Back to Journals » Clinical Ophthalmology » Volume 16
Real World Data Comparison of Standard Care vs SDM Laser Vision Protection Therapy for Prevention of Neovascular AMD
Authors Luttrull JK , Gray G
Received 24 March 2022
Accepted for publication 13 May 2022
Published 24 May 2022 Volume 2022:16 Pages 1555—1568
DOI https://doi.org/10.2147/OPTH.S366150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jeffrey K Luttrull,1 Gerry Gray2
1Ventura County Retina Vitreous Medical Group, Ventura, CA, USA; 2Regulatory Pathways, Inc, Laguna Beach, CA, USA
Correspondence: Jeffrey K Luttrull, Ventura County Retina Vitreous Medical Group, 3160 Telegraph Road, Suite 230, Ventura, CA, 93003, USA, Tel +1 805-6500664, Fax +1 805 650-0865, Email [email protected]
Purpose: To access the impact of regular periodic subthreshold diode micropulse laser (SDM) as Vision Protection Therapy on the rate of neovascular conversion of dry age-related macular degeneration (AMD).
Methods: Patient unidentified clinical data aggregated by Vestrum Health, LLC (VH) from 300 retina specialists across the United States was analyzed to examine the effect of a program of regular periodic panmacular low-intensity/high-density subthreshold diode micropulse laser as vision protection therapy (VPT) compared to standard care alone, on the incidence of neovascular conversion in patients with dry age-related macular degeneration (AMD), between January 4, 2016, and September 30, 2020, producing 392,250 eyes for study.
Results: After applying inclusion and exclusion criteria, eyes were matched by propensity scoring for key risk factors. This produced 830 eyes managed by standard care plus VPT, performed on average every 108 days per eye; and 8300 eyes managed with standard care alone (SCA) in a 1/10 ratio for comparison. Comparison found that VPT eyes had a markedly lower rate of neovascular conversion than SCA eyes (hazard ratio 13.04) overall, and for each propensity score matched quintile. VA worsened over time in the SCA group but improved in the VPT group.
Conclusion: Our findings suggest that, compared to standard care alone, VPT may markedly reduce the rate of neovascular conversion in AMD, the main cause of irreversible visual loss worldwide.
Keywords: age-related macular degeneration, dry, neovascular, neuroprotection, prevention, real-world data, reset, subthreshold micropulse laser, vision protection therapy
Introduction
Age-related macular degeneration (AMD) is the principal cause of irreversible visual loss worldwide, mainly affecting persons over 50 years of age. In over 90% of cases, this visual loss is the result of progression from early, or “dry” AMD, to advanced AMD characterized by the development of macular neovascularization (“wet” AMD). As the major cause of visual disability in adults, prevention of wet AMD is major public health priority.1
The current standard of care for dry AMD consists of antioxidant nutritional supplements including AREDS (age-related eye disease study) vitamins; a diet rich in green, leafy vegetables; lifestyle measures such as smoking cessation; and healthcare measures such as treatment of systemic hypertension.2,3 Implementation of such measures has been shown to reduce the risk of developing advanced AMD modestly, by approximately 4% per year.1–4
Panmacular low-intensity/high-density subthreshold diode micropulse laser (SDMTM) has been shown to be effective for treatment of diabetic macular edema, proliferative diabetic retinopathy and central serous chorioretinopathy. SDM has been shown to be safe for treatment of the fovea, and to improve retinal and visual function in dry AMD, inherited retinopathies, and open angle glaucoma (OAG), improve optic nerve function in OAG, and reverse tolerance to vascular endothelial growth factor (VEGF) injections in eyes with wet AMD.5–24 Vision Protection TherapyTM (VPT) describes a program of regular periodic SDM designed to maintain these treatment effects long-term, hoping to slow disease progression and reduce the risks of vision loss. Prior studies have found the VPT treatment strategy to slow age-related geographic atrophy by 47% per year and reduce conversion from dry to wet AMD by 95–98% per year.7,8 The current study compares the rates of neovascular conversion with standard care vs the addition of VPT in a large real-world population.16–19
Analysis of large-scale clinical datasets can provide meaningful insight into effectiveness of therapies in a “real world” setting. Appropriate matching of subjects can provide data that are balanced on observed covariates, removing many sources of bias. Although these methods are not a replacement for a well-conducted clinical trial, they nonetheless provide useful information that may encourage further study.25–28
Methods
Data Source
Vestrum Health, LLC (Naperville, Ill) (VH) is database that aggregates unidentified patient data from the electronic medical records systems (EMR) of approximately 300 geographically diverse retinal subspecialty practices across the United States. All study data for both comparison groups was obtained from the VH database. Data acquisition, filtering, application of study inclusions and exclusions, and propensity scoring was performed by VH. As this study is limited to analysis of patient unidentified electronic data provided by VH and did not involve human experimentation, patient informed consent was not required or obtained, and the study was exempted from investigational review board (IRB) review (WCG IRB). The study adheres to the Health Insurance Portability and Accountability Act and Helsinki Declaration for Medical Research. All study data and the statistical analysis may be found in the Supplemental Data, Tables 1–13 and Figures 1–16 that accompanies this report.
