Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Real-World Characterization of Women with Diagnosed Endometriosis Initiating Therapy with Elagolix Using a US Claims Database
Authors Surrey ES, Soliman AM, Johns B, Vora JB, Taylor HS , Agarwal SK
Received 28 May 2020
Accepted for publication 14 August 2020
Published 26 August 2020 Volume 2020:12 Pages 473—479
DOI https://doi.org/10.2147/CEOR.S264905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Eric S Surrey,1 Ahmed M Soliman,2 Beverly Johns,2 Jamie B Vora,3 Hugh S Taylor,4 Sanjay K Agarwal5
1Colorado Center for Reproductive Medicine, Lone Tree, CO, USA; 2Health Economics and Outcomes Research, AbbVie Inc, North Chicago, IL, USA; 3Healthcare Solutions, AbbVie Inc, North Chicago, IL, USA; 4Department of Obstetrics and Gynecology and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA; 5Center for Endometriosis Research and Treatment, University of California, San Diego, CA, USA
Correspondence: Eric S Surrey
Colorado Center for Reproductive Medicine, 10290 RidgeGate Circle, Lone Tree, CO 80124, USA
Tel +1 (303)-788-8300
Email [email protected]
Purpose: Elagolix is an oral gonadotropin-releasing hormone antagonist approved in the United States for the management of moderate to severe pain associated with endometriosis. We performed a real-world evaluation of the demographic and clinical characteristics of women diagnosed with endometriosis who were initiating elagolix therapy in the United States.
Patients and Methods: This retrospective cohort database analysis included women 18– 49 years of age with ≥ 1 pharmacy claim for elagolix between August 2018 and December 2019 from the Copyright © 2020 Truven Health Analytics LLC. All Rights Reserved. Women had continuous medical and pharmacy health plan enrollment during the baseline period (year immediately preceding the index date [date of earliest elagolix claim]) and had ≥ 1 medical claim with endometriosis (International Classification of Diseases [ICD]-9/10 code [617.x and N80.x]) on or before the index date. Baseline demographics, comorbidities, ICD code-based endometriosis anatomic site, endometriosis-related treatments, and pain symptoms were summarized descriptively.
Results: The study included 2083 patients with mean age at baseline of 33.2 ± 8.1 years. Comorbidities most commonly recorded were non-cancer, non-endometriosis pain (59.5%), including arthritis/joint pain (43.7%) and back/neck pain (31.7%), and mental disorder (40.7%), including anxiety (32.7%). The majority of endometriosis diagnosis codes recorded referred to unspecified location (52.3%) and pelvic peritoneum (23.0%); 61.0% of patients received a medical endometriosis-related treatment in the baseline period, with the most common treatments being contraceptives (various routes of administration, 40.2%) and progestins (31.7%). Additionally, 35.4% of the patients received an endometriosis-related surgery during baseline, with the most common being laparoscopy (33.2% of all patients). Opioids were used during the baseline period by 57.3% of the patients. For pain symptoms, 71.5%, 30.4%, and 19.3% of the patients had claims for pelvic pain, dysmenorrhea, and dyspareunia, respectively.
Conclusion: Endometriosis therapies were used by a significant proportion of patients with endometriosis in the year immediately preceding elagolix initiation.
Keywords: gonadotropin-releasing hormone antagonist, women’s health, clinical characteristics, demographics
Introduction
Endometriosis is a chronic, estrogen-dependent disease characterized by dysmenorrhea, non-menstrual pelvic pain, and dyspareunia that affects at least 176 million women worldwide.1–3 Additional symptoms women with endometriosis experience include infertility, pain at ovulation, constipation, painful urination, and chronic fatigue.1–3 As a consequence of these symptoms, women with endometriosis have a reduced health-related quality of life, psychological and social difficulties, and increased financial burdens.3–6
Both pharmacological and surgical treatments options are available for the treatment of endometriosis symptoms.1,7-10 Pharmacological treatment options include nonsteroidal anti-inflammatory drugs, pain medications (including opioids), hormonally active contraceptives (oral, intrauterine and intramuscular), progestins, and gonadotropin-releasing hormone (GnRH) agonists (including goserelin, leuprolide, nafarelin, buserelin, and triptorelin) and antagonists (cetrorelix [subcutaneous injection] and elagolix [oral]). Surgical options include lesion ablation or excision potentially involving concomitant procedures such as hysterectomy with or without oophorectomy as well as pre-sacral neurectomy. However, hysterectomies and presacral neurectomies are discouraged and should only be considered if all other treatment options have failed.9 As most of these pharmacologic and surgical treatments, with the exception of complete lesion excision, are associated with high recurrence rates and for many women, initial treatments provide temporary or no symptom relief, a variety of different treatments are needed.9
Elagolix is an oral, nonpeptide GnRH antagonist approved in the United States in 2018 for the management of moderate to severe pain associated with endometriosis.11 In the ELARIS Endometriosis I and II (NCT01620528 and NCT01931670) Phase III studies, elagolix, either at 200 mg twice daily or 150 mg once daily, significantly reduced dysmenorrhea (P<0.001) and nonmenstrual pelvic pain (P≤0.003) after 3 months of treatment compared with placebo for women with moderate or severe endometriosis.12 Significant improvements in dyspareunia (P<0.01) were observed with the higher elagolix dose relative to placebo after 3 months of treatment.
