Back to Journals » Cancer Management and Research » Volume 11
Real-World Data Of Osimertinib In Patients With Pretreated Non-Small Cell Lung Cancer: A Retrospective Study
Authors Mu Y, Xing P, Hao X, Wang Y, Li J
Received 2 July 2019
Accepted for publication 10 October 2019
Published 30 October 2019 Volume 2019:11 Pages 9243—9251
DOI https://doi.org/10.2147/CMAR.S221434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yuxin Mu, Puyuan Xing, Xuezhi Hao, Yan Wang, Junling Li
National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Junling Li
National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Number 17 Panjiayuan Nan Li, Chao Yang District, Beijing 100021, People’s Republic of China
Tel +86 13801178891
Email [email protected]
Purpose: Osimertinib is an oral, irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) targeted for both EGFR sensitizing mutations and T790M resistance mutation in patients with non-small-cell lung cancer (NSCLC). We assessed efficacy and safety of osimertinib in patients with pretreated NSCLC in a real-world setting.
Patients and methods: Ninety-four patients with advanced NSCLC who received osimertinib after progression of prior EGFR-TKIs or chemotherapy treatments were retrospectively collected.
Results: In patients evaluable for response analysis (n = 91), overall objective response rate (ORR) was 47.3%, and disease control rate (DCR) was 90.1%. Median duration of response (DoR) in responding patients was 12.5 months (95% confidence interval [CI], 10.7 to 14.3). Median progression-free survival (PFS) was 8.5 months (95% CI, 7.4 to 9.6) in 2nd line group, 9.1 months (95% CI, 6.6 to 11.6) in ≥3rd line group, and 8.6 months (95% CI, 7.2 to 10.0) in overall population. For subgroup analysis, DCR and median PFS were 91.9% and 8.6 months (95% CI, 7.2 to 10.0) in patients with detectable T790M mutation, respectively, while 80.0% and 3.2 months (95% CI, 0.5 to 5.9) for those without. Median PFS was significantly longer for T790M-positive patients co-occurring with exon19del than with L858R (17.9 months vs 7.3 months; P<0.001). Among 45 patients with metastases to the central nervous system (CNS), median systemic PFS was 8.8 months (95% CI, 6.9 to 10.7), while intracranial time to progression (iTTP) was not reached. Safety profile was acceptable, no adverse events (AEs) related deaths was observed.
Conclusion: Osimertinib was highly active in patients with pretreated advanced NSCLC who harbored EGFR T790M mutation, with manageable side-effects.
Keywords: osimertinib, non-small-cell lung cancer, efficacy, safety
Introduction
Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases.1 Approximately 10–15% of Caucasian patients and 30–40% of East Asian patients diagnosed with advanced NSCLC harbor activating epidermal growth factor receptor (EGFR) mutations.2 EGFR tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib and afatinib are recommended as standard first-line treatment for such patients based on several large-scale prospective clinical trials.3–5 EGFR T790M mutation has been identified as the most commonly mechanism of acquired resistance to first-line EGFR-TKIs which were found in approximately 60% of patients.6 Osimertinib is a third-generation oral, potent, and irreversible EGFR-TKI, which inhibits both EGFR activating mutation and T790M mutation.7 Phase I/II AURA trial reported osimertinib reached an objective response rate (ORR) of 61% and median progression-free survival (PFS) of 9.6 months among patients with T790M mutation.8 The phase III AURA3 study demonstrated the superiority of osimertinib over platinum-pemetrexed chemotherapy in patients with T790M positive advanced NSCLC after progression of first-line EGFR-TKI therapy.9 Data of osimertinib in treatment naive patients of AURA study10 and FLAURA trial11 also showed highly active of osimertinib in NSCLC patients with activating EGFR mutations. However, there was lack of real-world evidence to illustrate the effectiveness and safety of osimertinib which can reflect the current medical practice. We conducted this retrospective study to assess the real-world clinical impact of osimertinib in patients with advanced NSCLC in our Cancer Center.
