Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Real-World Economic Outcomes of Brexpiprazole and Extended-Release Quetiapine Adjunctive Use in Major Depressive Disorder
Authors Seetasith A , Greene M , Hartry A , Burudpakdee C
Received 19 June 2019
Accepted for publication 8 November 2019
Published 4 December 2019 Volume 2019:11 Pages 741—755
DOI https://doi.org/10.2147/CEOR.S220007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Arpamas Seetasith,1 Mallik Greene,2 Ann Hartry,3 Chakkarin Burudpakdee1
1Medical and Scientific Services, Real-World Evidence Solutions, IQVIA, Falls Church, VA 22042, USA; 2Health Economics and Outcomes Research, Otsuka Pharmaceutical Development And Commercialization, Inc., Princeton, NJ 08540, USA; 3Health Economics and Outcomes Research, Lundbeck, Deerfield, IL 60015, USA
Correspondence: Chakkarin Burudpakdee
IQVIA, 3110 Fairview Park Drive, Suite 400, Falls Church, VA 22042, USA
Tel +1 610 244 2025
Email [email protected]
Purpose: Major depressive disorder (MDD) is a chronic mental disorder with a substantial clinical and economic burden. Despite the efficacy of adjunctive atypical antipsychotics (AAP) for augmentation in patients with major depressive disorder (MDD) who failed first-line antidepressant therapy (ADT), little is known of the impact of AAP choices on healthcare resource use and costs in real-world practice. Therefore, this study compared real-world healthcare utilization and costs in patients with MDD treated with brexpiprazole or extended-release (XR) quetiapine as adjunctive treatment to ADT.
Patients and methods: Adults with MDD starting adjunctive treatment with brexpiprazole (n=844) or extended-release (XR) quetiapine (n=688) were identified in the adjudicated health plan claims data (07/2014 – 09/2016). Resource use and healthcare costs in the 6 months following treatment initiation were compared between non-matched populations, and between propensity score-matched groups, and by multivariable regression analyses.
Results: During follow-up, unadjusted all-cause hospitalization (6.6% vs 12.5%) and ED visits (17.0% vs 27.5%) were lower with brexpiprazole compared to quetiapine XR (both p<0.001). Brexpiprazole-treated patients had significantly lower mean medical costs (US$6,421 vs US$8,545, p=0.0123) but higher mean pharmacy costs (US$7,401 vs US$4,691, p<0.0001) than quetiapine XR-treated patients did. Total healthcare costs were not significantly different between the two cohorts. Propensity score-matched comparisons of 397 patients in each cohort showed no statistically significant difference in all-cause hospitalization, ED visits, and total healthcare costs; and significantly lower medical costs (US$5,719 vs US$8,602, p=0.0092) but higher pharmacy costs (US$7,091 vs US$5,091, p=0.0007) in brexpiprazole compared to quetiapine XR. In multivariable regressions, brexpiprazole was associated with 16.1% lower medical costs (p=0.0186) and 9.4% higher total healthcare costs (p=0.0463) as compared to quetiapine XR.
Conclusion: Significantly lower medical costs were observed in patients with MDD treated with brexpiprazole vs quetiapine XR.
Keywords: atypical antipsychotic agents, comparative effectiveness research, health care utilization, healthcare costs, propensity score matching
Introduction
Major depressive disorder (MDD) is a chronic mood disorder that affects over 300 million people or 4.4% of the world’s population.1 In the United States (US), 16 million adults or 6.9% of the adult population had at least one major depressive episode in 2012. The condition disproportionately affects female adults 35 years or older.2,3 MDD is a leading cause of the global disease burden in terms of disability, measured in terms of years lived with disability and disability-adjusted life years.4,5 In the US, MDD resulted in significant economic burden and almost 400 million disability days per year.2,6 In 2010, the incremental economic burden of individuals with MDD was estimated at US$210.5 billion, with 45%-47% attributable to direct costs, and 48%-50% to workplace costs.2
Pharmacotherapy plays an important role in MDD. The American Psychiatric Association recommends the use of an antidepressant medication, such as a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), tricyclic or tetracyclic antidepressant, or atypical antidepressant, as an initial treatment choice for patients with mild-to-moderate MDD.7 For patients with severe MDD, the combination of psychotherapy and antidepressant medication is recommended as an initial treatment of choice. For patients with minimal improvement after initial treatment, switching to an antidepressant from the same pharmacological class to one from a different class or augmenting the antidepressant with other agents such as lithium, thyroid hormone or an atypical antipsychotic medication (AAP) has shown to be effective in improving depressive symptoms.7 Results from STAR*D, a large clinical trial of 2,876 evaluable patients with at least moderate depression, show that only one-third of patients experience remission with their first antidepressant treatment, citalopram.8–10 Among patients who did not achieve sufficient response and switched to a different antidepressant, approximately 25% became symptom-free.8–10 The increased economic burden associated with inadequate treatment response and an increasing number of unsuccessful antidepressant trials,11–13 in addition to the limited responses and low remission rates, highlight the unmet need for effective treatment in patients with inadequate response to initial antidepressant therapy.