Study Groups
The study compares two cohorts of eyes with dry AMD active in the VH database between January 4, 2016 through September 30, 2020: one from a single vitreoretinal practice employing VPT in addition to standard care (recommendation of antioxidant vitamins, blood pressure control, smoking avoidance and healthy diet and lifestyle habits) in routine management of dry AMD, identified by selection of VPT as the comparator; and the control group consisting of the rest of the VH vitreoretinal practices that did not employ VPT for dry AMD, but instead relied on standard care alone (SCA). The study groups are thus identified as VPT vs SC alone (SCA).1–4
Study Endpoints
The primary endpoint of the current study was to compare the rate of conversion from dry to wet AMD between the VPT and SCA cohorts. Neovascular conversion was identified in the VH database by 2-factor identification including both a change in ICD-9 or ICD-10 diagnostic coding from dry to wet AMD; and initiation of anti-VEGF therapy. The date of conversion was defined as the earliest of the two events.
Visual acuity (VA) was a secondary endpoint. VA was scored by VH using an approximation of ETDRS measurement, according to the method of Gregori et al, by converting Snellen VA of 20/20, to 85 letters; 20/40 to 70 letters; 20/80 to 55 letters; 20/160 to 40 letters, etc.30
Inclusion and Exclusion Criteria
Inclusion criteria were an age of 50 years or more, and coding for the diagnosis of dry AMD. Exclusion criteria for all subjects included prior or current intra-vitreal injections of steroids or vascular endothelial growth factor (VEGF) inhibitors for any indication; neovascular AMD in the fellow eye; diabetes mellitus or retinal vascular occlusion as potential confounding causes of the need for intravitreal injections; or diagnoses of ocular histoplasmosis, high/degenerative myopia, and central serous chorioretinopathy as potential confounding causes of macular neovascularization. Eyes with prior or concurrent macular photocoagulation were also excluded.
Propensity Scoring
After application of inclusion and exclusion filters, eyes from the VPT and SCA cohorts were matched using propensity score methods based on patient characteristics.25–29 Propensity scores provide samples that are, in aggregate, balanced on all covariates included in the model. In a randomized clinical trial (RCT), presenting patients matching the study inclusion criterion are randomly distributed between study groups. Propensity scoring instead identifies subjects matching the study inclusion criteria in an existing population, such as the VH database. Whereas clinical trials match and randomize subjects prospectively, propensity scoring matches subjects retrospectively from an existing population.25–29 Major neovascular conversion risk factors including age, sex, AREDS vitamin use, diagnosis of systemic hypertension, and smoking were included in the propensity score modeling. As a patient encounter is a prerequisite for diagnosis and documentation of neovascular conversion, encounter frequency was also matched to balance the level of surveillance intensity and diagnostic opportunity between the groups. Using the resulting propensity scores, all VPT cohort eyes were nearest-neighbor matched with SCA cohort eyes in a 1/10 ratio (VPT/SCA) to create the analysis data set.25–29 For the SCA group, the date of study entry was the date of first diagnosis of dry AMD, and for the VPT group, the date of initial treatment. The R “Matchit” package was used to carry out the propensity score matching. After propensity score matching, subjects were divided into 5 strata based on propensity scores, and standard stratified analyses were applied. Propensity score stratification is analogous to a meta-analysis of a set of quasi-RCTs within each quintile stratum.27,28 Treatment assignment by the propensity score stratification process can be thought of as a random assignment conditional on observed covariates, such that the potential outcomes are independent of the treatment status.28 Use of 5 strata can remove approximately 90% of any bias that remains following propensity scoring. Additional strata do not significantly improve performance.28
Confidence intervals were calculated using bootstrap methods to account for correlation between eyes. The entirety of the statistical analyses and propensity scoring analysis and descriptive narrative of the analysis and process may be found in the Supplemental Data.