Materials And Methods
Data Source And Study Population
The clinical data of patients with advanced NSCLC who received osimertinib after progression of prior EGFR-TKIs or chemotherapy treatments were retrospectively collected from our Cancer Center from Mar 1, 2017 to Jul 1, 2018. Eligible patients were required to be histologically or cytologically confirmed, locally advanced or metastatic NSCLC (stage IIIB and IV), detected with EGFR mutation at least once during disease courses and received osimertinib for at least 3 weeks. The patients who received osimertinib for less than 3 weeks were excluded as they received osimertinib for a short time without tumor response evaluation before molecular testing, and then switched to other regimens once they got the result of molecular testing, according to their medical data. A total of 94 patients met selection criteria.
Assessments
Primary endpoints were disease control rate (DCR) and PFS, secondary objectives included ORR and safety. ORR, DCR and PFS were assessed using Response Evaluation Criteria in Solid Tumor (RECIST) criteria (version 1.1). DCR was calculated as the percentage of patients with response of complete response (CR), partial response (PR), or stable disease (SD) lasting ≥6 weeks before any disease-progression event, while ORR pointed to CR or PR. Radiographic scan was performed to assess the tumor response every 8 to 12 weeks, including CT for chest/abdomen and MRI for brain lesions according to medical records. All medical data were reviewed by a board-certified oncologist from our cancer center. PFS was defined as the time interval from the start of osimertinib treatment to progressive disease (PD) or death from any causes, whichever occurs first. Intracranial time to progression (iTTP) pointed to the time interval from the start of osimertinib to intracranial PD, regardless of extra-cranial response. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Statistics Analysis
The data cutoff was Jul 30, 2018. Statistical analyses were performed with SPSS 23.0 statistical software, P-values were derived from two-sided tests and alpha=0.05 was used as significant level for all statistical testing. ORR and DCR were compared using Chi-square tests and Fisher’s exact tests. PFS was analyzed using Kaplan–Meier method, survival curves of different subgroups were compared using log rank test.
Results
Patients And Characteristics
Ninety-four patients met selection criteria. Most patients but two received gene test prior to osimertinib, among whom 77 were T790M positive, 15 were T790M negative. A total of 59.6% (56/94) of the patients were women, 79.8% (75/94) were non-smokers, 56.4% (53/94) with Eastern Cooperative Oncology Group performance status (ECOG PS) 0, and 97.9% (92/94) had adenocarcinoma on histologic analysis. Most patients had received at least one prior EGFR-TKI (96.8%, 91/94), and 38.3% (36/94) had received prior cytotoxic chemotherapy. In the osimertinib ≥3rd treatment line group (n=38), in addition to EGFR-TKIs, 29 patients had received previous chemotherapy, most were pemetrexed-platinum-containing regimens (75.9%, 22/29), and 7/29 (24.1%) had received other anticancer regimens. Patient demographics and baseline characteristics were listed in Table 1.
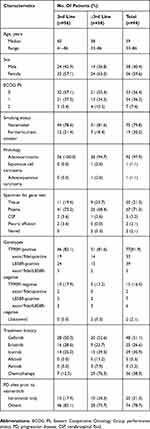 |
Table 1 Baseline Patient Demographic And Clinical Characteristics (n=94) |
Clinical Outcomes
At data cutoff, median duration of follow-up was 8.5 months, and 56 (59.6%) patients were still receiving osimertinib treatment.
Tumor Response
A total of 91 patients were evaluable for response analysis, 54 as 2nd line therapy and 37 as ≥3rd line. Of 91 patients, 43 (47.3%) had PR, 39 (42.9%) had SD, 9 (9.9%) had PD. Disease control was achieved in 82 of 91 patients (90.1%), and ORR was 47.3%. DCR was similar between the 2nd line and ≥3rd line treatment groups, and between patients detecting EGFR T790M from plasma ctDNA samples and those with positive tissue-based outcomes. ORR was higher in patients detecting EGFR T790M from tumor samples than those with plasma ctDNA samples, but was not statistically significant (Table 2). Tumor responses were significantly different in terms of various genotypes. Of 74 patients with detectable T790M mutation who could be evaluated for response, ORR and DCR were 51.4% and 91.9%, respectively. In contrast, among 15 patients without detectable T790M mutation, ORR and DCR were 26.7% and 80.0%, respectively. As for T790M co-occurring common EGFR sensitizing mutation status, ORR was 66.7% in the T790M(+)/exon19del(+) group, which was significantly higher than 36.1% in the T790M(+)/L858R(+) group (P=0.011). DCR was 100.0% in the T790M(+)/exon19del(+) group, compared with 86.1% in the T790M(+)/L858R(+) group (P=0.055). Of 43 patients with an objective response, most had initial response at the time of first follow-up scan, with a median time to response of 1.2 months (range: 0.7–6.7).