Abundant evidence from prospective, controlled clinical trials supports the use of adjunctive treatment of antidepressants with AAPs, including olanzapine, risperidone, aripiprazole, extended-release quetiapine, ziprasidone, lurasidone,14 and the recently approved drug, brexpiprazole,15 for patients with MDD.16,17 Multiple meta-analysis studies from published clinical trials (up until 2010) also show that the use of adjunctive AAPs was significantly more effective than placebo or antidepressant therapy alone in terms of achieving remission or clinical response.18–21 Use of adjunctive AAP in patients with MDD also shows significant reductions in all-cause, and MDD-related hospitalizations and ED visits despite increases in pharmacy fills and physician office visits,22 and early treatment – within the first year of first antidepressant therapy or within six months of evidence of inadequate therapy – is associated with significantly lower all-cause cost23 and greater reduction in hospitalization and overall medical costs22 compared to delayed treatment. Three AAPs, including aripiprazole (2007), extended-release quetiapine (2009), and brexpiprazole (2015), are approved by the FDA as an adjunctive treatment for MDD.24–26 Despite their efficacy, little is known of the impact of AAP choices for the treatment of MDD on healthcare resource use and costs in real-world practice.
Brexpiprazole (Rexulti®) is a serotonin-dopamine activity modulator that is a partial agonist at 5-HT1A and dopamine D2 receptor, and an antagonist at 5-HT2A and adrenergic alpha1B/2C receptors, all at similar potency.27,28 The drug was recently approved in the US as adjunctive therapy to antidepressant therapy (ADT) for the treatment of MDD and the treatment of schizophrenia in 2015.26 Extended-release quetiapine (Seroquel XR®) and its active metabolite, N-desalkyl quetiapine (norquetiapine) are partial agonists at 5-HT1A, and antagonists at 5-HT2A, 5-HT2c, dopamine D2, histamine H1, and adrenergic alpha1b receptors.29 The drug was approved in 2009 as adjunctive therapy to an antidepressant for the treatment of MDD and previously, for the treatment of schizophrenia, manic or mixed episode bipolar I disorder, and depressive episode bipolar disorder.25 In this study, we described real-world healthcare resource use and cost associated with adjunctive use of brexpiprazole in comparison to quetiapine XR in patients with MDD. Brexpiprazole was selected because of its recent approval for this indication while quetiapine XR was selected as a comparator as it is a branded product that is FDA approved for indications similar to brexpiprazole.
Materials and Methods
Database
This retrospective study utilized patient-level data from IQVIA Real-World Data – US Adjudicated Claims (formerly known as PharMetrics Plus) between July 2014 and September 2016. The database contains adjudicated medical, and pharmacy claims for more than 150 million unique enrollees from approximately 60 health plans across the US and are representative of the US commercially insured population based on age and sex. Data are de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA). No Institutional Review Board approval was required for this study.
Patient Selection
Figure 1 depicts the patient selection process. This study included adult patients with an MDD diagnosis who were newly initiated on brexpiprazole or quetiapine XR adjunctive therapy. Patients were included in the study if they had a pharmacy claim for brexpiprazole or quetiapine XR between July 10, 2015, and March 31, 2016 (selection window). The start of the selection window coincides with the approval date of brexpiprazole in the US. Patients were also required to have at least 14 days of medication supply for their index therapy, and at least six months of continuous health plan enrollment (both medical and pharmacy coverage) before and after the first claim for brexpiprazole or quetiapine XR (the index date). All patients were 18 years of age or older on the index date, and had at least one medical claim (outpatient or inpatient) for MDD (ICD-9: 296.2x, 296.3x, 311.x; ICD-10: F32.0-F32.5, F32.9, F33.x) on or before index date, and at least one additional medical claim for MDD on a different date at any time during the study period. Medication supply of index brexpiprazole or quetiapine XR had to overlap for at least 30 continuous days with an antidepressant to ensure adjunctive use to ADT. Patients with a diagnosis of schizophrenia, bipolar disorder, or dementia-related psychosis, or with a pharmacy claim for index therapy during the 6-month pre-index period were excluded from the study (to ensure they were newly treated with index therapy). Patients with both brexpiprazole and quetiapine XR pharmacy claims on index date were also excluded from the study.
 |
Figure 1 Study design. Abbreviation: Quetiapine XR, Extended-Release Quetiapine. |
Selected patients were categorized into two mutually exclusive cohorts, based on their index treatment. The hierarchical selection was applied to identify patients treated with brexpiprazole first and then quetiapine XR to maximize the number of patients in the brexpiprazole cohort. Patients were required to have six months of continuous medical and pharmacy coverage before their index date (pre-index period), and six months of continuous medical and pharmacy coverage following their index date (follow-up period).
