Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Real-World Case Studies Showing the Effective Use of Azelaic Acid in the Treatment, and During the Maintenance Phase, of Adult Female Acne Patients
Authors Layton AM, Dias da Rocha MA
Received 29 November 2022
Accepted for publication 1 February 2023
Published 24 February 2023 Volume 2023:16 Pages 515—527
DOI https://doi.org/10.2147/CCID.S396023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Alison M Layton,1 Marco Alexandre Dias da Rocha2
1Skin Research Centre, Hull York Medical School, University of Hull, Hull, UK; 2Federal University of São Paulo, São Paulo, Brazil
Correspondence: Marco Alexandre Dias da Rocha, Federal University of São Paulo, Rua Oscar Pinheiro Coelho, 115 casa 04. Caxingui, São Paulo, Brazil, Email [email protected]
Abstract: Acne Vulgaris is a chronic inflammatory skin disease, and one of the most prevalent inflammatory dermatoses among teenagers, affecting more than > 95% of boys and 85% of girls. Adult female acne (AFA) is a subtype of acne, pragmatically defined as affecting women over the age of 25. The clinical presentation of AFA may be distinguished from adolescent acne according to some key clinical and psychosocial characteristics. The etiopathogenic factors and the chronic clinical course that are implicated in AFA make management complex and challenging. A frequent tendency to relapse makes the requirement for maintenance therapy highly likely. Therefore, AFA typically requires a specific, tailored therapeutic approach. This paper presents six challenging case studies that demonstrate the efficacy of azelaic acid gel (AZA) in adult female acne. The six cases use AZA as monotherapy, as part of a combination regimen at treatment initiation, or as maintenance treatment (which is frequently required in this adult population). The positive outcomes achieved in this case series demonstrate that AZA can be efficacious, result in excellent patient satisfaction in mild to moderate adult female acne, and can be effective as a maintenance therapy.
Keywords: acne vulgaris, adult female acne, azelaic acid, skin conditions, inflammation
Introduction
Acne vulgaris (acne) is a chronic immune-inflammatory skin disease and one of the most prevalent skin conditions among teenagers, affecting more than >95% of boys and 85% of girls.1–3 Adult female acne (AFA) is recognised as a specific subtype of acne, defined pragmatically as affecting women over the age 25 years.4 It generally requires a specific therapeutic approach, with maintenance therapy as an essential element.4,5
Acne is multifactorial; its course and severity is affected by genetics, age of onset, lesion distribution, stress, hormones and skin manipulation.2,6 Smoking may make it worse, while endocrine disorders, (eg Cushing syndrome, polycystic ovary syndrome, congenital adrenal hyperplasia) and a number of medications (eg corticosteroids and some progestins), may be involved.2 Clinical presentation includes open and/or closed comedones and inflammatory lesions including papules, pustules and nodules.7
Often dismissed as self-limiting, high rates of acne are found in young adult women in their 20s and 30s. This suggests that acne is no longer only a disease of adolescent.8,9 Acne in adult women may persist as a continuum from adolescence or, less frequently, present for the first time at a later age. Approximately 20% of cases begin in adulthood.10 Even in persistent cases, it is common for acne to evolve between adolescence and adulthood, especially in lesion distribution and in relation to the number of comedonal and inflammatory lesions.11
For adult women, acne can have a profound impact on their wellbeing and quality of life.12–14 Notable consequences include personal rejection, diminished social confidence, suicidal ideation, and higher rates of unemployment compared with those unaffected.15 Furthermore, AFA may be associated with other skin diseases affecting the same site, including rosacea, melasma and seborrheic dermatitis. Co-existing diseases compound the complexity of treatment and the anxiety and psychosocial impact experienced by some patients. Additionally, affected women frequently present with a severe skin-picking habit causing hyperpigmentation and scarring.16
AFA can be distinguished from adolescent acne according to some key characteristics (Table 1). The diseases differ in terms of duration, the location and morphological characteristics of lesions, and the overall impact on a patient’s quality of life.8,14,16–18 As AFA often displays many inflammatory lesions, management is complex and challenging and the tendency for relapse is high; therefore maintenance therapy is an essential consideration.4 A real-life observational study demonstrated acne relapse rates affecting 39.9% of male and female ≤20-year-olds but 53.3% of male and female >20-year-olds.19 Such relapses were significantly associated with impaired quality of life, productivity loss and absenteeism.19 Consequently, the authors noted there is a “need to appropriately manage adult female acne patients, regardless of the acne grade”.19