 |
Table 2 Clinical Activity Summary Of Osimertinib |
PFS
At data cutoff, 53 of 94 (56.4%) patients had progressed or died. Median PFS was 8.6 months (95% CI, 7.2 to 10.0), 8.5 months (95% CI, 7.4 to 9.6), and 9.1 months (95% CI, 6.6 to 11.6) in the overall population, 2nd line group and ≥3rd line group, respectively. Subset analysis of PFS by T790M status showed a significant longer PFS in patients who was T790M positive compared with T790M negative population (median, 8.6 months [95% CI, 7.2 to 10.0] vs 3.2 months [95% CI, 0.5 to 5.9]; hazard ratio [HR], 0.51 [95% CI, 0.26 to 0.98]; P=0.041). Among patients with common EGFR sensitizing mutation exon19del or L858R, most received early-generation EGFR-TKIs prior to osimertinib. Of initial early-generation EGFR-TKIs treatment, subgroup analysis demonstrated a significant longer PFS in patients who harbored exon19del compared with L858R (median, 14.2 months [95% CI, 8.7 to 19.7] vs. 12.4 months [95% CI, 9.6 to 15.3]; HR, 0.53 (95% CI, 0.33 to 0.83); P=0.006). Similarly, of third-generation EGFR-TKI osimertinib, a significantly superior PFS was observed in patients harbored T790M mutation co-occurring with exon19del versus L858R (median, 17.9 months [95% CI, 5.4 to 30.4] vs. 7.3 months [95% CI, 4.8 to 9.8]; HR, 0.25 (95% CI, 0.11 to 0.54); P<0.001). Median PFS generally was statistically consistent across other subgroups analyzed: age at baseline (≤65 years vs. over 65 years), last treatment before osimertinib (EGFR-TKI vs. chemotherapy), and sample for detecting T790M status (tumor vs. plasma ctDNA). A nonsignificant trend toward longer PFS was observed in patients who were non-smokers compared with smokers (Table 2). Results of multivariate analysis for PFS showed that T790M/exon19del-positive contributed to significantly longer PFS (Table 3). The Kaplan-Meier curves of PFS was showed in Figure 1. Of 53 patients who had progressed of osimertinib at data cut-off, 30 (56.6%) continued osimertinib beyond PD (20 of osimertinib monotherapy, 5 combined with local therapy, 3 combined with bevacizumab, 1 combined with icotinib and 1 combined with chemotherapy). Twenty-three (43.4%) patients discontinued osimertinib, among whom 13 switched to chemotherapy, 6 received early-generation EGFR-TKIs and 4 received best supportive care.
 |
Table 3 Cox Regression For PFS |
OS
Data on overall survival (OS) was immature. At data cutoff, 21 patients (22.3%) had died, 1 with T790M(+)/exon19del(+), 14 with T790M(+)/L858R(+), and 6 with no detectable T790M mutation. One-year survival rate was 66.3%, a higher percentage of patients who were alive at data cutoff was observed in T790M(+)/exon19del(+) group (32/33, 97.0%) than in T790M(+)/L858R(+) group (25/39, 64.1%).
Osimertinib Activity In Patients With CNS Metastases
Forty-five patients had central nervous system (CNS) metastases at baseline, among whom 20 (44.4%) experienced local therapy to the brain (including surgery, radiotherapy and intracranial injection) prior to osimertinib (≤6 months: n=14; >6 months, n=6). PFS was not inferior in patients with CNS metastases than in those without (median, 8.8 months [95% CI, 6.9 to 10.7] vs. 7.8 months [95% CI, 5.9 to 9.7]; HR, 1.05 (95% CI, 0.61 to 1.81); P=0.851). Of patients with locally treated CNS metastases subgroup, median PFS was 8.5 months (95% CI, 2.9 to 14.2), compared with 9.1 months (95% CI, 6.5 to 11.8) of patients without (HR, 1.26 [95% CI, 0.57 to 2.80]; P=0.566). Events of CNS progression were observed in 10 patients (22.2%) before discontinuation of osimertinib or death, iTTP was not reached.