Study Measures
Baseline Characteristics
All baseline variables were collected from the index claim or claims during the 6-month pre-index period. These included patient demographic and clinical characteristics (age, sex, geographic region, payer type, plan type, index treatment prescriber specialty, Charlson comorbidity index, and comorbid conditions), treatment history (number of different antidepressant therapies [ADTs] and classes of ADT used prior to index therapy), and healthcare resource use and costs (MDD-related hospitalizations and ED visits, and total all-cause healthcare costs). MDD-related services and costs were identified from claims with an MDD diagnosis in any position.
Index AAP Treatments
Index daily dosing, number of fills, and days’ supply per fill during follow-up were reported. Index daily dosing was calculated from the index prescription (first AAP prescription) by multiplying the tablet strength determined from the NDC code for each medication with the quantity dispensed, then dividing it by the number of days’ supply.
Healthcare Resource Use and Costs
Healthcare resource use and costs were determined from the medical and pharmacy claims during follow-up. Medical costs (for services/products covered under a medical benefit) were reported separately from pharmacy costs (drug products covered under a pharmacy benefit). Medical services and costs were reported for all-cause hospitalizations, ED visits, physician office visits, infusion and injectable drugs administered in an outpatient setting, and other outpatient services.
Statistical Analysis
Unadjusted all-cause healthcare resource use and costs of patients with MDD who initiated brexpiprazole and those who initiated quetiapine XR during the 6-month follow-up were compared using the Chi-square test for proportions and t-test ANOVA for mean costs. To adjust for baseline differences between cohorts, we utilized two approaches. A propensity score matching approach was used to create two cohorts of similar values of propensity scores constructed from the following variables: age, sex, region, payer type, plan type, prescriber specialty, comorbidity index, baseline all-cause healthcare cost, and MDD-related ED visits and hospitalizations, comorbid anxiety and hyperlipidemia, and number of ADTs used during pre-index. Based on the propensity scores, brexpiprazole-treated patients were matched at 1:1 ratio to quetiapine XR-treated patients using a greedy nearest neighbor matching algorithm without replacement.30,31 Standardized differences (SDD) were calculated to measure the differences in covariates for unmatched and matched cohorts. Healthcare resource use and costs were then compared between the matched cohorts in a similar manner as the unadjusted analysis. Secondly, generalized linear models with a log link and gamma distribution were used to confirm the differences in all-cause medical costs between non-matched brexpiprazole and quetiapine XR cohorts. Demographic characteristics, baseline clinical characteristics, ADT treatment history, and baseline HRU and costs were included in the multivariable regression models. All costs were adjusted to the 2016 US dollar using the medical care component of the Consumer Price Index. A p value of <0.05 was considered statistically significant for all analyses. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Non-Matched Cohorts
Baseline Characteristics
A flow diagram depicting sample attrition is shown in Figure 2. From an initial pool of 3,433 patients with a pharmacy claim for brexpiprazole and 13,645 patients with a pharmacy claim for quetiapine XR, the final study population comprised of 844 patients newly treated with brexpiprazole and 688 patients newly treated with quetiapine XR.
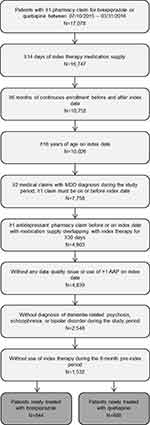 |
Figure 2 Sample attrition. Abbreviations: AAP, Adjunctive Atypical anti-Psychotic; MDD, Major Depressive Disorder; Quetiapine XR, Extended-Release Quetiapine. |
Baseline characteristics of the final study population are described in Table 1. Brexpiprazole-treated patients were slightly older than quetiapine XR-treated patients (mean (SD) age: 47.2 (12.7) years vs 44.7 (14.4) years. The majority of patients in both cohorts had commercial (57.5% vs 47.7%) or self-insured employer-sponsored (38.9% vs 29.4%) health insurance; however, the proportions of brexpiprazole-treated patients with Medicaid or Medicare health insurance were lower than that of quetiapine XR-treated patients (Medicaid: 3.0% vs 19.2%; Medicare: 0.6% vs 3.8% for brexpiprazole and quetiapine XR, respectively). Although most patients in both cohorts were enrolled in a PPO plan (87.0% vs 70.1%), there was a larger proportion of quetiapine XR-treated patients (27.3%) in an HMO plan, compared to brexpiprazole (7.7%). A larger proportion of brexpiprazole-treated patients was seen by a psychiatrist when starting the index treatment compared to those starting quetiapine XR (46.8% vs 30.4%, p<0.0001). Before starting the AAP treatment, patients treated with brexpiprazole appeared to have fewer comorbidities (comorbidity index: 0.48 vs 0.70), fewer MDD-related ED visits (0.07 vs 0.12) and MDD-related hospitalizations (0.09 vs 0.20) but incurred similar all-cause healthcare costs during the pre-index period (US$12,082 vs US$12,462) compared to quetiapine XR. Patients in both cohorts used, on average, 1.8 ADTs during the pre-index period. The most commonly used ADTs prior to brexpiprazole were atypical antidepressants (eg, bupropion, trazodone, nefazodone; 54.4%), followed by SNRIs (46.2%) and SSRIs (45.3%), while the most commonly used ADTs prior to quetiapine XR were SSRIs (57.9%), followed by atypical antidepressants (44.0%) and SNRIs (37.1%). Common comorbid conditions, including anxiety disorders, hypertension, hyperlipidemia, and diabetes, were similar in the two cohorts.