 |
Table 1 Characteristics of Adolescent and Adult Female Acne16,18,19,26 |
Adult female acne typically requires a specific therapeutic approach.5 Topical treatments are relevant for all degrees of disease but must be tailored to the patient’s clinical presentation and skin sensitivity. Proper skincare facilitates topical treatment and increases patient treatment adherence.20 Topical treatment can be combined with systemic therapy (including medication resulting in hormonal blockade), providing an important option for AFA patients. This strategy is effective both in patients with underlying endocrinological disease, and in those with no associated hormonal abnormalities.21
Hormonal treatment options include combined oral contraceptives (COCs). These may be considered when there are no contraindications: in adult females with acne who also require contraception or suffer menstrual irregularities and in patients when pregnancy is not planned.22,23 Spironolactone, a potassium-sparing diuretic that is used in the off-label treatment of AFA, has been shown to be effective.24
In this article we present a series of case studies that show the efficacy of azelaic acid (AZA) as monotherapy or in combination with other therapies. AZA is a well-known topical acne treatment option (20% cream or 15% gel) and has been recommended as potential first-line monotherapy for both non-inflammatory and inflammatory AFA.16 Studies suggest AZA has a triple action effect on acne, comprising a keratolytic effect, an anti-microbial action, and an anti-inflammatory effect – hence AZA can potentially target multiple pathophysiological mechanisms involved in adult female acne.25–27 While risks and benefits must be analysed on a case-by-case basis, AZA also offers a possible treatment option during pregnancy and lactation, adding to its potential utility in a female population of childbearing age.28
Methods
We present six case studies sourced from Prof. Dias da Rocha’s clinic in São Paulo, Brazil, involving adult female patients treated with either AZA monotherapy (AZA gel 15% twice daily) or a combination therapy including AZA (gel 15%).
Case Studies
Case 1: 25 Year-Old Adult Female with Mild Acne (Normoandrogenic) Treated with Monotherapy AZA Gel 15% Twice Daily
Initial Presentation
The patient sought care because she had persistent facial acne lesions from adolescence, which had only improved during a period when she took a COC. At the time of the consultation, she was 1 year post taking the oral contraceptive and trying to treat her skin with soaps and drying lotions and using topical salicylic acid for acne. The patient was very bothered by the presence of her acne. She had regular menses (29–31 days).
Clinical Examination
The presence of closed comedones and diffuse inflammatory papulopustular lesions on the face (diffuse) were observed and assessed as grade II acne based on Investigator’s Global Assessment (IGA). No truncal lesions were present.
Test Results
No hormonal abnormalities from laboratory tests were reported. Results from a transvaginal ultrasound were normal.
Working Diagnosis
Persistent adult female acne was diagnosed.
Care Plan
Taking into account the intensity and distribution of the patient’s acne, it was decided to introduce AZA 15% gel twice a day, sun protection in the morning and a gentle cleansing regime twice a day.
Length of Treatment/Number of Visits
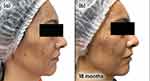
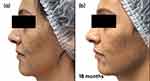
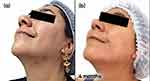
Follow-up visits occurred every 3 months, with guidance to maintain the initial treatment. Successive visits showed progressive improvement in inflammatory and comedonal lesions, with good adherence and absence of side effects (Figures 1a–c and 2a–c).
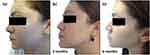 |
Figure 1 Case study 1 improvement on left-hand side of face, baseline to 6 months (a) Baseline (b) 3 months with AZA 15% gel twice daily (c) 6 months with AZA 15% gel twice daily. |
 |
Figure 2 Case study 1 improvement on right-hand side of face, baseline to 6 months (a) Baseline (b) 3 months with AZA 15% gel twice daily (c) 6 months with AZA 15% gel twice daily. |
Improvement
The improvement was considered very satisfactory by the physician and the patient (IGA 2 to IGA 0).
Why Treatment Ended
After the 6-month improvement, the patient did not return for follow-up.
Lessons Learned/Key Take-Away from Case
The case demonstrated effective use of AZA to control acne lesions in women with mild persistent acne and also confirmed the improvement takes time making it important to give realistic expectations and encourage longer term usage.
