Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Real-World Evidence of an Emollient Device for Atopic and Contact Dermatitis in Pediatric to Adult Patients – Data from a Post-Marketing Surveillance
Authors Hebert AA
Received 19 May 2022
Accepted for publication 11 August 2022
Published 7 September 2022 Volume 2022:15 Pages 1797—1803
DOI https://doi.org/10.2147/CCID.S364934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Adelaide Ann Hebert
UTHealth McGovern Medical School, Houston, TX, USA
Correspondence: Adelaide Ann Hebert, Email [email protected]
Purpose: Atopic and contact dermatitis have been attributed to skin barrier abnormalities, immune system dysfunction and inflammation leading to pruritus. As these factors may involve oxidative stress, emollient devices containing antioxidants furfuryl palmitate and tocopherol may help reduce itching and inflammation. In this study, a post-marketing questionnaire was carried out of 40 users of a novel emollient device containing furfuryl palmitate and tocopherol (Relizema™ cream), who purchased it from a pharmacy or health-care professional to ascertain the emollient device’s action on itching, flushing and moisturizing.
Patients and Methods: The post-marketing questionnaire, administered by trained pharmacy and health-care staff, collected data on age; diagnosis; number of times per day and for how many days the emollient device was applied; whether the product helped alleviate itching and flushing, and in what time frame if so; and how their skin felt after using the emollient.
Results: Most patients had atopic dermatitis (n = 25) or irritant contact dermatitis (n = 10); most were aged ≥ 19 years (n = 20) or between 3 and 12 years (n = 12). Most used the emollient device twice a day (n = 26) or more (n = 11) with 27 using it for ≤ 30 days. Patients predominantly reported that the emollient device “significantly improved” itching (n = 34) and flushing (n = 31) with a response being noted within 1 day (n = 17) or 1 week (n = 22). All users felt the product “protected and moisturized” their skin, the product texture was “pleasant” to “excellent” and the “non-fragrance” smell was “acceptable” to “excellent”.
Conclusion: Use of a novel emollient device containing ingredients aimed at managing atopic or contact dermatitis, including furfuryl palmitate and tocopherol, led to rapid improvements in itching and flushing. The emollient device was acceptable to the users.
Keywords: post-marketing surveillance, pediatric, adult, skin barrier repair, steroid-free
Introduction
Eczematous dermatitis is proposed to arise due to skin barrier abnormalities, immune system dysfunction and inflammation leading to pruritus.1,2 One factor underlying this causative trinity is oxidative stress that, in turn, leads to damage and death of keratinocytes.3,4 Oxidative stress arises from the generation and presence of reactive oxygen species (ROS) following external environmental insults, such as the irritants that induce contact dermatitis (CD),5 and endogenous factors, such as immune system reactivity in atopic dermatitis (AD). Factors that amplify oxidative stress include physical factors and associated inflammation.3
In support of this trinity in AD, upregulation of inflammatory agents, such as cytokines,6 represent markers of oxidative stress in skin biopsies,7 urine8 and serum,9 suggesting not only localized but also systemic changes in immune response. CD is known to also arise due to inflammatory cytokine production (as a direct response to the irritant for irritant CD and as a result of an immune system reaction for allergic CD) and recruitment of immune system cells, including leukocytes, T-lymphocytes and monocytes, to the site of inflammation.10,11 In CD, ROS are thought to have a role in upregulation of inflammatory signals that recruit immune system cells into tissues.10
The burden of dermatitis can be large and especially impact children and their caregivers. In a report by the National Eczema Association, quality of life, physical functioning, academic impacts, and financial burden, including medical costs and decreased productivity of the caregiver, were highlighted as being directly related to dermatitis in a child.12 As such, effective treatment is a key factor in relieving such burdens.