The characteristics of women who initiate elagolix treatment and their prior treatments are currently not known. To address this, we report on the demographic and clinical characteristics of women diagnosed with endometriosis who initiated therapy with elagolix using real-world data from a United States commercial claims database.
Patients and Methods
Study Design
This was a retrospective cohort database analysis using the IBM® MarketScan® Commercial Database for the period of August 2018 to December 2019. The MarketScan Commercial Database contains claims data from approximately 40 million employees and their dependents in the United States.6,13 The database consists of both inpatient and outpatient healthcare information, including pharmacy claims. It covers a variety of employee-sponsored private health insurance types, including health maintenance organization, point-of-service, and preferred provider organization plans.
All database records were de-identified and compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act (HIPAA) of 1996.13 Because of this, institutional review board approval was not required.
Study Population
Women 18 to 49 years of age who had ≥1 pharmacy claim for elagolix, as identified by the National Drug Code, between August 2018 and December 2019 were initially selected. The date of the first elagolix claim was considered the index date. Patients had to have continuous medical and pharmacy health plan enrollment for 12 months preceding the index date (baseline period). Additionally, patients had to have ≥1 medical claim with endometriosis based on International Classification of Diseases (ICD)-9/10 code (617.x and N80.x, Table 1) on or before the index date.
 |
Table 1 Endometriosis Codes Recorded by Elagolix Users (n=6088a) |
Outcomes
Comorbidities, ICD-code based endometriosis anatomic site claims, endometriosis-related medical and surgical treatments, opioid use, and pain symptoms reported during the baseline period were evaluated. Endometriosis-related medical treatments captured were contraceptives (combined hormonals, delivered through various types of administration including intramuscular, intrauterine, oral, topical, transdermal, and vaginal), progestins, GnRH agonists, and danazol. Endometriosis-related surgical treatments summarized were laparoscopy, hysterectomy, laparotomy, and oophorectomy. Endometriosis-related pain symptoms captured were pelvic pain, abdominal pain, dysmenorrhea, and dyspareunia. Comorbidities captured were non-cancer pain (ie, arthritis/joint pain, back/neck pain, migraine, and human immunodeficiency virus-associated pain), mental disorder (ie, anxiety, mood disorders, stress), cancer, infertility, opioid abuse, polycystic ovary syndrome, and uterine fibroids. The incidence of endometriosis-associated infertility and related therapies was not evaluated in this investigation.
Statistical Methods
Continuous variables were summarized by means and standard deviations (SD), and categorical variables were reported as percentages. All data analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Study Population
A total of 3954 patients with an elagolix pharmacy claim between August 2018 and December 2019 were identified from the MarketScan Commercial Database. Following further selection based on predefined inclusion criteria, data from 2083 women with endometriosis were included in the analysis (Figure 1). Mean age of these women at baseline was 33.2 ± 8.1 years with the most prevalent age group being 30–39 years (39.0%) (Table 2).
 |
Table 2 Study Population Demographics |
 |
Figure 1 Study population attrition. |
Baseline Comorbidities
During the 12-month baseline period, the most commonly recorded comorbidities were non-cancer, non-endometriosis associated pain (59.5%), defined as arthritis/joint pain (43.7%) or back/neck pain (31.7%), and mental disorder (40.7%), defined as anxiety (32.7%) or mood disorders (25.5%) (Figure 2). The endometriosis diagnosis codes that were most often documented in the endometriosis-associated medical claim referred to unspecified location (52.3%) and pelvic peritoneum (23.0%) (Table 1).