Case 2: 26 Year-Old Female Adult with Mild Acne (Normoandrogenic) Plus Skin Picking. The Patient Was Treated with AZA Gel Twice Daily for 6 Months
Initial Presentation
The patient reported long-standing facial acne lesions, which worsened around the menstrual period. She had tried topical and oral antibiotics which resulted in transient improvement. She did not want to take COCs due to interference with her libido. She had regular menses (28 days). She also stated that she could not stop manipulating her skin when new lesions appeared.
Clinical Examination
Presence of diffuse excoriated comedonal and inflammatory papulopustular lesions on the face associated with post-inflammatory hyperchromia (PIH). No truncal lesions were present and the acne was grade as IGA 2.
Test Results
No hormonal abnormalities from laboratory tests were reported. Results from a transvaginal ultrasound were normal.
Working Diagnosis
Persistent inflammatory adult female acne with skin picking both leading to hyperpigmentation (PIH).
Care Plan
Taking into account the intensity and distribution of the patient’s acne, it was decided to introduce AZA 15% gel twice a day, sun protection in the morning and a gentle cleansing regime twice a day.
Length of Treatment/Number of Visits
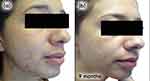
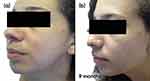
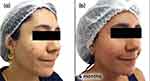
Follow-up visits occurred every 3 months with guidance to maintain the initial treatment. Progressive improvement in inflammatory and comedonal lesions with good adherence and absence of side effects (Figures 3a–c, 4a–c) was observed. We also noticed an important improvement in the hyperchromia.
 |
Figure 3 Case study 2 improvement on right-hand side of face, baseline to 6 months (a) Baseline (b) 3 months with AZA 15% gel twice daily (c) 6 months with AZA 15% gel twice daily. |
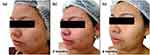 |
Figure 4 Case study 2 improvement on left-hand side of face, baseline to 6 months (a) Baseline (b) 3 months with AZA 15% gel twice daily (c) 6 months with AZA 15% gel twice daily. |
Improvement
The improvement was considered very satisfactory by the physician and the patient (IGA 2 to IGA 0).
Lessons Learned/Key Take-Away from Case
The case demonstrated the effective use of AZA to control acne lesions in women with mild persistent acne. A substantial reduction in pigmentation was also observed. Improvement takes time making it important to give realistic expectations and encourage longer term usage.
Case 3: 33 Year-Old Female Adult with Moderate Acne Plus Acne Induced Pigmentation (Normoandrogenic) Treated with Spironolactone* Plus AZA Gel Twice Daily (9 Months)
Initial Presentation
The patient reported a history of acne since early adolescence, which could not be sufficiently controlled using only topical prescribed medications. After a while, a COC was introduced, and the acne completely cleared. At age 25 years of age, she developed a deep vein thrombosis and needed to stop the hormonal contraceptive and changed to using a copper intrauterine device. The patient noted that during the use of hormonal contraceptives she noticed the emergence of melasma. She had regular menses (28 days). She was very worried about dark spots on her skin.
Clinical Examination
Presence of inflammatory papulopustular and nodular lesions on the face, more concentrated at the malar zone; lower third and neck associated with pigmentation. Acne was graded as IGA 3 and malar and frontal melasma was noted. No truncal lesions were evident.
Test Results
No hormonal abnormalities from laboratory tests were reported. Results from a transvaginal ultrasound were normal.
Working Diagnosis
Persistent adult female moderate acne/acne induced pigmentation and melasma.
Care Plan
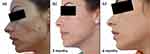
Considering the intensity and distribution of acne, it was decided to introduce spironolactone 50mg twice a day plus AZA 15% gel twice a day, sun protection in the morning and a gentle cleansing regime for 3 months. After initial improvement during an initial treatment phase of 9 months (Figure 5a and b, Figure 6a and b), the oral drug was reduced to 25mg twice a day for a further 9 months as part of a maintenance phase (Figure 7a and b, Figure 8a and b).
 |
Figure 5 Case study 3 improvement on both sides of face, baseline to 9 months. (a) Baseline (b) 9 months with spironolactone 50mg twice a day plus AZA 15% gel twice a day. |
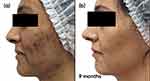 |
Figure 6 Case study 3 improvement on both sides of face, baseline to 9 months (a) Baseline (b) 9 months with spironolactone 50mg twice a day plus AZA 15% gel twice a day. |
Improvement
The improvement was considered very satisfactory by the physician and the patient (IGA 3 to IGA 0).