Emollients, traditionally containing an occludent to reduce evaporation and an humectant to aid stratum corneum hydration, have been found very advantageous for treating and controlling skin conditions including AD and CD.13–15 While basic emollient therapy can alleviate dry skin, additional active ingredients can also target the causes of dry skin, associated inflammation and skin barrier disruption.15 The usual reaction of a cell to oxidative stress is to produce antioxidants that “mop up” the reactive species and/or prevent their formation. However, if the level of oxidative stress is much higher than can be controlled by endogenous antioxidants, cell damage and death can occur.4,10 Topical antioxidant therapy may be able to redress this balance and limit cell death and AD/CD exacerbation. One such antioxidant is furfuryl palmitate, the ester of furfuryl alcohol and palmitic acid, which can inhibit the production of reactive singlet oxygen (1O2) from oxygen (O2).16,17 Furfuryl palmitate has been an active ingredient in emollients for the past decade and such creams have been suggested to be, according to one review of furfuryl palmitate studies, “a valuable alternative to topical corticosteroids in mild-medium severity skin disorders”.17
A number of studies have shown the efficacy of furfuryl palmitate-containing emollients in treating AD,17–20 with actions including reductions in itching,20 improvement in inflammation19 and overall reduction of AD signs and symptoms.17 These gains have been shown in some studies to be equivalent to topical corticosteroid treatment.18 An open-label, uncontrolled study of the use of an emollient containing furfuryl palmitate and tocopherol (vitamin E), among other AD/CD-targeting ingredients, in 40 patients with AD or CD found that 28 days’ twice-daily administration led to statistically significant reductions in disease severity scores, from mild-to-moderate to almost clear, and in pruritus intensity scores, as well as significant increases in quality-of-life indices.21 Studies have also shown antioxidant-containing emollients have a good tolerability profile.17
The objective of this study was to gather efficacy data from the use of a marketed prescription or over-the-counter emollient device (Relizema™ cream, RELIFE Menarini Group, Florence, Italy) enriched with antioxidants including furfuryl palmitate and tocopherol.
Materials and Methods
This study was carried out in the Republic of Ireland, where the emollient device was launched in 2019. As this was a post-marketing questionnaire asking for feedback from users, not a post-market clinical follow-up investigation, European Union Medical Device Regulations did not require ethics committee approval (MDR Annex XIV, Part B, Section 6.2).22 Patients younger than 18 years of age were included with their parent or guardian’s consent. Privacy rights of all participants were observed.
The novel emollient device formulation key ingredients include furfuryl palmitate and tocopherol, and other antioxidants, as well as glyceryl stearate, glycerin and lipids, including caster seed oil (ricinus communis seed oil) and ethyl ester (or ethyl linolenate).
Study participants purchased the cream of their own volition, though this may have been recommended by a health-care professional (HCP) (pharmacist, pharmacy assistant, nurse, general practitioner dermatologist), and were not provided specific instructions at the point of sale. The packaging contains the suggestion to apply to “the affected area two or three times a day as needed.” Study participants were not provided with any incentive to take part in the study. Inclusion criteria for study participation was the presence of atopic dermatitis, irritant contact dermatitis, allergic contact dermatitis or erythema of unspecified etiology, confirmed by the health-care professional completing the questionnaire.
User feedback was via a pre-set questionnaire, read to patients using the cream by one of the above HCPs who were trained in administering the questionnaire (Appendix 1). Data was captured on the role of the HCP filling in the form, user age bracket (0−3 months, 3−6 months, 6−36 months, 3−12 years, 13−18 years, 18+ years) and indication (AD; contact irritant dermatitis, contact allergic dermatitis, erythema, other). Users were also asked how many times a day they applied the cream (once, twice, more than twice, other) and how long the product was used for (<30 days, 30 days, >30 days, for 30 days and now every few days as required).