 |
Table 1 Baseline Characteristics Before and After Matching |
Brexpiprazole and Quetiapine XR Use
Patients in the brexpiprazole cohort were initiated on either 1 mg (45.5%), 2 mg (39.1%), or 0.50 mg (11.8%) per day. Over one-third of patients (34.6%) in the quetiapine XR cohort was initiated on low-dose 50 mg quetiapine XR per day; 30.4% were initiated on 150 mg quetiapine XR per day. Other starting daily doses of quetiapine XR were 300 mg (11.0%), 200 mg (9.7%), and 100 mg (7.7%). During follow-up, patients filled 3.9 (SD 2.4) prescriptions for brexpiprazole, with a mean (SD) of 31.4 (7.6) days per brexpiprazole fill, and 3.7 (SD 2.6) prescriptions for quetiapine XR, with a mean (SD) of 33.1 (13.2) days per quetiapine XR fill (Table 2). Based on the number of pharmacy fills, patients were treated with brexpiprazole or quetiapine XR for approximately 4 months.
 |
Table 2 Index Dosing and Use of Index Therapy During Follow-Up |
Healthcare Resource Use
Healthcare resource use after starting AAP treatment is summarized in Table 3. There was a lower proportion of patients with an all-cause hospital stay and ED visit for any reason during the 6-month post-index period in the brexpiprazole cohort (hospitalization: 6.6% vs 12.5%, p<0.0001; ED visit: 16.9% vs 27.5%, p<0.0001, for brexpiprazole and quetiapine XR, respectively). The mean numbers of all-cause hospitalizations (0.10 vs 0.21, p=0.0002) and ED visits (0.30 vs 0.55, p<0.0001) per patient during follow-up were also lower with brexpiprazole. Almost all patients (>97%) in both cohorts had at least one physician office visit during follow-up. The higher mean number of all-cause physician office visits (14.89 vs 12.57, p=0.0008) was observed with brexpiprazole compared to quetiapine XR. Pharmacy fills (35.61 vs 35.03, p=0.6146) were similar between the two cohorts.
 |
Table 3 Healthcare Resource Utilization Over 6-Month Follow-Up |
Healthcare Cost
Medical and pharmacy costs, stratified by treatment, during follow-up in the non-matched cohorts are summarized in Figure 3A. Little difference existed in the mean total healthcare costs (medical and pharmacy costs) per patient between brexpiprazole and quetiapine XR. The mean (SD) total healthcare costs were US$13,821 (US$15,543) for brexpiprazole and US$13,235 (US$22,293) for quetiapine XR. The mean (SD) medical costs were US$6,421 (US$13,055) for brexpiprazole and US$8,545 (US$19,939) for quetiapine XR, revealing $2,214 (95% CI: US$2,124, US$3,785) lower medical costs in patients treated with brexpiprazole compared to quetiapine XR. The mean (SD) pharmacy costs were US$7,401 (US$7,564) for brexpiprazole and US$4,691 (US$8,314) for quetiapine XR, or US$2,710 (95% CI: 1,914, US$3,506) higher in patients treated with brexpiprazole. Medical costs were further assessed for inpatient and outpatient care (Figure 3B). Hospitalization cost was $1,299 (95% CI: US$135, US$2,464) lower with brexpiprazole. The costs associated with ED visits and other outpatient services were also lower with brexpiprazole compared to quetiapine XR; the cost associated with ED visits was US$182 (95% CI: US$52, US$311) lower, and that associated with other outpatient services was US$907 (95% CI: US$71, US$1,744) lower. The cost of physician office visits was higher in patients treated with brexpiprazole (US$336 [95% CI: US$101, US$507]).
 |
Figure 3 Mean healthcare costs over 6-month follow-up (non-matched). (A) Total, medical, and pharmacy costs. (B) Components of medical costs. |
The lower medical cost in the brexpiprazole cohort was confirmed in the regression model controlling for baseline characteristics and healthcare resource use. Medical cost of a brexpiprazole-treated patient was 16.1% lower than that of a quetiapine XR-treated patient (exponentiated coefficient = 0.839; 95% CI: 0.725, 0.971; p=0.0186). The total healthcare cost of a brexpiprazole-treated patient was 9.4% higher than that of a quetiapine XR-treated patient (exponentiated coefficient = 1.094; 95% CI: 1.001, 1.196; p=0.0463). Other factors significantly associated with lower medical and total costs included “other” payer type (reference: commercial payer), ‘other plan type (reference: HMO), epilepsy and liver disease comorbid conditions, and higher MDD-related office visits prior to treatment initiation. Older age, high comorbidity index, and a higher number of all-cause office visits were associated with higher costs (Table 4).