Lessons Learned/Key Take-Away from Case
This case demonstrated the effective use of AZA in combination with oral spironolactone to control moderate acne lesions in women with persistent acne. A substantial reduction in acne induced pigment observed and maintained Improvement takes time making it important to give realistic expectations and encourage longer term usage.
Case Study 4: 34 Year-Old Adult Female with Moderate Acne and Acne Induced Pigmentation (Normoandrogenic) Treated with COC, Spironolactone, and AZA Gel 15% Twice Daily for 9 Months
Initial Presentation
The patient had a history of acne since early adolescence, which could not be sufficiently controlled using topical prescribed medications alone. She took several courses of oral antibiotics (cyclines) and used isotretinoin at 28 years of age for 9 months (120mg/kg - cumulative dose). After a while, the acne returned and became intense again. She had regular menses (28 days). The patient was very worried about inflammatory lesions and depressed scars.
Clinical Examination
Inflammatory papulopustular and nodular lesions on the face, more concentrated on lower third and the neck (“U” type acne) associated with some comedonal lesions and atrophic scars were present. The acne was graded as IGA 3. Presence of inflammatory papulopustular lesions were evident on the trunk.
Test Results
No hormonal abnormalities from laboratory tests were reported. Results from a transvaginal ultrasound were normal.
Working Diagnosis
Persistent adult female moderate acne with acne induced pigment and scarring was diagnosed.
Care Plan
Considering the intensity and distribution of acne, it was decided to introduce COC (ethinylestradiol 0.02mg plus drospirenone 3mg) plus spironolactone 25mg twice a day, plus AZA gel 15% twice daily.
Length of Treatment/Number of Visits
Follow-up visits occurred every 3 months, showing progressive improvement in inflammatory and comedonal lesions with good adherence. The patient experienced facial tingling in the first weeks that later disappeared; after 9 months, good results were observed (Figure 9a and b, Figure 10a and b). The patient currently is maintained on the contraceptive and AZA without the emerge of new acne lesions.
Improvement
The improvement was considered very satisfactory by the physician and the patient (IGA 3 to IGA 0).
Lessons Learned/Key Take-Away from Case
The case demonstrated effective use of AZA combined with spironolactone and COC to control moderate acne lesions in women with persistent acne.
Case 5: 36 Year-Old Adult Female with Moderate Acne. The Patient Was Treated with Oral Contraceptive (Ethinylestradiol Plus Chlormadinone) Plus AZA 15% Twice a Day for 4 Months
Initial Presentation
The patient reported a history of persistent acne, which could not be sufficiently controlled using topical prescribed medications alone. She had irregular menses. She experienced generalized anxiety and was receiving treatment from a psychiatrist.
Clinical Examination
Inflammatory papulopustular and nodular lesions on the face, more concentrated on the lower third of the face and neck (“U” type acne) associated with atrophic scars and acne induced pigment were present. The acne was graded as IGA 2. Inflammatory papulopustular lesions were present on the trunk.
Test Results
Increased free testosterone and the presence of multiple ovarian cysts on ultrasound were reported.
Working Diagnosis
We diagnosed persistent adult female moderate acne, acne induced pigment in a patient with Polycystic Ovarian Syndrome (PCOS).
Care Plan
Considering the intensity and distribution of acne, it was decided to introduce an oral contraceptive (ethinylestradiol plus chlormadinone) plus AZA 15% gel twice a day, sun protection in the morning and a gentle cleansing regime for 4 months. After an initial improvement (Figure 11a and b, Figure 12a and b), the same treatment was continued as maintenance without the appearance of new lesions.
Improvement
The improvement was considered very satisfactory by the physician and the patient (IGA 2 ➔ IGA 0). We also noticed an improvement in the acne induced pigment.
Lessons Learned/Key Take-Away from Case
The case demonstrated effective use of AZA with COC to control moderate acne lesions in women with persistent acne.
Case 6: 29 Year-Old Adult Woman with Moderate Acne Resistant to Spironolactone Treatment (Normoandrogenic)
Initial Presentation
The patient had a history of late onset acne, which was not sufficiently controlled using topical prescribed medications alone. She took several courses of oral antibiotics (cyclines) with minimal improvement. The patient had a levonorgestrel intrauterine device in situ. She was very worried about inflammatory lesions. She had been diagnosed with depression.