Regarding symptoms, users were asked when they first noticed improvements in their skin condition following product application/use (after the first day, within the first week, after 1 week, after 2/3 weeks, more than 3 weeks) and whether the product helped to reduce/alleviate the signs and/or symptoms of itching and (on a separate scale) flushing, on a scale of 1 (got worse) to 5 (significantly improved). The user was then asked to comment if they had the feeling their skin was protected and moisturized (no difference, felt somewhat more protected and moisturized, felt very protected and moisturized); if they found the texture of the product was pleasant (fairly pleasant, pleasant, very pleasant, excellent) and if the non-fragrance smell was acceptable (fairly acceptable, acceptable, very acceptable, excellent).
The user was finally asked if they used the cream in combination with other products (drug, cosmetic, food supplement, other, none), whether the product labelling was clear and easy to understand (Yes/No, comments), whether they found the packaging and tube easy to use (Yes/No, comments) and if they had any other comments.
The questionnaire was constructed by the study sponsor. No personal identifying data was collected from questionnaire respondents. The questionnaire was administered, and data collected, by pharmacy staff and health-care professionals who were trained on the product characteristics by RELIFE Territory Leads (RTL). Pharmacies involved in the study were contacted by RTLs who explained the user feedback data collection process. Pharmacies were chosen to collect the data as pharmacy staff are likely to be in touch with the end user buying the product and are the most likely to receive feedback from the end user when they return to purchase either another product or the emollient device again. All pharmacies and health-care professionals who stock/prescribe the emollient device were contacted and introduced to the questionnaire before deciding whether or not they would be involved in the study. Once a pharmacy had agreed to take part in the study, the RTL contacted them periodically to obtain questionnaire results from staff during the study period. Pharmacy staff did not receive payment for their involvement in the study but did receive a sample gift pack of the product and thank you card, both of nominal value and not linked to the number of questionnaires completed.
This investigation was intended to achieve at least 40 questionnaire respondents. For participants’ responses to the questionnaire, descriptive statistics were calculated and frequency data was constructed.
Results
Data Acquisition
The surveillance was carried out between 15 November to 22 December 2020. A total of 40 questionnaires were administered, 16 by pharmacists, nine by pharmacy assistants, nine by nurses, five by general practitioners and one by a dermatologist.
Patient Characteristics
Figure 1 shows diagnosis by age group. Of the 40 patients questioned, 25 had AD, 10 had irritant CD, three had allergic CD and two had erythema of unspecified etiology. Half of the patients (n = 20) were aged 18 or older, three were 13−18 years, 12 were 3−12 years, three were 6−36 months and one was 3−6 months (Figure 1).
 |
Figure 1 Diagnosis by age. N=39 as age not noted in one questionnaire. |
Product Application
Most patients applied the product twice a day (n = 26) or more than twice a day (n = 11). Two patients only applied the product once a day, one patient did not answer this question. For most patients, the product was used by itself (n = 35), with two patients saying they used it with an unstated cosmetic and two with a steroid (application method not stated) and two with another dermatological cream.
Half of the patients (n = 20) used the product for less than 30 days, with seven patients using it for 30 days exactly. Twelve patients used it for 30 days, then every few days as required, with one patient using the product for over 30 days.
Results of Product Use
Patients were asked to rate how signs of itching or flushing were impacted by use of the cream, on a scale of 1−5. Table 1 shows that, of those who answered this question, most patients rated both itching and flushing as being “significantly improved.”
 |
Table 1 Did the Product Help to Reduce/Alleviate Signs and/or Symptoms Listed Below |
From Figure 2 it can be seen that in response to being asked “When did you notice improvements in your skin condition?”, most patients chose “after the first day of application”, or “within the first week of application”, one chose “after 1 week of application”, and none chose “after 2−3 weeks” or “more than 3 weeks” of application.
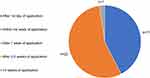 |
Figure 2 When did the user first notice improvements in their skin condition?. |
Users were also asked if they “had the feeling that their skin felt protected and moisturized after using the product?”. On a 1 (no difference) to 3 (very protected and moisturized) scale, 34 patients chose that their skin “felt very protected and moisturized” after using the product with six saying their skin “felt somewhat protected and moisturized” (score of 2).
