were considered statistically significant. Perceptions of patients and caregivers to N8-GP were statistically compared with their perceptions of previous treatments (compared with no treatment for HA if no previous treatment). Significance testing (Z-test for two proportions and t-test for two means) was performed on comparative samples ≥30 with a confidence level of 95%. Given the relatively small sample size for each country, the statistical analysis was conducted on the global data across countries.
Results
A total of 62 participants completed the survey (46 patients and 16 caregivers). The mean patient age was 29 years, mean time since diagnosis of HA was 28 years, and the majority of patients were male (98%, n=61) (Table 1). The participants who responded to the survey had been on N8-GP treatment for an average of 1.4 years, and the majority were previously on another prophylactic treatment (n=58) for a mean duration of 6.5 years (Figure S1A). Sixty percent (60%, n=37) of patients had non-severe HA (FVIII activity 1–40%), while the remaining 40% (n=25) had severe HA (FVIII activity <1%) (Figure S1B; Table S1). A relatively large proportion of patients (61%, n=28) were currently employed and approximately a quarter (26%, n=12) were students (Table 1). The mean caregiver age was 44 years, the majority of whom were female (94%, n=15), and all were living with the patients. Most of the caregivers were the patients’ mothers, with the remaining 6% (n=1) being legal guardians (Table 2). For patients on N8-GP, a larger proportion (76%, n=47) were receiving prophylactic treatment, and a quarter (24%, n=15) were being treated on demand with most of the severe patients on prophylaxis (96%, n=24) (Figure S1C & Table 3). During their previous treatments, 74% (n=44) were treated prophylactically with additional on-demand doses, 23% (n=14) were treated on-demand and 3% (n=2) treated with prophylaxis only (Figure S1C). Most previous treatment comprised recombinant FVIII concentrates (73%, n=44), of which 16% (n=10) received different EHL products (Efmoroctocog alfa or Damoctocog alfa pegol) (Table S2).
 |
Table 1 Patient Demographic Details |
 |
Table 2 Caregiver Demographic Details |
 |
Table 3 Mode of Treatment |
Patient Current Health Status
All patients who were able to complete the survey themselves responded to the questions (n=46). Most of the patients reported no issues with mobility (ie, walking, 78%, n=36), self-care (85%, n=39), or in performing usual activities (61%, n=28) (Figure 1A). Nearly two thirds of patients had experienced problems with pain (67%, n=31), with “slight problems with pain” being the largest category (48%, n=22). Just over half (56%, n=26) reported experiencing problems with anxiety/depression (Figure 1A). Both non-severe and severe patients reported no issues with self-care (85%, n=22 non-severe; 85% n=17 severe) with non-severe patients feeling more anxious/depressed (69%, n=18) (Figure 1B). The overall average health score was 0.83. Moreover, when asked to rate their health on that day on a scale from 0 to 100, with 100 being the best score, the median score was 75 (Figure 1).
Patient Satisfaction and Preference for N8-GP Over Previous Treatments
Most of the participants (73%, n=45) accepted the need for constant life-long treatment for hemophilia (Figure S1D) and the main reasons to start on N8-GP were less frequent injections (65%, n=40), improved QoL (60%, n=37), and convenient treatment (53%, n=33) (Table S3).
The majority of participants and subgroups expressed satisfaction with N8-GP (95%, n=59) compared with previous treatments (42%, n=26; p<0.001), with the main reasons for satisfaction including lower frequency of injections (46%, n=27) and improved QoL (14%, n=8; Figure 2A and B; Figure S2A and B). Importantly, lower frequency of injection drove most satisfaction among patients with severe HA compared with non-severe patients (87% vs 61%, p=0.038; Figure 2C). Patients on prophylaxis and on-demand treatment were equally satisfied with improved QoL (Figure 2D).
Participants preferred N8-GP over their previous treatments (91%, n=53 vs 9%, n=6, p<0.001) (Figure 3A) due to its treatment convenience (79%, n=42), improved product characteristics (45%, n=24), and positive outcomes/feelings (23%, n=12) (Figure 3B). A large proportion (58%, n=31) felt that the lower frequency of injections contributed to the convenience of taking N8-GP (Figure 3B). Patients with severe HA (96%, n=24) and those who were on prophylaxis (93%, n=41) predominantly preferred N8-GP over previous treatments (Figure 3C).