Clinical Examination
Comedonal and inflammatory papulopustular and nodular lesions more concentrated in the malar zone and lower third of the face were noted. The acne was graded as IGA 3. No truncal lesions were observed.
Test Results
No hormonal abnormalities from laboratory tests were reported. Results from a transvaginal ultrasound were normal.
Working Diagnosis
Persistent adult female moderate acne.
Care Plan
Considering the intensity and distribution of acne, it was decided to introduce spironolactone 50mg twice a day plus tretinoin 0.05% gel at night. After 6 months, the patient showed little improvement (Figure 13a). The spironolactone was therefore changed to bicalutamide 50mg, 3 times a week for 3 months, alongside AZA 15% gel twice a day. After the 3 months, the bicalutamide was reduced it to 50mg twice a week with good results (Figure 13b and c). No abnormalities were observed from laboratory tests taken every month.
Improvement
After the introduction of bicalutamide combined with AZA improvement in inflammatory and comedonal lesions was noted and good adherence achieved, Good results were observed after 6 months (Figure 13b and c). The patient is currently maintained on this treatment regime. We also noted improvement in acne induced pigment. The improvement was considered very satisfactory by the physician and the patient (IGA 3 to IGA 0).
Lessons Learned/Key Take-Aways
This case demonstrated effective use of AZA combined with oral bicalutamide in a case resistant to oral spironolactone.
Discussion
When evaluating management options for adult female acne, clinicians will be sensitive to the need to take both clinical and non-medical factors into account. These considerations include: a patient’s medical history, in particular their response to previous treatments, familial factors and any predisposition to scarring and acne induced hyperpigmentation; the presence or absence of any underlying endocrine abnormalities; and the extent, severity, lesion type(s), degree of seborrhoea and duration of their condition. Other medical factors, such as the increased potential for irritation in ageing skin, the failure rate of standard acne therapy, the frequency of relapses that require long-term maintenance therapy – often for years – as well as a patient’s particular needs and preferences, their use of cosmetics, childbearing status and the overall cost of treatment, are all pertinent.8,12,16,29
Two factors contribute to skin sensitivity in adult acne patients, making it difficult to tolerate aggressive topical treatments. The first is the aging process, which gradually reduces the concentration of ceramides of the skin barrier, and the second is acne itself which also contributes to this change. With a smaller and altered lipid film, there will be greater loss of transepidermal water, worsening in the control of permeability, thus facilitating the triggering of irritative processes. We can therefore conclude that we should use mild skin care products, preferably cleansers that maintain the physiological pH of the epidermis, non-comedogenic moisturizers that aid in the barrier repair process, and topical medications with less potential to cause dermatitis, such as AZA.4,30–32
Considering the chronicity of acne in adult patients, doctors face a complex challenge in every case. In addition to the issues relating to skin sensitivity, there is often the presence of other dermatoses co-located to the acne, including rosacea, melasma and photoaging. As observed in Case Three, after a long treatment course for the acne and acne induced hyperpigmentation, which result in therapeutic success, using the combination of a hormonal blocker associated with AZA, the patient still suffered from melasma, which remained an important complaint and which negatively impacted their quality of life.33–35
Treatments that target as many pathophysiological aspects of acne as possible and, in particular, impact associated inflammation should be instituted as soon as possible to reduce inflamed lesions and their consequences, such as acne-induced hyperchromia and atrophic scars.35 AZA has been shown to have a multimodal mechanism of action. After dermal administration, AZA penetrates all layers of the skin and has a specific affinity for dysfunctional melanocytes involved in pigmentary changes.29,36 Furthermore, over-activity of TLR-2 has been shown to have a role in the pathogenesis of acne; therefore, the ability of AZA to target and inhibit TLR-2 activity, responsible for inflammation, further supports its efficacy in treating acne.25 In addition to its anti-acne actions, AZA has an important complementary role in treating acne-induced hyperchromia. Many of the cases we present demonstrated an improvement in acne induced hyperpigmentation with AZA treatment. The drug may reduce pigment through bleaching activity by inhibiting tyrosinase. This mechanism is selective, acting only on the hyperpigmentation without leading to perilesional hypochromia. This renders it safe in all phototypes and differentiates it from other topical bleaching creams such as hydroquinone.37
As acne is characterized by a chronic evolution with frequent relapses requiring long-term maintenance therapy,8 some clinicians go as far as to regard maintenance therapy mandatory after every successful acne treatment.38,39 This practice stems from the need to minimize the likelihood of relapse after treatment, and when recurrences of acne are frequent. When thinking about the duration of maintenance, therapy factors such as efficacy, tolerability and patient adherence should be considered.4,16 AZA could be considered for maintenance therapy following resolution of all forms of acne. Topical 20% AZA cream has been shown to prolong the recurrence-free interval.39 In this case series, maintenance therapy with AZA often continued following the initial improvement of acne and acne-related signs.