 |
Table 4 Regression Analysis Evaluating the Impact of Brexpiprazole vs Quetiapine XR Adjunctive Treatment on Medical Cost and Total Healthcare Cost in The Non-Matched Cohorts |
Matched Cohorts
Baseline Characteristics
A total of 397 patients in each treatment group were matched. Following the match, the two cohorts were well-balanced on all baseline characteristics (Table 1). The mean age of patients in the matched cohorts was 46 years; the majority were female (67%), with similar health insurance payers and plan types. The largest proportion of patients had commercial health insurance (58%) and were enrolled in a PPO plan (86.4% of brexpiprazole cohort and 84.6% of quetiapine XR cohort). Matched cohorts had a mean comorbidity index of 0.5, a mean of 0.09 (brexpiprazole) and 0.07 (quetiapine XR) MDD-related ED visits per patient, and a mean of 1.2 MDD-related hospital stay per patient before starting the index treatment.
Brexpiprazole and Quetiapine XR Use
Patients in the brexpiprazole matched cohort were initiated on either 1 mg (43.6%), 2 mg (40.6%), or 0.5 mg (12.1%) per day. Low dose quetiapine XR (50 mg per day) was used as the starting dose in 35.3% of matched quetiapine XR-treated patients. Other starting daily doses of quetiapine XR in the matched cohort were 150 mg (30.2%), 300 mg (11.8%), 200 mg (10.1%), and 100 mg (8.1%). During follow-up, patients had 3.7–3.9 pharmacy fills for the respective AAP, with a mean medication supply of 32–34 days for each fill (Table 2). Based on the number of pharmacy fills, patients were treated with brexpiprazole or quetiapine XR for approximately 4 months.
Healthcare Resource Use
No significant differences in hospitalization, ED visits, or physician office visits were found between the two treatment cohorts. The proportion of patients with at least one hospital stay was 6.5% for brexpiprazole and 9.8% for quetiapine XR. The mean number of hospitalizations per patient was 0.10 and 0.14 for brexpiprazole and quetiapine XR, respectively. ED visits were numerically, but not significantly lower in the brexpiprazole cohort (18.6% vs 21.9% of patients with at least one ED visit and 0.33 vs 0.38 ED visits per patient for brexpiprazole and quetiapine XR, respectively). The mean number of office visits per patient were numerically higher in brexpiprazole-treated patients (13.47 vs 13.28) (Table 3).
Healthcare Cost
Consistent with the non-matched analysis, there was no statistical difference in the mean total healthcare cost per patient over follow-up between the two matched cohorts. The mean (SD) total costs were estimated at US$12,810 (US$12,760) for brexpiprazole and US$13,693 (US$22,845) for quetiapine XR. The mean (SD) medical costs were US$5,719 (US$10,440) for brexpiprazole and US$8,602 (US$19,378) for quetiapine XR, or US$2,884 (95% CI: US$721, US$5,046) lower for brexpiprazole compared to quetiapine XR. The mean (SD) pharmacy costs were US$7,901 (US$6,278) for brexpiprazole and US$5,091 (US$9,944) for quetiapine XR, equating to US$2,001 (95% CI: US$845, US$3,156) higher for brexpiprazole compared to quetiapine XR. Medical and pharmacy costs by treatment are summarized in Figure 4A.
 |
Figure 4 Mean healthcare costs over 6-month follow-up (matched). (A) Total, medical, and pharmacy costs. (B) Components of medical costs. |
Costs associated with inpatient and outpatient care among matched patients are reported in Figure 4B. Compared to quetiapine XR, brexpiprazole-treated patients had lower costs associated with hospitalization (US$1,182 lower, 95% CI: -US$56, US$2,420) and outpatient care (US$1,701 lower, 95% CI: US$159, US$3,244); however, only cost associated with other outpatient services was statistically different.
Discussion
This study characterized patients with MDD treated with brexpiprazole shortly after FDA approval in 2015 and assessed the impact of early adoption of brexpiprazole on healthcare resource use and costs compared to quetiapine XR. Our study includes analyses of both non-matched and PS-matched patient cohorts. The purpose of the analysis of the non-matched cohorts was to describe how both medications are utilized in the real-world setting as adjunctive treatment as intended by healthcare providers without restricting the populations; therefore, no adjustments were made for baseline differences in cohorts. Two adjusted analyses were performed: regression analysis of the non-matched cohorts and propensity-score matched comparisons. To the best of our knowledge, this is the first study comparing healthcare resource use and costs among patients with MDD newly treated with adjunctive atypical brexpiprazole compared to quetiapine in the real-world setting.