VPT Group Case Selection
As previously reported, SDM in the VPT group was offered to patients with dry AMD of at least AREDS category 2 or higher.7 Thus, patients in the VPT group with early AMD of low-risk AREDS 0–1 were not treated. Untreated eyes with early AMD were included in the VPT group to balance VPT and SCA group demographics, as the SCA group also included eyes with early AMD and inclusion of ICD 9 codes precluded distinction of eyes early from intermediate AMD and exclusion of low-risk (untreated) early AMD eyes from the VPT group would have skewed the conversion risk toward the VPT group, by including only eyes with more advanced AMD and a higher risk of neovascular conversion.1–4 Review of retinal images and reading center analysis of the very large number of eyes and encounters reported in this analysis was beyond the logistical and financial scope of this study.
VPT Treatment
VPT consisted of regular periodic panmacular SDM treatment performed in a maintenance program intended to maximize treatment benefits. SDM is a highly uniform and standardized approach to microsecond pulsed laser therapy that uses identical laser parameters in all eyes.5–21 SDM is characterized by a long laser wavelength (810nm) and low duty cycle (5%) to maximize safety (defined as reliably sublethal to the RPE); the same treatment area (panmacular, which includes all retina between the major vascular arcades), and the same number of laser spot applications in every eye.21 In the current study, use of published SDM parameters known to be both safe and effective in all eyes were employed.5–21 This use of “fixed” laser parameters is permitted by the exceptionally wide therapeutic range of SDM which far exceeds the influences of individual eye specific variations in media opacity, fundus pigmentation, and retinal thickness, making SDM treatment reliably safe and thus infinitely repeatable.13,16,20,21 The periodicity of treatment was informed by clinical data, demonstrating prompt improvements in retinal function following SDM treatment (generally within 24 hours), persisting at maximum levels through 3–4 months, before gradually returning to baseline levels by approximately 6–9 months post treatment.6,9 Thus, VPT was performed every 3–4 months to maintain therapeutic effectiveness.7,8
Results
Demographics
Search of the VH database produced 392,250 eyes coded for dry AMD. Application of inclusion/exclusion filters and nearest-neighbor propensity scoring matching produced 830 eyes in the VPT cohort and 8300 eyes in the SCA cohort for comparison. The average interval for panmacular SDM treatment in the VPT group was 108 days per eye (Tables 1–4).
 |
Table 1 Initial Filtering of Vestrum Dry AMD Database |
 |
Table 2 Variables Used to Perform Propensity Score Matching |
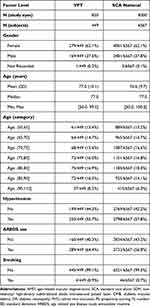 |
Table 3 Demographics by Study Group, After Propensity Score Matching |
 |
Table 4 Follow-Up and Treatment Summary by Study Group, After Propensity Score Matching |
Frequency of Neovascular Conversion
Comparison of the propensity-score matched study groups using a stratified Cox proportional hazards model found a markedly lower rate of neovascular conversion in the VPT group compared to the SCA group overall, and in each propensity score quintile (hazard ratio 13.04; 95% bootstrap CI [5.5, 18.5]) (Figure 1) (Table 5–6) (Supplemental data, Appendix). The cumulative probability of neovascular conversion in the VPT group was 3.9% after 4 years from dry AMD diagnosis, compared to 30.3% in the SCA group (Tables 5–6) (Figure 2). All diagnostics indicated that the Cox model provides an acceptable fit (Supplemental Data, Table 6).
 |
Table 5 Kaplan-Meier Test Between Groups, Stratified by Propensity Score Quintiles |
 |
Table 6 Summary of Overall Survival by Group (Summary of Hazard Ratio Between the Two Groups (Unstratified Kaplan–Meier Estimates), SCA (Standard Care Alone) vs VPT (Vision Protection Therapy) |
Visual Acuity
VA over time showed a trend for worsening in the SCA group and improvement in the VPT group (Figure 3). Due to the volatility in the smaller VPT group, statistical comparison was not performed.