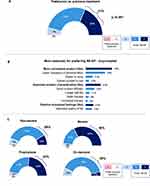 |
Figure 3 Preference for N8-GP over previous treatments. (A) Preference for N8-GP over previous treatments (n=53, 91%). (Patients rating 4 or 5 on a 5-point scale; Q38a). (B) Main unprompted reasons for preferring N8-GP (Q38b). Net indicates combined scores for parameters falling within main reasons. Base: patients who preferred N8-GP to previous treatments (n=53) (Please refer to Appendix 1 for examples of data analysis). (C) Preference for N8-GP for patient subgroups: Non-severe (n=29, 88%) and severe (n=24, 96%); prophylaxis (n=41, 93%) and on-demand (n=12, 86%). (Please refer to supplementary materials for examples of data analysis). Tx: Treatment. Base: All patients with a previous treatment (n=58). |
Impact of Hemophilia and N8-GP Treatment on QoL of Patients
The impact of hemophilia on routine activity levels and QoL was assessed in different patient groups. Compared with adults, the overall QoL was affected for the majority of adolescents due to hemophilia (caregivers, n=12, [71%] vs adults, n=16, [36%]), as well as day-to-day routine activities (caregivers, n=12 [71%] vs adults, n=11 [24%]) and levels of stress and anxiety (caregivers, n=9 [53%] vs adults, n=11 [24%]) (Table 4). Compared to patients with severe hemophilia, the overall QoL (non-severe, n=23, [62%] vs severe, n=5, [20%]) and day-to-day routine (non-severe, n=23, [54%] vs severe, n=3, [12%]) was affected for most of the non-severe patients due to hemophilia. (Table 4). The impact of hemophilia on QoL did not differ significantly between the prophylaxis and on-demand patient groups (Table 4).
 |
Table 4 Impact of Hemophilia on Day-to-Day Life |
Most of the participants experienced that N8-GP improved their overall QoL (n=52, 84%), daily living (n=51, 82%), and joint health (n=40, 65%) with a mean increase in QoL score from 65.9 to 74.8 (p<0.01) compared with the previous treatments (refer to survey questions Q26 and Q35 in Supplementary Materials) (Figure 4A and B). The QoL score improved across all patient groups: adults and adolescents; non-severe and severe; prophylaxis and on-demand compared with the previous treatments (Table S4).
Performance and Long-Term Impact of N8-GP
N8-GP was perceived to be more convenient in terms of frequency of injections in patients with severe HA (80%, n=20) and patients on prophylaxis (81%, n=38). One of the convenient factors similarly scored by all participant groups was storage flexibility (76% non-severe [n=28]; 56% severe [n=14]; 66% prophylactic [n=31] and 73% [n=11] on-demand) (Figure 5A and B). In particular, in countries such as Italy and Germany, the patients’ expectations for ability to store N8-GP at RT were achieved (88% and 64%, respectively) (Table S5). Patients and caregivers expressed their satisfaction through verbatim responses indicating that they were content with simple storage without the need for refrigeration and the convenience with the short treatment setup (Table S6).
Long-Term Impact of N8-GP on Patients’ Lives
When asked about the long-term impact of N8-GP on patients’ lives, apart from improving QoL (79%, n=49) and daily routine (68%, n=42), most participants agreed that they felt they had “independence to live like a person without hemophilia” (63%, n=39), “the ability to go on a holiday” (60%, n=37) and “feelings of self-worth and self-confidence” (56%, n=35) (Figure 6A). Compared with their feelings towards their previous treatment, 82% (n=51) felt more “optimistic and hopeful”, 76% (n=47) felt “being free or unbound”, 73% (n=45) felt “in control of hemophilia”, and 66% (n=41) have started “to think less about hemophilia” (Figure 6B).
Discussion
Summary of Findings
This is the first RWE survey study addressing patients’ experience with N8-GP. This study reports novel insights on treatment preference, satisfaction, and QoL of patients on N8-GP in comparison with their previous treatments. A large proportion of patients included in the survey reported higher levels of satisfaction and preference for N8-GP compared with their previous treatments. Most of the patients with severe HA expressed satisfaction due to the lower frequency of injections, while satisfaction for non-severe patients was driven by improved QoL. Important reasons for N8-GP preference included “more convenient” (79%, lower frequency of injections and easier to store), “improved product characteristics” (45%, efficacy, safety, longer half-life), and “overall improvement in QoL” (23%). Patients perceived their QoL to be lower prior to starting treatment with N8-GP with an overall increase of QoL score after starting N8-GP. Additionally, N8-GP was perceived to be better than previous treatments in the aspects of frequency of injections and flexibility of storage. Furthermore, patients stated that N8-GP had a positive long-term impact on “overall QoL”, “daily routine”, “ability to live like a person without hemophilia”, “plan for holidays”, and felt optimistic and in control of hemophilia.