Osimertinib Activity In Patients With Co-Occurring Mutation
Of 77 patients with detectable T790M mutation prior to osimertinib, 5 patients harbored co-occurring mutations at baseline including ROS1 exon36 mutation, EGFR exon7 mutation,EGFR exon18 G719C mutation, EGFR exon19 V742I mutation, KRAS mutation and MET amplification. Of these patients co-occurring with uncommon mutations, 2 achieved PR, 3 achieved SD, and events of progression were observed in 2 patients at data cutoff, PFS ranged from 2.7 months to 7.5 months (Table 4).
 |
Table 4 Osimertinib Activity In T790M-Positive Patients With Co-Occurring Mutation |
Safety
The most common AEs were rash (30.9%), fatigue (29.8%), and stomatitis (25.5%), and majority of AEs were grade 1 or 2 in severity. Eleven events of grade 3 were occurred, no grade 4 AEs or AE-related death were reported. AEs leading to dose reduction or drug discontinuation were not observed, but 4 (4.3%) patients experienced a dose interruption (one because of grade 3 stomatitis, one because of grade 2 dizziness, one because of grade 3 platelet count decreased, and one because of grade 3 alanine aminotransferase [ALT] increased and aspartate aminotransferase [AST] increased). Summary of AEs were listed in Table 5.
 |
Table 5 Adverse Events (n=94) |
Discussion
In our study, osimertinib showed a high ORR (47.3%) and DCR (90.1%) with superior median time to response of 1.2 months and median PFS of 8.6 months in patients with pretreated NSCLC. Our data suggested that osimertinib is promising in patients with advanced NSCLC, especially for those harboring T790M mutation or those with CNS metastases.
The primary endpoint DCR was 90.1% of our results in overall population and range from 80.0% to 100.0% in subgroups. This finding was consistent with prospective clinical trials of AURA,8 AURA extension,12 AURA 213 and AURA 39 studies. While ORR and PFS were inferior than those randomized studies. AURA 213 and AURA extension12 phase II study demonstrated ORR of 60–70% and median PFS of 9.9–12.3 months. The AURA 3 phase III study9 reported a similar ORR of 71% and PFS of 10.1 months. The lower ORR and PFS in our study possibly were attributed to the reasons below: Our study population had characteristics that were different from the global population of patients. 38/94 (40.4%) patients in our study treated with osimertinib as ≥3rd line therapy and 17/94 (18.1%) without detectable T790M mutation or unknown, while osimertinib act as second line treatment in AURA 3 trial9 and study population were T790M-positive. Besides, patients with CNS metastases accounts for about 33–41% of overall population in AURA 2,13 AURA extension12 and AURA 3,9 and only patients with CNS metastases could be enrolled if the disease was asymptomatic, stable, and not requiring corticosteroids for at least 4 weeks before osimertinib in these clinical trials. In addition, it is possible that lacking of blinded independent central review (BICR) and evaluation of efficacy of osimertimib by different treating oncologists in our retrospective study may have impacted ORR outcomes, and for this reason, we used DCR and PFS as our primary objectives.
Prior to osimertinib, the standard recommendation for patients who progress after first-line EGFR-TKIs is chemotherapy, alternative treatment strategies explorations included afatinib, afatinib plus single-agent chemotherapy, and afatinib in combination with cetuximab. Those studies were associated with ORR of 7–34% and PFS of 2–6 months, with a high rate of toxic effects.14–18 The ORR, PFS, and safety profile with osimertinib in both the clinical trials8,12,13 and our retrospective study were superior compared with historical results. These data support osimertinib for the treatment of patients with T790M-positive advanced NSCLC after progression of prior EGFR-TKI therapy.
In our study, we noted a superior outcome in patients with co-occurring T790M and exon19del mutations than those with co-occurring T790M and L858R mutations documented before the osimertinib initiation. This was in line with previous evidence for greater clinical benefits of early-generation EGFR-TKIs in patients harboring exon19del versus L858R mutations.19 The reasons are not clear, one possibility is that exon19del are more efficiently inhibited by EGFR-TKIs. However, in vitro studies do not support this hypothesis.20 Continued analyses are needed to answer this question.