Discussion
In an ageing world, prevention of age-related vision loss is an increasingly important public health priority.1 Age-related macular degeneration is the single most common cause of irreversible vision loss worldwide. In the vast majority of cases, this vision loss is the result of conversion from “dry” to “wet”, or neovascular, AMD. Standard care of dry AMD to reduce the risks of visual loss has consisted of use of antioxidant vitamins, smoking avoidance, and advice to maintain a healthy diet and lifestyle. While these are goods in and of themselves, the effect of these measures on reducing the risk of vision loss once AMD has developed is modest.4 Neovascular conversion from dry AMD remains the main cause of irreversible vision loss. While standard care reduces the risk of neovascularization slightly, standard care does not slow disease progression, or progression of age-related geographic atrophy.1–4 In the current study, VPT reduced the per-eye daily risk of conversion from dry to wet, or neovascular, AMD 13.04 times better than standard care alone. Over the 4.75 year study window, eyes in the VPT group gained visual acuity on average, while in eyes managed by standard care alone VA gradually worsened. Three things may explain these findings: misrepresentation; significant unidentified risk factors and/or population differences between the study groups; or that VPT is highly effective at preventing neovascular conversion and thus visual loss from AMD.31,32
Regarding the first, there does not appear to be evidence of misrepresentation or unexplained data anomalies.32 Regarding the second, that of unrecognized confounding variables, comparison of the population from a one practice to a pool of over 300 other similar practices is inherently problematic, potentially introducing bias due to patient characteristics or other idiosyncrasies of the single practice, other than VPT treatment, compared to the control practices. However, the same VH patient unidentified database was the single source of all study data, and the VPT group practice was the only VH audited practice to employ VPT. This difference also allows for a clear binary distinction between treatment groups - VPT - yes or no? As the populations are well-matched for all known major conversion risk factors, inclusion and exclusion criteria, and derived from similar retinal subspecialty referral-based practices offering otherwise similar services, it is unlikely that unrecognized confounding risk factors would be present that would be sufficient to alter the results significantly, as any such unidentified or unknown confounding risk factor would need to negate the observed hazard ratio (HR) of 13.04, which would require the presence of an unknown confounding AMD risk factor greater than any currently known.1–4,7
As noted, a limitation arising from the design of the current study is the absence of sub-categorization of dry AMD severity. This is unavoidable as the study time window included ICD 9 coding, which did not subtype AMD beyond “dry” or “wet”. Alternative use of a retinal reading center was beyond both the financial and logistical scope of the current study. However, several factors mitigate the absence of AMD subtyping. First, all study data is from retinal subspecialty referral practices in the United States, suggesting similar patient populations. All study data was derived patient unidentified from the same aggregated EMR database, minimizing the possibility of selection bias. Patient age is the primary risk factor for AMD and main determinant of AMD severity, and patient ages and all other major risk factors identifiable in the aggregated EMR database are well matched by propensity scoring. Such matching and the large study population of 9130 eyes would be expected to balance dry AMD severity between the well-matched study groups. This is illustrated by the high degree of concordance between the study groups overall, propensity score matching for each primary risk factor, and for each propensity scored substratum quintile1–4,25–29 (Figure 1) (Supplemental Data). Therefore, it is reasonable to conclude that the advantage of the VPT group is due to the effectiveness of VPT, rather than skewing of the study populations. While the results of this study are remarkable, we note that the robust results we find in the current study are fully consistent will all prior reports of SDM for various indications, including AMD.5–24
The exceptionally high acceptance rate and compliance with panmacular SDM for dry AMD, previously reported, mitigated treatment selection bias in the VPT group.7 Eyes with early, low risk dry AMD were not generally treated with VPT due to the low risk of neovascular conversion.7 These eyes were retained in the VPT group to achieve demographic, and thus risk factor, balance with the SCA group, which also contained all eyes coded for dry AMD, and thus eyes with early AMD as well, as stratification of dry AMD subtypes based on ICD 9 coding was not possible. The low risk of conversion in eyes with early AMD is reflected in the lack of conversion events in any of the untreated eyes in the VPT group. Because of the low risk of neovascular conversion in early AMD, use of AREDS vitamin prophylaxis is also not generally recommended, because it is not helpful.2 Thus, we note that AREDS vitamins use in both groups (64% in the VPT and 57% in the SCA groups, respectively) are also similar, suggesting similar proportions of eyes with early vs intermediate and advanced dry AMD.2 The 32% of untreated eyes in the VPT group approximates the 36% of eyes in the group not reporting use of AREDS vitamins, consistent with VPT not being recommended to eyes with low-risk AMD.2 Thus, retention of untreated eyes in the VPT appears to have contributed to demographic and risk factor balance with the SCA group.