In the non-matched analysis, we observed that early users of brexpiprazole are different from patients treated with quetiapine XR. We found patients treated with brexpiprazole to be slightly younger than patients treated with quetiapine XR. Relative to quetiapine XR-treated patients, fewer brexpiprazole-treated patients had Medicare or Medicaid as a primary payer; and fewer were enrolled in an HMO insurance plan (most patients in both treatment groups were enrolled in a PPO plan). Moreover, early brexpiprazole prescribing, compared to quetiapine XR, was more commonly seen among psychiatrists. In general, before index treatment initiation, patients who started brexpiprazole had a lower comorbidity index score, fewer MDD-related ED visits, fewer MDD-related hospitalizations, but similar healthcare costs, compared to patients who initiated quetiapine XR. Such differences in demographic and baseline clinical characteristics between brexpiprazole- and quetiapine XR-treated patients may be due to channeling bias, in which newer drugs were preferentially prescribed to patients with different prognoses.32
Despite differences in baseline characteristics among the non-matched brexpiprazole and quetiapine XR cohorts, we found that patients in both treatment cohorts were treated for a similar amount of time before discontinuing their adjunctive therapy (approximately 4 months) during the 6-month follow-up period. During this period after treatment initiation, we found all-cause hospitalization and ED visits among brexpiprazole-treated patients to be significantly lower than that of quetiapine XR-treated patients. Notably, we observed a significantly higher number of physician office visits and associated costs among patients treated with brexpiprazole, which could be due to increased monitoring by physicians when prescribing a newly available medication. Additionally, it is possible that higher physician office visits may have contributed to reductions in hospitalizations or ED utilization by providing early detection of major depressive episodes and may be one reason why the lower total medical cost is observed with brexpiprazole relative to quetiapine XR (US$6,421 vs US$8,545).
In contrast to the medical cost, we found pharmacy cost to be 57.8% higher with brexpiprazole (US$7,401 vs US$4,691). This was likely due to the higher acquisition cost of brexpiprazole (US$25 per day) compared to quetiapine XR (US$1.15 per day) at the time of the study (based on Wholesale Acquisition Cost (WAC) reported by Wolters Kluwer Medi-Span Price Rx®).33 Despite the higher pharmacy cost, our findings report similar total healthcare costs among patients treated with brexpiprazole or quetiapine XR (US$13,821 vs US$13,235). Additional studies are needed to confirm this hypothesis. However, these findings in our real-world populations without matching can be informative by providing an understanding of how healthcare providers are utilizing these medications, describes the treated population, and services and costs to payers and healthcare providers. Given the wide range of available augmentation pharmacotherapies for the treatment of MDD, real-world evidence such as data from this study can help inform evidence-based decisions when providers and payers are selecting augmentation strategies for patients with MDD.
In the adjusted analysis using regression models, we were able to confirm the significantly lower medical cost (16.1% lower) in patients treated with brexpiprazole during the 6-month follow-up period compared to those treated with quetiapine XR. However, our regression results revealed significantly higher total healthcare costs with brexpiprazole (9.4% higher).
In the analysis of matched patients, we compared healthcare resource use and costs between brexpiprazole- and quetiapine XR-treated patients who had similar demographic and clinical characteristics, MDD severity measured by number of MDD-related hospitalizations and MDD-related ED visits, and healthcare resource use and costs prior to treatment initiation, all of which could potentially influence our outcomes measures during the follow-up period.30,31 Results from the matched analysis were consistent with those from the non-matched analysis. With well-balanced cohorts, we observed lower trends in hospitalizations, and ED visits, significantly lower mean total medical costs, and significantly higher mean pharmacy costs with brexpiprazole, relative to quetiapine XR. It is worth noting that despite the large magnitude of difference in the mean hospitalization costs between brexpiprazole-treated patients and their matched quetiapine XR-treated patients (US$1,166 vs US$2,346; 50.3% lower), the difference did not reach statistical significance. Such observed differences that did not reach a statistically significant level could be due to the small sample size (N=397 in each cohort), which reduces the power of our analysis. An ad hoc power analysis determined that 514 patients would be needed, assuming an alpha of 0.05, beta of 0.2, and power of 0.8. Future research can be conducted to help expand our understanding of brexpiprazole and quetiapine XR treatment effects on healthcare resource use and costs when larger samples of treated patients become available.
The costs per patient over a 6-month period estimated in our study are slightly higher than that reported in another study.34 Wu et al, 2011 estimated the 6-month total medical costs of a patient with MDD starting escitalopram and citalopram to be US$4,410 and US$5,752, respectively. The higher estimated costs in our study may be related to differences in the patient populations; individuals requiring augmentation with atypical antipsychotics in our study may represent a more severe population with higher healthcare resource utilization, compared to patients initiating escitalopram and citalopram in the Wu study.34
The results of this study should be interpreted with appropriate consideration to limitations inherent to administrative claims database studies. First, data included in the analysis do not contain information related to treatment choice for the atypical antipsychotic and cannot ascertain if patients took the atypical antipsychotics as prescribed. Second, administrative claims data are collected for reimbursement purposes and can be subject to potential misclassification, coding error, and data entry error. Furthermore, the database consists of only commercially insured patients; therefore, the study results may not be generalizable to other insured populations such as Medicare or Medicaid. Given the substantial prevalence of depressive symptoms in older adults,35 particularly those living in nursing homes,36 and Medicaid-covered adults and youth,37,38 further research in the Medicare and Medicaid population and long-term care residents may be warranted. Moreover, only direct medical and pharmacy costs are available in the database; indirect costs associated with productivity loss, which can account for as much as 67% of the total cost of care, are not available in the database.3 Finally, findings from this study provide information on treatment patterns and resource use; however, the treatment paradigm and clinical practice may change over time as prescribers become more familiar with brexpiprazole. Nonetheless, the longitudinal claims records do allow for a more complete view of resource utilization and costs of healthcare services in patients with MDD.