 |
Figure 2 Comorbidities reported in the year prior to first elagolix prescription (n=2083). |
Endometriosis-Related Treatments and Surgeries
Endometriosis-related medical and surgical treatments were recorded by 61.0% and 35.4% of patients, respectively, during the baseline period, with 72.9% (n=1519) having either treatment (Figure 3). The mean time between the last endometriosis-related medical and surgical treatment and the index date was 52.5 days (SD 82.8). The most common documented endometriosis-related medical treatments were contraceptives (various routes of administration, 40.2%), progestins (31.7%), and GnRH agonists (8.0%). Although not indicated in the claim, a proportion of the progestin use may have been as add-back with other agents (namely GnRH agonists). Laparoscopy (33.2%) was the most common endometriosis-related surgical treatment recorded. Interestingly for 27% of women, elagolix was the first documented treatment in the index year.
 |
Figure 3 Endometriosis-related medical treatments and surgeries reported in the year prior to first elagolix prescription (n=2083). |
Opioid Use and Pain Symptoms
Opioid use was documented for 57.3% (n=1193) of patients during the baseline period. Pain symptoms that were most often recorded were pelvic pain (71.5%), abdominal pain (46.5%), dysmenorrhea (30.4%), and dyspareunia (19.3%) (Figure 4).
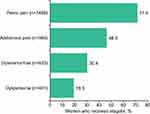 |
Figure 4 Pain symptoms reported in the year prior to first elagolix prescription (n=2083). |
Discussion
In this report, we evaluated the demographics and clinical characteristics of women with endometriosis and their related treatments in the year prior to initiating elagolix therapy. Using a large, United States commercial insurance claims database, we determined that almost two-thirds of women had prescribed medical treatments and one-third had surgical treatments to relieve their endometriosis-related symptoms. The high proportion of women who underwent medical and surgical procedures and subsequently needed to initiate elagolix therapy approximately 2 months following the respective procedure highlights the unmet medical need for these patients. This is consistent with previous studies indicating the lack of adequate response with current treatment regimens.9,14-16 In a systematic literature review of medical treatment responses for endometriosis-associated pain, 11–19% had no reduction in pain with treatment while 5–59% had remaining pain.14 Additionally, discontinuation rates of 5–16% were noted due to lack of efficacy or adverse events.14 In a World Endometriosis Research Foundation ENDOCost study of women with diagnosed endometriosis, 60% still experienced chronic pain despite treatment.15 For women who underwent surgery to relieve endometriosis symptoms, 30% and 61% required further surgery after 5 years for all age groups and for those aged 19–29 years, respectively.16 Finally, elagolix was the first documented treatment for 27% of women with endometriosis. This suggests that, prior to the index year, other therapies may have failed and no available therapy was helpful before the approval of elagolix. Alternatively, elagolix may be viewed by some clinicians as the optimal first-line treatment for a subset of patients with particular pain or symptom profiles, although there have been no reports on the first-line use of elagolix.
Another finding which reflects the need for additional medical options is the high proportion, almost 60%, of women with endometriosis who use opioids. Although generally prescribed for chronic pain, they are addicting and lead to misuse, resulting in a significant number of overdose deaths in the overall population, approximately 47,000 in the United States in 2018.17,18 Our results are consistent with a recent retrospective analysis from 2006 to 2016 of women with endometriosis, aged 18–49 years, which found that 79.3% had opioid prescriptions filled.19 Besides its association with higher morbidity, opioid use has also resulted in greater healthcare utilization and costs among women with endometriosis.20
The primary comorbidities women had in this study were either pain- or mental health-related. The mental health-related comorbidities are most likely associated with the burden of endometriosis symptoms. This psychological impact of endometriosis has been well characterized and has been shown to affect various aspects of mental health; including anxiety, depression, emotional distress, feelings of powerlessness, and self-esteem.3–5 These findings emphasize the need for additional therapeutic options to treat not only the physical but also the psychological consequences of the disease.
Certain limitations were associated with this study. As this study involved only commercially insured, reproductive-aged women, the reported findings may not be generalizable to the overall population of women with endometriosis. Endometriosis-related symptoms not recorded as a diagnosis on a medical claim may not have been captured. This particularly may have resulted in an underestimation of the percentage of patients with dysmenorrhea. This database did not capture whether women received a surgically confirmed endometriosis diagnosis. As treatment of women for clinically suspected endometriosis is common,21 it cannot be confirmed if these women had surgically recognized disease. Because the use of over-the-counter medications and other self-management techniques were not usually captured in claims data, this was not included in the analysis. The definition of contraceptives used was broad and included agents that were not therapeutic treatments for endometriosis. Therefore, the use of contraceptives for endometriosis-related medical treatment may have been overestimated. As comorbidity prevalence was based only on claims with comorbidity diagnostic codes which occurred before and not after endometriosis diagnosis, true comorbidity prevalence may not have been reflected in this analysis. Another potential limitation was that a disproportionate percentage of patients were from the southern region of the country and could represent a confounding variable. Nevertheless, this study had a number of strengths, including the utilization of a large sample of patients with geographically and socioeconomically diverse backgrounds from a United States commercial insurance database. The use of such a claims database avoided the potential of reporting bias.