Conversion of the VA data from Snellen to an ETDRS approximation was necessitated by the aggregated EMR data source. However, all VA data was treated identically and only used comparatively within the study. The volatility of the VAs from the smaller VPT group precluded comparison of VA outcomes between the study groups. It is notable, however, that over the 4.75 year study period, there was a trend of decreasing VA in the SCA group, and increasing VA in the VPT group. At study conclusion, VPT eyes averaged several lines better VA than the SCA group. (Figure 3) This suggests a possible VA benefit over time in the VPT group from the VPT long-term preventive maintenance strategy. Further study will be instructive in this regard.
The study inclusions and exclusions, as well as two-factor confirmation of neovascular conversion, requiring both a coding change from dry to wet AMD and initiation of anti-VEGF therapy, were designed to minimize inclusion of false-positive conversion events. Because of these restrictions, neovascular conversions in excluded eyes were not determined. Thus, our data likely underestimates the actual rates of neovascular conversion due to dry AMD in the overall VH database. However, by applying appropriate filters and eye-level matching with propensity scoring, the current study appears to accurately reflect the comparative rates of neovascular conversion in our matched study populations, indicating a marked risk reduction associated with VPT.7,8 Because of the robust advantage of VPT, alternative, less restrictive definitions of neovascular conversion (eg, either single-item ICD coding change, or initiation of anti-VEGF therapy) would not substantively alter our results.
It is interesting to compare the results of the current study with the results of nanosecond laser (2RT) for dry AMD.33 For dry AMD, SDM VPT appears to improve all outcomes, while in the 2RT RCTs, nanosecond laser had no overall effect, and worsened outcomes in high-risk eyes.33 Nanosecond 2RT laser is photodisruptive to the RPE.24,33 SDM, by contrast, is sublethal to the retina and RPE and thus has no known adverse treatment effects clinically, in vivo, or in vitro.5–21 In the 2RT study, the main cause of worsening was incitement and progression of geographic atrophy after nanosecond laser photodisruption of the RPE. Geographic atrophy was not an endpoint in the current study.8,30 However, VPT managed eyes in the current study fared substantially better than SCA eyes. While the reasons for this difference between VPT and 2RT is unclear, it is worth noting that the biophysical effects of SDM, and thus VPT, and 2RT are fundamentally different. 2RT destroys the retinal pigment epithelium (RPE) while SDM is preserves and revitalizes it. This would seem to be an important difference.5–24,33–41 Laser damage to the retina, such as is necessary to trigger debridement of drusen, is pro-inflammatory, pro-neoangiogenic, worsens retinal function, and compromises the ability of the retina to resist neovascularization, as demonstrated in the “laser for drusen” studies.14 SDM, by contrast, is homeotrophic (functionally restorative) to the RPE, is anti-inflammatory, improves retinal function, and is anti-neoangiogenic, thus improving the ability of the retina to resist choroidal neovascularization.5–24,34–41 A prerequisite for slowing disease progression and reducing adverse events such as visual loss in chronic progressive disease, such as neovascular conversion and vision loss in AMD, is prompt, and then maintained, improvement in retinal function.5,6,11 This is the basis of the VPT strategy, and, for that matter, any effective preventive treatment. Absent adverse treatment effects, the effects of SDM are manifold and uniformly therapeutic and restorative. A few include normalization of RPE cytokine expression/balance including decreased VEGF and increased pigment epithelial derived factor levels; normalized antioxidant balance and tissue nitrous oxide levels; decreased markers of disease-driving chronic inflammation; improved mitochondrial function, anti-apoptosis, increased markers of reparative acute inflammation, and restorative local and systemic immune response modulation.6,7,11,34–41 Such effects, maintained by regular periodic treatment, may account for our findings.7,8 While intravitreal anti-VEGF drugs have improved the management and visual prognosis of wet AMD, conversion to neovascular or “wet” AMD from dry AMD continues to be the main cause of age-related visual loss, decreased quality of life, loss of independence in older adults, and a tremendous economic and social healthcare burden worldwide.1–4 Prevention of neovascular conversion would thus be the most effective measure to reduce visual loss and visual disability due to AMD.1–4,42–44 In the current study, VPT appears to be highly effective in this regard.