It is worth noting that in the exploratory matched analysis, we constructed propensity scores from a selected, but limited set of baseline variables (matching factors). The purpose of matching is to ensure similar baseline distribution of matching factors between the two treatment cohorts and to reduce selection bias,39 mimicking that of a randomized clinical trial.40 However, over- or under-adjustment bias could result from the selection of matching factors. As previously discussed, the significance level is related to sample size and overmatching diminishes sample size, leading to reduced statistical power to detect true differences between the two matched cohorts.30 Over-adjustment and unnecessary adjustment can also mask true differences and bias results toward the null.41–43 On the other hand, differences in unadjusted or other unobserved variables (ie, residual confounding bias) may continue to exist in the matched cohorts and confound study results.44–46 For example, despite factoring out the differences in patient baseline clinical characteristics with CCI and proxies for MDD severity, the more severely ill patients (other conditions not considered in the CCI calculation or not MDD-related) might develop on a different trajectory of severity from the healthier individuals. Similarly, differences regarding health insurance may be related to a different social background – unmeasurable in our data – which might have influenced healthcare resource use and costs.
Conclusions
These findings provide evidence that treatment with adjunctive brexpiprazole was associated with significantly lower medical costs, particularly hospitalization-associated costs, but higher pharmacy costs compared to patients treated with quetiapine XR. These results were consistent after adjusting for differences in baseline characteristics. As newer atypical antipsychotics become available for the treatment of MDD, the use of these drugs may help reduce the clinical burden to patients, and the economic burden to payers. Future research with larger populations should be performed to confirm the benefits of long-term use of adjunctive atypical antipsychotics on healthcare resource use and costs in patients with MDD.
Abbreviations
AAP, atypical antipsychotics; ADT, antidepressant therapy; CCI, Charlson Comorbidity Index; ED, emergency department; FDA, the Federal Food & Drug Administration; HMO, Health Maintenance Organization; HRU, healthcare resource utilization; ICD-9/10, the International Classification Diseases, 9th/10th Revision; MDD, major depressive disorder; NDC, National Drug Code; PPO, preferred provider organization; Quetiapine XR, Extended-Release quetiapine; SD, standard deviation; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; US, United States.
Ethical Disclosure
This study was based on secondary, de-identified data which comply with the Health Insurance Portability and Accountability Act (HIPAA). Institutional Review Board approval was not required for this study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the commercially owned, proprietary nature of the datasets, but are available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge Kainan Sun of IQVIA for assisting programming, Jenny Tse of IQVIA for assisting medical writing, and Xiaohui Zhao of IQVIA for assisting formatting. The study was funded by Otsuka Pharmaceutical Development and Commercialization, Inc and Lundbeck, USA. Medical writing of the manuscript was funded by Otsuka Pharmaceutical Development and Commercialization, Inc and Lundbeck, USA.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Arpamas Seetasith (AS) and Chakkarin Burudpakdee (CB) were contracted by Otsuka Pharmaceutical Development and Commercialization, Inc. to conduct this study. Mallik Greene (MG) is an employee of Otsuka Pharmaceutical Development and Commercialization, Inc. Ann Hartry (AH) is an employee of Lundbeck, USA. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Depression and other common mental disorders-global health estimates. 2017; Available from: http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf.
2. Rai D, Zitko P, Jones K, Lynch J, Araya R. Country-and individual-level socioeconomic determinants of depression: multilevel cross-national comparison. Br J Psychiatry. 2013;202(3):195–203. doi:10.1192/bjp.bp.112.112482
3. Greenberg PE, Fournier -A-A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. doi:10.4088/JCP.14m09298
4. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi:10.1371/journal.pmed.1001547
5. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
6. Merikangas KR, Ames M, Cui L, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Arch Gen Psychiatry. 2007;64(10):1180–1188. doi:10.1001/archpsyc.64.10.1180
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder third edition. Am J Psychiatry. 2010;167(10):1.
8. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi:10.1176/appi.ajp.163.1.28
9. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–1242. doi:10.1056/NEJMoa052963
10. National Institute of Mental Health. Questions and answers about the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study – all medication levels. 2006; Available from: https://www.nimh.nih.gov/funding/clinical-research/practical/stard/allmedicationlevels.shtml.
11. Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26(1):83–97. doi:10.1002/da.v26:1
12. Knoth RL, Bolge SC, Kim E, Tran Q-V. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188–e196.
13. Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65(3):341–347. doi:10.4088/JCP.v65n0309
14. Suppes T, Silva R, Cucchiaro J, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2015;173(4):400–407. doi:10.1176/appi.ajp.2015.15060770
15. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224–1231. doi:10.4088/JCP.14m09688
16. Wang P, Si T. Use of antipsychotics in the treatment of depressive disorders. Shanghai Arch Psychiatry. 2013;25(3):134.
17. Shelton RC, Papakostas GI. Augmentation of antidepressants with atypical antipsychotics for treatment‐resistant major depressive disorder. Acta Psychiatr Scand. 2008;117(4):253–259. doi:10.1111/acp.2008.117.issue-4
18. Papakostas GI, Shelton RC, Smith J, Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry. 2007;68:826–831. doi:10.4088/JCP.v68n0602
19. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980–991. doi:10.1176/appi.ajp.2009.09030312
20. Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S. Second‐generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010;(12):CD008121.
21. Chen J, Gao K, Kemp DE. Second-generation antipsychotics in major depressive disorder: update and clinical perspective. Curr Opin Psychiatry. 2011;24(1):10–17. doi:10.1097/YCO.0b013e3283413505
22. Seetasith A, Greene M, Hartry A, Burudpakdee C. Changes in healthcare resource use and costs associated with early versus delayed initiation of atypical antipsychotic adjunctive treatment in major depressive disorder. J Med Econ. 2018;21(9):888–901. doi:10.1080/13696998.2018.1484373
23. Yermilov I, Greene M, Chang E, Hartry A, Yan T, Broder MS. Earlier versus later augmentation with an antipsychotic medication in patients with major depressive disorder demonstrating inadequate efficacy in response to antidepressants: a retrospective analysis of US claims data. Adv Ther. 2018;35(12):2138–2151. doi:10.1007/s12325-018-0838-2
24. Prescribing information for Abilify. Otsuka American Pharmaceutical, Inc., Rockville, MD.
25. Prescribing information for Seroquel XR. AstraZeneca Pharmaceuticals LP., Wilmington, DE.
26. Prescribing information for Rexulti. Otsuka American Pharmaceutical, Inc., Rockville, MD.
27. Maeda K, Lerdrup L, Sugino H, et al. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605–614. doi:10.1124/jpet.114.213819
28. Das S, Barnwal P, Winston AB, Mondal S, Saha I. Brexpiprazole: so far so good. Ther Adv Psychopharmacol. 2016;6(1):39–54. doi:10.1177/2045125315614739
29. Peuskens J. The management of schizophrenia: focus on extended-release quetiapine fumarate. Neuropsychiatr Dis Treat. 2011;7:549. doi:10.2147/NDT
30. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
31. Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. doi:10.1161/CIRCOUTCOMES.113.000359
32. Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10(4):577–581. doi:10.1002/(ISSN)1097-0258
33. Kluwer W Medi-span price Rx. Available from: https://www.wolterskluwercdi.com/price-rx/. Accessed May 29, 2019.
34. Wu EQ, Greenberg PE, Ben-Hamadi R, Yu AP, Yang EH, Erder MH. Comparing treatment persistence, healthcare resource utilization, and costs in adult patients with major depressive disorder treated with escitalopram or citalopram. Am Health Drug Benefits. 2011;4(2):78.
35. World Health Organization. Mental health of older adults. 2017; Available from: http://www.who.int/mediacentre/factsheets/fs381/en/.
36. Kramer D, Allgaier A-K, Fejtkova S, Mergl R, Hegerl U. Depression in nursing homes: prevalence, recognition, and treatment. Int J Psychiatry Med. 2009;39(4):345–358. doi:10.2190/PM.39.4.a
37. Richardson LP, DiGiuseppe D, Garrison M, Christakis DA. Depression in Medicaid-covered youth: differences by race and ethnicity. Arch Pediatr Adolesc Med. 2003;157(10):984–989. doi:10.1001/archpedi.157.10.984
38. Centers of Medicare & Medicaid Services. Physician and mental health condition prevalence and comorbidity among fee-for-service medicare-medicaid enrollees. 2014; Available from: https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/Downloads/Dual_Condition_Prevalence_Comorbidity_2014.pdf.
39. Mansournia MA, Hernán MA, Greenland S. Matched designs and causal diagrams. Int J Epidemiol. 2013;42(3):860–869. doi:10.1093/ije/dyt083
40. Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708. doi:10.1093/eurheartj/ehr031
41. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass). 2009;20(4):488. doi:10.1097/EDE.0b013e3181a819a1
42. Porta M. A Dictionary of Epidemiology. Oxford university press; 2014.
43. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Vol. 3. Wolters Kluwer Health/Lippincott Williams & Wilkins Philadelphia; 2008.
44. Brookhart MA, Stürmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(60):S114. doi:10.1097/MLR.0b013e3181dbebe3
45. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a monte carlo study. Stat Med. 2007;26(4):734–753. doi:10.1002/(ISSN)1097-0258
46. Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–655. doi:10.1093/aje/kwm165
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