Our study investigated the efficacy of osimertinib, regardless of T790M status. Patients with non-T790M-mediated resistance counted for approximately 40% of cancers.6 For patients without detectable T790M mutation, osimertinib was associated with relatively low response rate and PFS, especially for those had received an EGFR-TKI as the last treatment regimen before osimertinib.8 Other approaches to address cancers that are resistant to EGFR-TKIs with non-T790M-dependent resistance mechanisms was chemotherapy, with similarly limited efficacy.14 Confirmation of T790M status was carried out mostly by using plasma ctDNA samples in our study. Previous studies have demonstrated the feasibility of detecting EGFR T790M from plasma ctDNA samples, the results were equivalent to patients with positive tissue-based outcomes.9 However, a biopsy sample for patients with a plasma T790M-negative result after PD of first-line EGFR-TKI was still needed considering the false negative rates with plasma ctDNA T790M testing, as knowledge of truly T790M status is important both for the clinical practice and prognosis prediction.
Patients with EGFR mutated NSCLC have a much higher risk of developing CNS metastases.21 Osimertinib had demonstrated greater penetration of the Blood Brain Barrier (BBB) than gefitinib or afatinib in preclinical studies,22 and promising intracranial efficacy in patients with advanced NSCLC.9,23,24 In our study, the benefit of osimertinib in the subgroup of patients with CNS metastases was not inferior than those without. We also reported an encouraging systemic PFS of 9.1 months of CNS metastases patients without local treatment to the brain before or during osimertinib therapy, which showed a potential that this strategy may avoid patients from the long-term complications of brain radiation.25
Our study had several strengths. The data were relatively new, and we included elderly patients, patients with ECOG PS > 1 and patients with symptomatic CNS metastases, which were usually excluded from prospective clinical trials. The selection of early-generation EGFR-TKIs in the first-line setting and the available samples for confirmation of mutation status reflects actual current real-world medical practice. The limitations of the study included its single-center, retrospective design, relatively small sample size, the lack of BICR, and the relatively short follow-up time to obtain OS and iTTP. Thus, a long-term follow-up and multicenter study would be required. Additionally, AEs of osimertinib in our study were retrospectively extracted from the medical records, which may introduce potential documentation bias, especially for non-laboratory findings.
In conclusion, our study shows that osimertinib provides encouraging clinical activity with a manageable safety profile in patients with pretreated advanced NSCLC, especially for whom T790M-mediated drug resistance had developed.
Ethical Statement
The Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College approved this study (approval no. 15-144/1071). This study was conducted compliance with the Declaration of Helsinki, and we ensure the confidentiality of patient data. Since it was a retrospective study, some patients had already died before this study, and we were not able to get their informed consent. Besides, we didn’t use any specific information on patients in this study. We get informed consent exemption approved by the ethics committee.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289.
2. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.
3. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
4. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X
5. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi:10.1200/JCO.2012.44.2806
6. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.CCR-12-2246
7. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi:10.1158/2159-8290.CD-14-0337
8. Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi:10.1056/NEJMoa1411817
9. Mok TS, Wu Y, Ahn M, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
10. Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(9):841–849. doi:10.1200/JCO.2017.74.7576
11. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
12. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study Phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi:10.1200/JCO.2016.70.3223
13. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17(12):1643–1652. doi:10.1016/S1470-2045(16)30508-3
14. Yoshida T, Kuroda H, Oya Y, et al. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer. 2017;109:89–91. doi:10.1016/j.lungcan.2017.05.001
15. Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4(9):1036–1045. doi:10.1158/2159-8290.CD-14-0326
16. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. doi:10.1016/S1470-2045(15)00121-7
17. Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–538. doi:10.1016/S1470-2045(12)70087-6
18. Schuler M, Yang JC, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol. 2016;27(3):417–423. doi:10.1093/annonc/mdv597
19. Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(13):3908–3914. doi:10.1158/1078-0432.CCR-06-0462
20. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi:10.1126/science.1099314
21. Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi:10.1016/j.lungcan.2015.01.020
22. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi:10.1158/1078-0432.CCR-16-0399
23. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;JCO2018783118.
24. Xing P, Mu Y, Hao X, Wang Y, Li J. Data from real world to evaluate the efficacy of osimertinib in non-small cell lung cancer patients with central nervous system metastasis. Clin Transl Oncol. 2019;21:1424–1431. doi:10.1007/s12094-019-02071-5
25. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi:10.1001/jama.2016.9839
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