Previous Pathfinder Data Supporting Current results
Interestingly, the survey results were in line with previous pathfinder studies that reported positive outcomes regarding treatment with N8-GP. An analysis of HRQoL in patients with HA on a long-term N8-GP regimen demonstrated that HRQoL scores were maintained or further improved with N8-GP, with a high level of treatment satisfaction.13 Importantly, the data that support findings for treatment satisfaction are derived from low median ABR reported in the pathfinder2 clinical trial.14 In exit interview findings from adolescents and adults participating in the pathfinder8 trial, respondents reported that N8-GP was effective in the management and control of bleeds, with improvements in physical functioning, energy levels, frequency of doses, and treatment burden level.15 In the current study, majority of patients agreed that N8-GP offers joint protection to enable stable joint health (65%).
Challenges and Unmet Needs of Patients
The major challenges for patients with HA are their inability to undertake different physical activities and the perceived burden of the disease. Though previous illustrative cases from the pathfinder clinical trials demonstrated improved physical activity levels, bleeding rates and treatment adherence,16 there are still unmet needs for the patients, which was also evident from this study. Less than half (44%, n=22) acknowledged that N8-GP had an impact on reducing pain (Figure S3A) and some economic factors such as days missed at school/college (31%, n=8) and ability to work in more demanding roles (19%, n=5; Figure S3B). With advances in hemophilia care, there is much focus on HRQoL factors. The Hemophilia Experiences, Results and Opportunities (HERO) study in North America reported that approximately 30% of patients with hemophilia experienced severe pain associated with increase in age (>40 years) as well as lower employment and HRQoL.17 A cross-national survey of 364 participants conducted in central Europe demonstrated that frequent difficulties in daily life fell into domains such as mobility, pain, unexpected bleeding, and uncertainty in patient ability to perform activities, which highlights key aspects of patients’ unmet needs in hemophilia18 in line with these findings. The findings from this study demonstrated that 56% of patients completing the survey themselves experienced anxiety and depression (Figure 1), which is higher than the observations from the Cost of Hemophilia in Europe: a Socioeconomic Survey (CHESS) study) that showed 31% of respondents experienced moderate to severe anxiety and depression with compromised employment and productivity compared with the general population.19
Advantages and Limitations of the Study
The advantages of this study include the ability to have both the patients’ and caregivers’ perspective on experiences with N8-GP, the international nature of the study, and how the survey was designed and implemented to capture the real-world experience of people on N8-GP. Some of the limitations of the study include the uncertainty of the data associated with patient and caregiver interpretation of the questions used in the survey, and the relatively small sample size with a mixed cohort of patients with varying degree of exposure such as patients treated on-demand. Selection and recall bias is an additional limitation of the study that represents patients who were on N8-GP for more than 3 months in some countries, while most of the patients were exposed over more than 6 months in other countries.
Conclusion
Overall, this study presents a perceived patient experience with N8-GP for the management of HA and its impact on QoL. The advantages of lower frequency of injections, easier storage and improved QoL offered by N8-GP drove patient satisfaction and preference over previous treatments among patients with HA. Patients reported that the storage flexibility of N8-GP was the most important factor in improving their QoL, allowing them to plan travel without storage concerns. Despite having positive experiences with treatment, some patients perceived an emotional burden and felt that their QoL was less than that of the average person without hemophilia. This reinforces the need for future advancement in hemophilia treatment, which could be valuable to address the remaining unmet needs of these patients.
Abbreviations
HA, Hemophilia A; QoL, quality of life; HRQoL, health-related quality of life; ABR, annualized bleeding rates; FVIII, factor VIII; SHL, standard half-life; RWE, real-world evidence; EHL, extended half-life; EQ-5D-5L, EuroQol-5 Dimension; EQ-VAS, EuroQol-visual analogue scales; HERO, The Hemophilia Experiences, Results and Opportunities; CHESS, Cost of Hemophilia in Europe: a Socioeconomic.
Acknowledgments
The authors would like to thank the patients and caregivers who completed the survey. This study was funded by Novo Nordisk Health Care and medical writing support was provided by Dr. Ambika Shivakant Kurbet, PhD of Novo Nordisk India Pvt Limited, Global Medical Affairs, Bengaluru, India. We also thank Ashfield MedComms GmbH (Mannheim, Germany, an Inizio company) for editorial support, which was funded by Novo Nordisk. We thank Masha Eletskaya and Florence MacIver Bulbrook from Lumanity for data collection and analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
AN has received investigator-initiated grant funding from Takeda (Shire), Chugai and Bayer; and has received honoraria from Sanofi, Takeda, Chugai, Bayer, Fujimoto, KMB, Pfizer, JB, Novo Nordisk, Sekisui Medical and CSL. AS does not have any obligations towards any of the companies involved in the study or the development of the medication. HE has received research support, honoraria, or consultation fees from Bayer Vital, BioMarin, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, and Sobi. JW is an employee of Novo Nordisk. The authors report no other conflicts of interest in this work.