In relevant cases, topical therapy with AZA, can be co-prescribed with hormonal blockers, such as COCs and/or spironolactone. These drugs can reduce androgenic activation on sebaceous glands, be it by endocrine or intracrine mechanisms. This concept is valid for both the acute inflammatory active phase of acne and also the maintenance phase. Indeed, this is demonstrated by the successful combination therapy shown in a number of the cases presented here. Topical AZA can also be used alongside other systemic agents, including oral isotretinoin. As shown in some of our cases, this combined approach may allow systemic medications to be reduced or withdrawn as the acne resolves, with topical therapy then being used as the mainstay of maintenance treatment.21
Conclusion
Acne remains the most common inflammatory dermatosis seen worldwide. It is very burdensome both clinically and psychosocially. The prevalence in adult females appears to be increasing.
Experience in clinical practice as demonstrated by these case reports confirms that topical treatment with AZA has an important role to play in the acute management and maintenance of adult female acne and the resulting hyperchromia that may avoid the use of antibiotics while also treating the post hyperpigmentation through its anti-inflammatory mechanism of action. AZA offers an efficacious, well tolerated, non-antimicrobial agent for mild to moderate adult female acne, therefore aligning to all current guidance recommending non-antibiotic agents as a means of reducing antimicrobial resistance.
Acknowledgments
The patients in this manuscript have given written informed consent to publication of their case details, including images. No institutional approval was required.
Funding
The authors provided independent evidence and expert advice which was collated into the final manuscript by a medical writer funded by Leo Pharma.
Disclosure
Professor Alison M Layton reports grants from National Institute For Health Research, British Skin Foundation, and MRC; personal fees from Galderma Pharma, Galderma Pharma, LEO Pharma, La Roche-Posay, L’Oreal, Viatris, Novartis, Origimm Biotechnology, Beirsdorf, and Wiley, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Albuquerque RGR, Rocha MAD, Bagatin E, Tufik S, Andersen ML. Could adult female acne be associated with modern life? Arch Res. 2014;306:683–688.
2. Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500–506. doi:10.1136/pgmj.2006.045377
3. Tuchayi SM, Makrantonaki E, Gancevicience R, Dessinioti C, Feldman S, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi:10.1038/nrdp.2015.29
4. Bagatin E, Proença Freitas TH, Rivitti-Machado MC, Medeiros Ribeiro B, Nunes S, Dias da Rocha MA. Adult female acne: a guide to clinical practice. An Bras Dermatol. 2019;94:62–75. doi:10.1590/abd1806-4841.20198203
5. Vera N, Patel N, Cardwell LA, Saleem M, Feldman SR. Chemical pharmacotherapy options for managing adult acne. Exp Opin Pharmacother. 2017;18:263. doi:10.1080/14656566.2017.1282460
6. Baldwin H, Tan J. Effects of diet on acne and its response to treatment. Am J Clin Dermatol. 2021;22:55–65. doi:10.1007/s40257-020-00542-y
7. Nast A, Dréno B, Bettoli V. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–1268. doi:10.1111/jdv.13776
8. Dréno B. Treatment of adult female acne: a new challenge. J Eur Acad Dermatol Venereol. 2015;29(Suppl. 5):14–19. doi:10.1111/jdv.13188
9. Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. doi:10.2147/AHMT.S55832
10. Vargas-Diez E, Hofmann M, Bravo B, Malgazhdarova G, Katkhanova OA, Yutskovskaya Y. Azelaic acid in the treatment of acne in adult females: case reports. Skin Pharmacol Physiol. 2014;27(Suppl. 1):18–25. doi:10.1159/000354889
11. Dumont-Wallon G, Dreno B. Specificity of acne in women older than 25 years. Presse Med. 2008;37(Pt. 1):585–591. doi:10.1016/j.lpm.2007.07.014
12. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(Suppl. 1):3–8. doi:10.1159/000354887
13. Karimkhani C, Dellavalle R, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153:406–412. doi:10.1001/jamadermatol.2016.5538
14. Gallitano SM, Berson DS. How acne bumps cause the blues: the influence of acne vulgaris on self-esteem. Int J Womens Dermatol. 2018;4:12–17. doi:10.1016/j.ijwd.2017.10.004
15. Dias da Rocha MA, Guadanhim LRS, Sanudo A, Bagatin E. Modulation of Toll Like Receptor-2 on sebaceous gland by the treatment of adult female acne. Dermatoendocrinol. 2017;9:e1361570. doi:10.1080/19381980.2017.1361570
16. Dréno B, Layton A, Zouboulis C, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063–1070. doi:10.1111/jdv.12061
17. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474–485. doi:10.2147/CCID.S137794
18. Dreno B, Bagatin E, Blume-Peytavi U, Rocha M, Gollnick H. Female type of adult acne: physiological and psychological considerations and management. J Dtsch Dermatol Ges. 2018;16:1185–1194.