Product Characteristics
Patients were asked to rate on a 1 (“fairly pleasant”) to 4 (“excellent”) scale if they found the texture of the product pleasant. Of the 40 respondents, 16 rated the product’s pleasantness as “excellent”, nine as “very pleasant” and 15 as “pleasant.”
Patients were also asked about the “non-fragrance” smell on a scale of 1 (“fairly acceptable”) to 4 (“excellent”). Responses were: eight rated it as “Excellent;” 11 as “Very acceptable;” 14 as “Acceptable” and seven as “Fairly acceptable.”
When asked if the information on the product labelling, packaging and tube was clear and easy to understand, all users said it was.
Discussion
AD and CD are common skin conditions in both children and adults.5,23,24 These forms of dermatitis are associated with a number of burdens for the individual, such as impacts on sleep and quality-of-life factors including social interactions and career choice, as well as potential financial and loss of productivity burdens for the caregivers of children with dermatitis.12,25 Accordingly, a relatively fast-acting, easy to use, over-the-counter emollient with a targeted antioxidant ingredient such as furfuryl palmitate could be advantageous to help in AD and CD alleviation and control.
In this study, over half the respondents had AD (n = 25), which occurred across all of the age groups (Figure 1). In AD, there are indications of both an increase in oxidative stress26 and a decrease in antioxidant production.4 Studies regarding a variety of emollients containing furfuryl palmitate and other antioxidants that can address these issues have previously been shown these to be advantageous.17–21,27 Control of AD and skin barrier restoration as early as possible is important as, especially in infants, this may help prevent development of more severe and chronic forms of AD.28 A quarter of the questionnaire respondents (n = 10) had irritant CD, with a further three having allergic CD. Both types of CD also involve inflammatory and immune system components10,11 and thus, local control of these, as provided by an antioxidant cream, could be advantageous.
One of the key findings of this questionnaire is that two important aspects of AD and CD – itching and redness (flushing) – were found to be improved or significantly improved by use of the novel emollient device. This provides further indication that an emollient containing antioxidants and other AD/CD targeting ingredients can aid in alleviation of these skin conditions. However, as it is known that basic emollients themselves can provide dermatitis relief,29 it cannot be discounted here that the humectant, occludent and other antioxidant components of the cream led or contributed to these results. Further studies could compare this emollient device to a suitable basic emollient and/or a placebo cream.30
Patient satisfaction with a treatment is a vital factor for continued use.31 One way a treatment is advantageous is if it works quickly as the longer a treatment takes to work, the more a patient will be dissatisfied and possibly discontinue treatment, whatever the eventual result could be. In this study, almost half of the users noticed an improvement in skin condition after the first day, with over half seeing improvements within the first week. Time to notice improvements was not analyzed by skin condition. As allergic CD is usually more delayed than irritant CD, and as AD stems from a different set of factors from CD, it would be interesting to further investigate whether the time to seeing benefits from the furfuryl palmitate-containing emollient used here differs by type of dermatitis experienced. Knowledge of this could help when advising patients regarding how long to potentially wait before seeing a response, which could aid treatment adherence.31
Standard instructions for application of an emollient are twice daily use32 and indeed most questionnaire respondents used the cream twice a day or more. Another finding was that half of the respondents used the cream for less than 30 days. This is another aspect that could be further investigated, to understand if patients ceased using the cream as the skin condition completely cleared or because the cream failed to work as anticipated. This would be an important distinction to understand the use of an antioxidant-containing emollient and whether this was coincident with the type of dermatitis experienced, CD or AD, and the severity and extent of the skin condition.