References
1. Gringeri A, Ewenstein B, Reininger A. The burden of bleeding in haemophilia: is one bleed too many? Haemophilia. 2014;20(4):459–463. doi:10.1111/hae.12375
2. Okaygoun D, Oliveira DD, Soman S, Williams R. Advances in the management of haemophilia: emerging treatments and their mechanisms. J Biomed Sci. 2021;28(1):64. doi:10.1186/s12929-021-00760-4
3. Mannucci PM. Hemophilia therapy: the future has begun. Haematologica. 2020;105(3):545–553. doi:10.3324/haematol.2019.232132
4. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26(Suppl 6):1–158. doi:10.1111/hae.14046
5. Chowdary P. Extended half-life recombinant products in haemophilia clinical practice - expectations, opportunities and challenges. Thromb Res. 2020;196:609–617. doi:10.1016/j.thromres.2019.12.012
6. McCall M, Koerner P, Miller R, Radi M. Comparison of extended to standard half-life recombinant factor VIII therapy in patients with hemophilia A on prophylactic therapy. J Drug Assess. 2019;8(sup1):46–47. doi:10.1080/21556660.2019.1658327
7. Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemost. 2015;13(Suppl 1):S176–S179. doi:10.1111/jth.12929
8. Stennicke HR, Kjalke M, Karpf DM, et al. A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models. Blood. 2013;121(11):2108–2116. doi:10.1182/blood-2012-01-407494
9. Lentz SR, Kavakli K, Klamroth R, et al. Turoctocog alfa pegol (N8-GP) in severe hemophilia A: long-term safety and efficacy in previously treated patients of all ages in the pathfinder8 study. Res Pract Thromb Haemost. 2022;6(2):e12674. doi:10.1002/rth2.12674
10. Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res. 2021;12(3):171–174. doi:10.4103/picr.picr_62_21
11. Baumfeld Andre E, Reynolds R, Caubel P, Azoulay L, Dreyer NA. Trial designs using real-world data: the changing landscape of the regulatory approval process. Pharmacoepidemiol Drug Saf. 2020;29(10):1201–1212. doi:10.1002/pds.4932
12. Wong ELY, Xu RH, Cheung AWL. Health-related quality of life among patients with hypertension: population-based survey using EQ-5D-5L in Hong Kong SAR, China. BMJ Open. 2019;9(9):e032544. doi:10.1136/bmjopen-2019-032544
13. Kearney S, Raffini LJ, Pham TP, et al. Health-related quality-of-life and treatment satisfaction of individuals with hemophilia A treated with turoctocog alfa pegol (N8-GP): a new recombinant extended half-life FVIII. Patient Prefer Adherence. 2019;13:497–513. doi:10.2147/PPA.S196103
14. Giangrande P, Andreeva T, Chowdary P, et al. Clinical evaluation of glycoPEGylated recombinant FVIII: efficacy and safety in severe haemophilia A. Thromb Haemost. 2017;117(2):252–261. doi:10.1160/TH16-06-0444
15. Waldman LT, Busk AK, Lee XY, Brod M. Exploring the treatment experiences of adolescent and adult patients with haemophilia A: exit interview findings from the pathfinder 8 trial (PO059). Haemophilia. 2022;28(S1):25–126. Presented at Annual Congress of European Association for Haemophilia and Allied Disorders.
16. Klamroth R, Hampton K, Saulyte Trakymienė S, Korsholm L, Carcao M. Illustrative cases from the pathfinder clinical trials of patients with hemophilia A treated with turoctocog alfa pegol (N8-GP). Patient Prefer Adherence. 2021;15:2443–2454. doi:10.2147/PPA.S326282
17. Forsyth AL, Witkop M, Lambing A, et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. 2015;9:1549–1560. doi:10.2147/PPA.S87659
18. Banchev A, Batorova A, Faganel Kotnik B, et al. A cross-national survey of people living with hemophilia: impact on daily living and patient education in Central Europe. Patient Prefer Adherence. 2021;15:871–883. doi:10.2147/PPA.S303822
19. O’Hara S, Castro FA, Black J, et al. Disease burden and remaining unmet need in patients with haemophilia A treated with primary prophylaxis. Haemophilia. 2021;27(1):113–119. doi:10.1111/hae.14171
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