In an era of increasingly available and finely granulated data and computational analysis, the use of “real world” data (RWD) is becoming more common and informative.25–27,31,45,46 While randomized clinical trials indicate how well an invention can work in an idealized setting, RWD reveals how the same intervention translates to application by clinicians in the community, arguably the most important measure of efficacy, and certainly the most relevant to patients. In real-world application, VPT appears safe and highly effective at preventing visual loss from neovascular conversion in dry AMD. Panmacular SDM is a technically simple to perform, uniform, and standardized procedure employing the same laser settings and parameters, treatment field, and number of laser spot applications in every eye, regardless of treatment indication or individual patient variables, including fundus pigmentation.20,21 It is thus easily transferrable. The current study corroborates prior studies of SDM in dry AMD, suggesting that SDM VPT may be the first procedural intervention to reduce the risk of visual loss in dry AMD, and first treatment of any kind to be significantly more effective than standard care alone.7,8
As a study of RWD, the current study is retrospective and thus has limitations compared to prospective study. However, we note that interventions studied by RWD generally suffer in comparison RCTs, which represent idealized and highly controlled settings. Thus, the robust nature of the results of the current study of RWD are notable.45,46 Further, RWD can supply important information that cannot be obtained, or may be missed, in RCTs.47 Thus, emergency regulatory approval of the various coronavirus vaccines was conferred based on RCT data, while full regulatory approval required RWD confirmation. Approval of brolucizumab for neovascular AMD was based on several large RCTs. RWD caused it to be promptly abandoned in most clinical use over safety concerns not identified in the RCTs.44,45,48 In “Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials” (2014), the Cochrane collaboration found no difference in the results of RCTs compared to those from large RWD studies with robust results, such as in the current study.46 Further study is desirable and will certainly follow. However, the large size and robust findings of the current study predict validation.
The current standard of care for dry AMD consists of lifestyle and dietary counselling along with use of antioxidant vitamins. While laudable, the benefits of standard care for dry AMD are modest.1–4,7 Conversion from dry to neovascular, or wet, AMD remains the most important cause of irreversible visual loss worldwide. The current study suggests that improvement in retinal function by low-intensity/high-density subthreshold diode microsecond pulsed laser, maintained in a program of regular periodic retreatment as vision protection therapy, can safely and significantly reduce the risk of neovascular conversion compared to standard care alone. Confirmed by further study, VPT would establish a new benchmark for dry AMD therapies.49–51
Authors Declaration and Warranties
The authors certify and warrant that:
The submitting author has been authorized by all co-authors to submit the research article; and
- They are the sole author(s) of the article and are legally able and entitled to submit the article and authorize Dove Medical Press (DMP) to publish the research article. If the law requires that the article be published in the public domain, the author(s) will notify DMP at the time of submission.
- The research article is original, has not already been published in any other journal (medical, or otherwise) or is not currently under consideration for publication by another journal, and does not infringe any existing copyright or any other rights prescribed by law;
- The article contains nothing that is unlawful, defamatory, or which would, if published, constitute a breach of contract or of confidentiality;
- Due care, diligence and all other requisite investigations were carried out in the preparation of the research article(s) to ensure its accuracy. To the best of their knowledge all statements contained in it purporting to be factual are true and correct.
Author Contributions
The listed authors warrant that each author:
- Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
- Have drafted or written, or substantially revised or critically reviewed the article.
- Have agreed on the journal to which the article will be submitted.
- Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
- Agree to take responsibility and be accountable for the contents of the article.
Disclosure
Dr. Luttrull reports the following:
Ojai Retinal Technologies, LLC Managing member, equity;
Retinal Protection Sciences, LLC: Managing member, equity;
Vision Protection Institutes, LLC: Managing member, equity;
Vision Protection TherapyTM and SDMTM are registered trademarks of Ojai Retinal Technologies, LLC.
Dr. Gray is a consultant for Regulatory Pathways, Inc. The authors report no other conflicts of interest in this work.
References
1. Wan LW, Xinyi S, Xiang L, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systemic review and meta-analysis. Lancet Glob Health. 2014;2(2). doi:10.1016/S2214-109X(13)70145-1
2. Ferris FL
3. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report number 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi:10.1001/archopht.119.10.1417
4. American Academy of Ophthalmology preferred practice pattern – update; 2015. Available from: https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015.