The findings here are very positive but further studies could be conducted with an enhanced methodology, for instance, by having patient input into the questionnaire design.30 There exists a potential bias in the questionnaire in that the manufacturer disseminated the questionnaires in selected pharmacies; future studies could involve an independent contract research organization.30 Numbers of patients were very low in this study due to the recent introduction of this non-drug product to the market. Further studies with a larger number of participants could add to the current body of knowledge. It is of note that some one of the ingredients, tocopherol,33 may cause contact dermatitis. However, this reaction was not noted here. Clinical trials of this novel emollient device for dermatitis are currently underway.34,35
Conclusion
This questionnaire provided further evidence that the use of this novel emollient device formulated with antioxidants and other AD and CD-targeting ingredients can reduce itchiness and flushing associated with AD and CD from as early as after 1 day of use.
Acknowledgments
Help with manuscript preparation was carried out by Eleanor Roberts PhD, Beeline Science Communications Ltd, funded by RELIFE Menarini Group.
Disclosure
Adelaide Ann Herbert has received honorarium as a manuscript reviewer for the study sponsor. She reports grants from Pfizer, Arcutis, Galderma and Ortho Dermatologics paid to UTHealth McGovern Medical School at Houston, Texas, USAol, personal fees Pfizer, Arcuits, Ortho Dermatologics, Galderma, Beiersdorf: honoraria, personal fees from Regeneron-Sanofi, Ortho Dermatologics and GSK paid to her for DSMB, during the conduct of the study. The author reports no other conflicts of interest in this work.
References
1. Hebert AA. Oxidative stress as a treatment target in atopic dermatitis: the role of furfuryl palmitate in mild-to-moderate atopic dermatitis. Int J Womens Dermatol. 2020;6(4):331–333. doi:10.1016/j.ijwd.2020.03.042
2. Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70(1):3–11. doi:10.1016/j.jdermsci.2013.02.001
3. Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:2721469. doi:10.1155/2016/2721469
4. Sivaranjani N, Rao SV, Rajeev G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J Clin Diagn Res. 2013;7(12):2683–2685.
5. Sedó-Mejía G, Soto-Rodríguez A, Pino-García C, Sanabria-Castro A, Monge-Ortega OP. Contact dermatitis: clinical practice findings from a single tertiary referral hospital, a 4-year retrospective study. World Allergy Organ J. 2020;13(7):100440. doi:10.1016/j.waojou.2020.100440
6. Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14(5):289–301. doi:10.1038/nri3646
7. Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003;149(2):248–254. doi:10.1046/j.1365-2133.2003.05417.x
8. Tsukahara H, Shibata R, Ohta N, et al. High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: relationship with oxidative stress. Metabolism. 2003;52(12):1601–1605. doi:10.1016/S0026-0495(03)00310-X
9. Amin MN, Liza KF, Sarwar MS, et al. Effect of lipid peroxidation, antioxidants, macro minerals and trace elements on eczema. Arch Dermatol Res. 2015;307(7):617–623. doi:10.1007/s00403-015-1570-2
10. Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21(4):164–169. doi:10.1179/1351000215Y.0000000015
11. Kim DH, Byamba D, Wu WH, Kim TG, Lee MG. Different characteristics of reactive oxygen species production by human keratinocyte cell line cells in response to allergens and irritants. Exp Dermatol. 2012;21(2):99–103. doi:10.1111/j.1600-0625.2011.01399.x
12. Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi:10.1016/j.jid.2016.07.012
13. Brasch J, Becker D, Aberer W, et al. Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), the Working Group for Occupational and Environmental Dermatology (ABD) of the DDG, the Medical Association of German Allergologists (AeDA), the Professional Association of German Dermatologists (BVDD) and the DDG. Allergo J Int. 2014;23(4):126–138. doi:10.1007/s40629-014-0013-5
14. Raone B, Ravaioli GM, Dika E, Neri I, Gurioli C, Patrizi A. The use of emollients for atopic eczema. Austin J Allergy. 2015;2(1):1018.
15. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. doi:10.1111/jdv.14888
16. Linetsky M, Ortwerth BJ. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem Photobiol. 1997;65(3):522–529. doi:10.1111/j.1751-1097.1997.tb08598.x
17. Pigatto PD, Diani M. Beneficial effects of antioxidant furfuryl palmitate in non-pharmacologic treatments (prescription emollient devices, PEDs) for atopic dermatitis and related skin disorders. Dermatol Ther (Heidelb). 2018;8(3):339–347. doi:10.1007/s13555-018-0239-0
18. Lauriola M, Pigatto P, Pedrelli V A single-center, randomized, perspective, investigator blinded controlled trial to examine efficacy and safety of a furpalmate cream in comparison to topical corticosteroid in atopic dermatitis of hands of 40 adult patients.
19. Nemelka O, Bleidel D, Fabrizi G, et al. Experimental survey of a new topical anti-oxidant based on furfuryl palmitate in the treatment of child’s and baby’s dermatitis with eczema: results from a multicenter clinical investigation. Minerva Pediatr. 2002;54(5):465–474.
20. Patrizi A, Raone B, Raboni R, Neri I. Efficacy and tolerability of a cream containing AR-GG27® (sorbityl furfural palmitate) in the treatment of mild/moderate childhood atopic dermatitis associated with pityriasis alba. A double-blind, placebo-controlled clinical trial. G Ital Dermatol Venereol. 2012;147(6 Suppl 1):1–8.
21. Hebert AA, Pellacani G, Micali G. Advancing breakthrough innovative treatment in paediatric dermatitis and various skin disorders. Eur Med J. 2021;9:2–9.
22. Medical Device Regulation. Annex XIV. Available from: https://www.medical-device-regulation.eu/2019/08/14/annex-xiv/.
23. Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. doi:10.1038/jid.2010.251
24. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151(7):743–752. doi:10.1001/jamadermatol.2014.5432
25. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534. doi:10.1038/jid.2013.446
26. Tsukahara H, Shibata R, Ohshima Y, et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003;72(22):2509–2516. doi:10.1016/S0024-3205(03)00145-0
27. Tripodi S, Di Rienzo Businco A, Panetta V, et al. Lack of efficacy of topical furfuryl palmitate in pediatric atopic dermatitis: a randomized double-blind study. J Investig Allergol Clin Immunol. 2009;19(3):204–209.
28. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134(4):818–823. doi:10.1016/j.jaci.2014.08.005
29. Boralevi F, Saint Aroman M, Delarue A, et al. Long-term emollient therapy improves xerosis in children with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014;28(11):1456–1462. doi:10.1111/jdv.12314
30. Spelsberg A, Prugger C, Doshi P, et al. Contribution of industry funded post-marketing studies to drug safety: survey of notifications submitted to regulatory agencies. BMJ. 2017;356:j337. doi:10.1136/bmj.j337
31. Prakash B. Patient satisfaction. J Cutan Aesthet Surg. 2010;3(3):151–155. doi:10.4103/0974-2077.74491
32. Wollenberg A, Schnopp C. Evolution of conventional therapy in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30(3):351–368. doi:10.1016/j.iac.2010.06.005
33. Warshaw EM, Ruggiero JL, DeKoven J, et al. Patch Testing With Tocopherol and Tocopherol Acetate: the North American Contact Dermatitis Group Experience, 2001 to 2016. Dermatitis. 2021;32(5):308–318. doi:10.1097/DER.0000000000000706
34. Relife S.r.l. Clinical investigation on the efficacy and safety of relizema cream in paedriatric patients (YOUNG). NCT05259774. Available from: https://clinicaltrials.gov/ct2/show/NCT05259774?term=NCT05259774&draw=2&rank=1.
35. Relife S.r.l. Clinical investigation on the efficacy and safety of relizema ecofoam. Available from: https://clinicaltrials.gov/ct2/show/NCT05001139?term=NCT05001139&draw=2&rank=1.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