Conclusion
In this study of women with endometriosis who initiated elagolix, we found that a significant proportion of these women used endometriosis therapies in the 12 months immediately prior to initiation. These findings indicate a substantial unmet medical need for this patient population. Further research is needed to evaluate the potential impact of elagolix initiation on these treatment patterns longitudinally.
Abbreviations
GnRH, gonadotropin-releasing hormone; HIPAA, Health Insurance Portability and Accountability Act; ICD, International Classification of Diseases.
Acknowledgments
Medical writing services were provided by Alan Saltzman, PhD, of JK Associates Inc. (a member of the Fishawack Group of Companies), Conshohocken, PA; this support was funded by AbbVie, Inc.
Disclosure
Eric S. Surrey has served in a consulting role for AbbVie and DOT Laboratories, received research grants from AbbVie, and served on the speakers’ bureau for AbbVie and Ferring Laboratories. Ahmed M. Soliman, Beverly Jones, and Jamie B. Vora are AbbVie employees and have stock/stock options. In addition, Dr Ahmed M. Soliman has patents, US Patent App. 16/105,440 pending and US Patent App. 16/105,396, pending. Hugh S. Taylor has had his research supported from a grant received by Yale University from AbbVie. He also reports that Yale University owns IP related to his work on testing for endometriosis. It is unrelated to the material in this manuscript. Sanjay K. Agarwal has served in a consulting role on research for AbbVie and has received research support from AbbVie. The authors report no other conflicts of interest in this work.
References
1. Johnson NP, Hummelshoj L, World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568. doi:10.1093/humrep/det050
2. Adamson GD, Kennedy S, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J Endometr. 2010;2(1):3–6. doi:10.1177/228402651000200102
3. Soliman AM, Coyne KS, Zaiser E, Castelli-Haley J, Fuldeore MJ. The burden of endometriosis symptoms on health-related quality of life in women in the United States: a cross-sectional study. J Psychosom Obstet Gynaecol. 2017;38(4):238–248. doi:10.1080/0167482X.2017.1289512
4. Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–639. doi:10.1093/humupd/dmt027
5. Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women’s lives: a qualitative study. BMC Womens Health. 2014;14:123. doi:10.1186/1472-6874-14-123
6. Soliman AM, Surrey E, Bonafede M, Nelson JK, Castelli-Haley J. Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Adv Ther. 2018;35(3):408–423. doi:10.1007/s12325-018-0667-3
7. National Guidance Alliance (UK). Endometriosis: diagnosis and management. London: National Institute for Health and Care Excellence (UK); 2017. Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096947/.
8. Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223–236.
9. As-Sanie S, Black R, Giudice LC, et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. 2019;221(2):86–94. doi:10.1016/j.ajog.2019.02.033
10. Barra F, Grandi G, Tantari M, et al. A comprehensive review of hormonal and biological therapies for endometriosis: latest developments. Expert Opin Biol Ther. 2019;19:343–360. doi:10.1080/14712598.2019.1581761
11. OrilissaTM [package insert]. North Chicago, IL: AbbVie Inc; 2019.
12. Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. doi:10.1056/NEJMoa1700089
13. IBM Watson Health. Data brochure. IBM MarketScan research databases for life sciences researchers. 2019. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO.
14. Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. 2017;108(1):125–136. doi:10.1016/j.fertnstert.2017.05.004
15. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685. doi:10.1093/humrep/det284
16. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292. doi:10.1097/AOG.0b013e3181758ec6
17. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses-a U.S. epidemic. 2012. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm.
18. Centers for Disease Control and Prevention. Opioid overdose. 2020. Available from: https://www.cdc.gov/drugoverdose/epidemic/index.html.
19. Lamvu G, Soliman AM, Manthena SR, Gordon K, Knight J, Taylor HS. Patterns of prescription opioid use in women with endometriosis: evaluating prolonged use, daily dose, and concomitant use with benzodiazepines. Obstet Gynecol. 2019;133(6):1120–1130. doi:10.1097/AOG.0000000000003267
20. As-Sanie S, Soliman AM, Evans K, Erpelding N, Lanier R, Katz NP. Healthcare utilization and cost burden among women with endometriosis by opioid prescription status in the first year after diagnosis: a retrospective claims database analysis. J Med Econ. 2020;23:371–377. doi:10.1080/13696998.2019.1707212
21. Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82(5):453–461. doi:10.1159/000452660
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