19. Dreno B, Bordet C, Seite S, Taieb C. Acne relapses: impact on quality of life and productivity. J Eur Acad Dermatol Venereol. 2019;33:937–943. doi:10.1111/jdv.15419
20. Nast A, Dreno B, Bettoli V, Degitz K, Erdmann R, Finlay AY. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26:1–29. doi:10.1111/j.1468-3083.2011.04374.x
21. Bagatin E, Dias da Rocha MA, Proença Freitas TH, Sousa Costa C. Treatment challenges in adult female acne and future directions. Expert Rev Clin Pharmacol. 2021;14:687–701. doi:10.1080/17512433.2021.1917376
22. Katsambas A, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? Facts and controversies. Clin Dermatol. 2010;28(1):17–23. doi:10.1016/j.clindermatol.2009.03.006
23. Lakshmi C. Hormonal Therapy in acne. Indian J Dermatol Venereol Leprol. 2013;79:322–337. doi:10.4103/0378-6323.110765
24. Rocha MA, Bagatin E. Adult-onset acne: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2018;11:59–69. doi:10.2147/CCID.S137794
25. Zeichner J. New insights into azelaic acid. Practical dermatology; 2013:45–46. Available from: practicaldermatology.com/pdfs/pd0313_ClinicalFocus.pdf.
26. CCDS. Skinoren® 20% Cream v1.0. LEO Pharma; 2022.
27. Sieber MA, Hegel JK. Azelaic acid: properties and mode of action. Skin Pharmacol Physiol. 2014;27(Suppl. 1):9–17. doi:10.1159/000354888
28. Fluhr JW, Degitz K. Antibiotics, azelaic acid and benzoyl peroxide in topical acne therapy. J Dtsch Dermatol Ges. 2010;8:24–30. doi:10.1111/j.1610-0387.2009.07169.x
29. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281–290. doi:10.2165/00128071-200607050-00002
30. Pappas A, Kendall AC, Brownbridge LC, Batchvarova N, Nicolaou A. Seasonal changes in epidermal ceramides are linked to impaired barrier function in acne patients. Exp Dermatol. 2018;27:833–836. doi:10.1111/exd.13499
31. Dias da Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018;310:181–185. doi:10.1007/s00403-017-1795-3
32. Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288(12):765–770. doi:10.1007/BF02505294
33. Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282–289. doi:10.1111/bjd.16481
34. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305–318. doi:10.1007/s13555-017-0194-1
35. Tan J, Thiboutot D, Gollnick H, et al. Development of an atrophic acne scar risk assessment tool. J Eur Acad Dermatol Venereol. 2017;31:1547–1554. doi:10.1111/jdv.14325
36. Breathnach AS, Robins E, Nazzaro-Porro M, Passi S, Picardo M. Hyperpigmentary disorders--mechanisms of action. Effect of azelaic acid on melanoma and other tumoral cells in culture. Acta Derm Venereol. 1989;143:62–66.
37. Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586–590.
38. Katsambas AD, Stefanaki C, Cunliffe WJ. Guidelines for treating acne. Clin Dermatol. 2004;22:439–444. doi:10.1016/j.clindermatol.2004.03.002
39. Gollnick HP, Graupe K, Zaumseil RP. Comparison of combined azelaic acid cream plus oral minocycline with oral isotretinoin in severe acne. Eur J Dermatol. 2001;11:538–644.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.