5. Luttrull JK, Chang DB, Margolis BWL, Dorin G, Luttrull DK. Laser re-sensitization of medically unresponsive neovascular age-related macular degeneration: efficacy and implications. Retina. 2015;35(6):1184–1194. doi:10.1097/IAE.0000000000000458
6. Luttrull JK, Margolis BWL. Functionally guided retinal protective therapy as prophylaxis for age-related and inherited retinal degenerations. A pilot study. Invest Ophthalmol Vis Sci. 2016;7(1):265–275. doi:10.1167/iovs.15-18163
7. Luttrull JK, Sinclair SH, Elmann S, Glaser BM. Low incidence of choroidal neovascularization following subthreshold diode micropulse laser (SDM) for high-risk AMD. PLoS One. 2018;13(8):e0202097. doi:10.1371/journal.pone.0202097
8. Luttrull JK, Sinclair SH, Elmann S, Chang DB, Kent D. Slowed progression of age-related geographic atrophy following subthreshold laser. Clin Ophthalmol. 2020;14:2983–2993. doi:10.2147/OPTH.S268322
9. Luttrull JK, Kent D. Modern retinal laser for neuroprotection in open-angle glaucoma. Samples JR, Ahmed IIK, editors. New Concepts in Glaucoma Surgery. Amsterdam: Kugler Publications; Sept 2019: 1.
10. Luttrull JK. Low-intensity/high-density subthreshold diode micropulse laser (SDM) for central serous chorioretinopathy. Retina. 2016;36(9):1658–1663. doi:10.1097/IAE.0000000000001005
11. Luttrull JK. Improved retinal and visual function following subthreshold diode micropulse laser (SDM) for retinitis pigmentosa. Eye. 2018;32(6):1099–1110. doi:10.1038/s41433-018-0017-3
12. Luttrull JK, Samples JR, Kent D, Lum BJ. Panmacular subthreshold diode micropulse laser (SDM) as neuroprotective therapy in primary open-angle glaucoma. Glaucoma Res. 2018;2020:281–294.
13. Luttrull JK, Musch MC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular edema. Br J Ophthalmol. 2005;89(1):74–80. doi:10.1136/bjo.2004.051540
14. Luttrull JK, Kent D. Laser therapy to prevent choroidal neovascularization. In: Choroidal Neovascularization. Chhablanni J, editor. Springer Verlag; 2020. doi:10.1007/978-981-15-2213-0_30
15. Luttrull JK, Spink CJ, Musch DA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye. 2008;22(5):607–612. doi:10.1038/sj.eye.6702725
16. Luttrull JK, Dorin G. Subthreshold diode micropulse photocoagulation as invisible retinal phototherapy for diabetic macular edema.A review. Curr Diabetes Rev. 2012;8(4):274–284. doi:10.2174/157339912800840523
17. Kozak I, Luttrull JK. Modern retinal laser therapy. Saudi J Ophthalmol. 2015;29(2):137–146. doi:10.1016/j.sjopt.2014.09.001
18. Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina. 2012;32(2):375–386. doi:10.1097/IAE.0b013e3182206f6c
19. Luttrull JK, Sinclair SD. Safety of transfoveal subthreshold diode micropulse laser for intra-foveal diabetic macular edema in eyes with good visual acuity. Retina. 2014;34(10):2010–2020. doi:10.1097/IAE.0000000000000177
20. Chang DB, Luttrull JK. Comparison of subthreshold 577nm and 810nm micropulse laser effects on heat-shock protein activation kinetics: implications for treatment efficacy and safety. Transl Vis Sci Technol. 2020;9(5):23. doi:10.1167/tvst.9.5.23
21. Keunen JEE, Battaglia-Parodi M, Vujosevic S, Luttrull JK. International retinal laser society guidelines for subthreshold laser treatment. Transl Vis Sci Technol. 2020;9(9):15. doi:10.1167/tvst.9.9.15
22. Lavinsky D, Cardillo JA, Melo LA
23. Chen G, Tzekov R, Li W, et al. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema. A meta-analysis of randomized controlled trials. Retina. 2016;36(11):2059–2065. doi:10.1097/IAE.0000000000001053
24. Chhablani J, Roh YJ, Jobling AI, et al. Restorative retinal laser therapy: present state and future directions. Surv Ophthalmol. 2018;63(3):307–328. doi:10.1016/j.survophthal.2017.09.008
25. Kim SC, Schneeweiss S. When randomized clinical trials and real-world evidence say the same: tocilizumab and its cardiovascular safety. Arthritis Rheumatol. 2019. doi:10.1002/art.41092
26. Jupiter DC. Propensity score matching: retrospective randomization? J Foot Ankle Surg. 2017;56(2):417–420. doi:10.1053/j.jfas.2017.01.013
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41
28. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
29. Greenhouse JB. Commentary: cornfield, epidemiology and causality. Int J Epidemiol. 2009;38(5):1199–1201. doi:10.1093/ije/dyp299.
30. Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi:10.1097/IAE.0b013e3181d87e04
31. Wang RY, Strong DM. Beyond accuracy: what data quality means to data consumers. J Manag Inf Syst. 1996;12(4):5–53.
32. Sheehan JG. Fraud, conflict of interest, and other enforcement issues in clinical research. Cleve Clin J Med. 2007;74(Suppl 2):S63–9. doi:10.3949/ccjm.74.suppl_2.s63
33. Guymer RH, Wu Z, Hodgson LAB; Laser Intervention in Early Stages of Age-Related Macular Degeneration Study Group, et al. Subthreshold nanosecond laser intervention for age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2018. doi:10.1016/j.ophtha.2018.09.015
34. Frizziero L, Calciati A, Midena G, et al. Subthreshold micropulse laser modulates retinal neuroinflammatory biomarkers in diabetic macular edema. J Clin Med. 2021;10(14):3134. PMID:34300297. doi:10.3390/jcm10143134
35. Inagaki K, Shuo T, Katakura K, Ebihara N, Murakami A, Ohkoshi K. Sublethal photothermal stimulation with a micropulse laser induces heat shock protein expression in ARPE-19 Cells. J Ophthalmol. 2015;729792:2015.
36. Midena E, Bini S, Martini F, et al. Changes of aqueous humor Muller cells’ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina. 2018. doi:10.1097/IAE.0000000000002356
37. Iwami H, Pruessner J, Shariaki K, Brinkmann R, Miura Y. Protective effect of a laser-induced sub-lethal temperature rise on RPE cells from oxidative stress. Exp Eye Res. 2014;124:37–47. doi:10.1016/j.exer.2014.04.014
38. Hattenbach LO, Beck KF, Pfeilschifter J, Koch F, Ohrloff C, Schake W. Pigment epithelium- derived factor is up regulated in photocoagulated human retinal pigment epithelial cells. Ophthalmic Res. 2005;37(6):341–346. doi:10.1159/000088263
39. Caballero S, Kent DL, Sengupta N, et al. Bone marrow-derived cell recruitment to the neurosensory retina and retinal pigment epithelial cell layer following subthreshold retinal phototherapy. Invest Ophthalmol Vis Sci. 2017;58(12):5164–5176. doi:10.1167/iovs.16-20736
40. De Cilla S, Vezzola D, Farruggio S, et al. The subthreshold micropulse laser treatment of the retina restores the oxidant/antioxidant balance and counteracts programmed forms of cell death in the mice eyes. Acta Ophthalmol. 2018;97(4):e559–e567. doi:10.1111/aos.13995
41. Flaxel C, Bradle J, Acott T, Samples JR. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina. 2007;27(5):629–634. doi:10.1097/01.iae.0000249561.02567.fd
42. Ciulla TA, Huang F, Westby K, et al. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2018;2(7):645–653. doi:10.1016/j.oret.2018.01.006
43. Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients. A real-world analysis of 49, 485 eyes. Ophthalmol Retina. 2019;4(1):19–30. doi:10.1016/j.oret.2019.05.017
44. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441–448. PMID: 33630045; PMCID: PMC7907988. doi:10.1001/jamaophthalmol.2020.7085
45. Schilsky RL. Finding the evidence in real-world evidence: moving from data to information to knowledge. JACS. 2017;224(1):1–7.
46. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014;2014(4). doi:10.1002/14651858.MR000034.pub2
47. Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials Review. New Eng J Med. 1983;309(22):1358–1361. doi:10.1056/NEJM198312013092204
48. Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170(4):477–480.
49. Solomon SD, Lindsley K, Vedula SS, Ksystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;4(3):CD005139. doi:10.1002/14651858.CD005139.pub4
50. Mitchell P, Annemans L, White R, et al. Cost effectiveness of treatments for wet age-related macular degeneration. Pharmacoeconomics. 2011;29(2):107–131. doi:10.2165/11585520-000000000-00000
51. Rosenfeld PJ, Feuer WJ. Warning: do not treat intermediate AMD with laser therapy. Am J Ophthalmol. 2019;125(6):839–840.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



