Back to Journals » Clinical Ophthalmology » Volume 18
Real-World Analysis of the Efficacy of Bimatoprost Sustained-Release Glaucoma Implant Where American Indians Comprise the Largest Minority Population
Authors Sarkisian Jr SR, Mitchell EC
Received 2 December 2023
Accepted for publication 15 March 2024
Published 23 March 2024 Volume 2024:18 Pages 917—927
DOI https://doi.org/10.2147/OPTH.S452159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Steven R Sarkisian Jr,1 Evann C Mitchell2
1Research & Clinical Trials, Oklahoma Eye Surgeons, Oklahoma City, OK, USA; 2College of Medicine, University of Oklahoma, Oklahoma City, OK, USA
Correspondence: Steven R Sarkisian Jr, Research & Clinical Trials, Oklahoma Eye Surgeons, 5600 N Portland Ave, Oklahoma City, OK, 73112, USA, Tel +1405 943-4413, Fax +1405 942-0115, Email [email protected]
Purpose: To assess the effectiveness and safety of bimatoprost sustained release (SR) glaucoma implant as a treatment for open-angle glaucoma and ocular hypertension in a real-world private practice setting with a significant American Indian population.
Methods: This retrospective study included 156 eyes from adult patients who received a single injection of bimatoprost implant between June 2020 and May 2022 at the Oklahoma Eye Surgeons. Patients were stratified by baseline intraocular pressure (IOP) (≥ 21 mmHg versus IOP< 21 mmHg). The co-primary endpoints were changes in the mean IOP and the number of topical IOP-lowering medications from baseline to Month 6.
Results: At 6 months, eyes with baseline IOP≥ 21 mmHg had a significantly lower mean IOP (19.85± 8.01 versus 26.25± 4.84 mmHg; p< 0.0001) and the mean number of IOP-lowering medications (1.04± 1.44 versus 1.38± 1.50; p=0.048) compared with baseline. One year after implantation, 73.58% of eyes had a ≥ 20% reduction in IOP, 41.51% were medication-free and 30.19% were receiving at least one fewer medication. Among eyes with baseline IOP< 21 mmHg, there was a significant reduction in the mean number of IOP-lowering medicines by Month 6 (0.61± 1.03 versus 1.93± 1.21 at baseline; p< 0.0001), with no change in IOP. At 12 months, 24.27% of eyes had a ≥ 20% decrease in IOP, 43.69% of eyes did not require any medications and 63.11% had at least one fewer medication compared with baseline. An analysis using Welch’s two-sample t–test showed no significant differences in the outcomes between the overall population and the American Indian population (number of eyes, 23).
Conclusion: Bimatoprost SR glaucoma implant lowered IOP in eyes with high, uncontrolled baseline IOP, while it reduced the number of medications in eyes with a controlled baseline IOP. No clinically meaningful and statistically significant differences in the efficacy of bimatoprost were observed in patients of American Indian descent.
Keywords: intraocular pressure, open-angle glaucoma, ocular hypertension, prostaglandin analog, intracameral implant, ethnicity/race
Introduction
Glaucoma is a chronic optic neuropathy characterized by progressive degeneration of the optic nerve and retinal nerve fiber layer. It is the leading cause of irreversible vision loss worldwide and is associated with poor quality of life. Based on the anatomy of the anterior chamber angle of the eye, glaucoma can be classified as primary open-angle glaucoma (POAG) or primary angle-closure glaucoma (PACG). POAG is the most common form of glaucoma with a global prevalence of around 3%, while PACG is less prevalent and disproportionately affects individuals of Asian descent.1,2
There are many known risk factors for glaucoma, including older age, elevated intraocular pressure (IOP), ethnicity, and family history of glaucoma. IOP (normal range, 10–21 mmHg) is currently the only modifiable risk factor shown to slow or halt visual field loss, and medical reduction of IOP is the only proven treatment to preserve vision in patients with glaucoma.3–7 Current glaucoma treatments for lowering IOP primarily include pharmacotherapy (topical and oral agents), laser therapy, and incisional surgery.8 Topical medications such as prostaglandin analogs and beta-blockers are often the frontline treatment, especially in early disease. Standard practice is to prescribe an additional hypotensive medication when the first drug has not lowered a patient’s IOP to an optimal level. However, the efficacy and safety of single-agent therapy may differ when given together with another therapy and thus the combination regimen may not necessarily exert an additive effect, with a potentially less favorable safety profile.9,10 As glaucoma is usually a bilateral but asymmetric disease, its management can be highly individualized.
The effectiveness of pharmacotherapy is often limited due to poor compliance. Studies indicated that fewer than 50% of patients adhere to their prescribed antiglaucoma medications at one year, with adherence rates further declining beyond 12 months.11–13 Sustained-release (SR) drug delivery devices have emerged as an approach to minimize compliance challenges in glaucoma patients. Bimatoprost implant (Durysta, Allergan plc, Dublin, Ireland) is an intracameral, biodegradable implant that was approved by the United States Food and Drug Administration (FDA) to lower IOP in patients with open-angle glaucoma or ocular hypertension in March 2020. This was based on the results from randomized Phase III ARTEMIS 1 and ARTEMIS 2 trials demonstrating a 30%-reduction in the IOP from baseline through the 12-week primary efficacy period with bimatoprost implant. Compared with timolol, the single-administration bimatoprost implant met the primary endpoint of non-inferiority after 12 weeks and showed a favorable safety profile.14,15
Ethnicity/race is another established risk factor for glaucoma, with individuals of African and Latino/Hispanic origin being four- to six-fold more likely to develop POAG than individuals from other groups.1,16–19 These ethnic variations in the prevalence of glaucoma can be attributed to significantly thinner corneas, larger optic nerves, and more rapid disease progression in individuals of African descent compared with a matched group of Americans of European descent, as shown in the prospective, observational African Descent and Glaucoma Evaluation Study Groups (ADAGES) study.20 In the Hispanic population, findings from the Los Angeles Latino Eye Study (LALES) suggested that the optic nerves of Hispanics are more susceptible to glaucoma damage due to the high prevalence of diseases affecting blood vessels, such as diabetes, compared with individuals of European ancestry.16
Studies have also shown a high prevalence of glaucoma in North American Native individuals (~6%), with normotension glaucoma, a common form of POAG, being frequent in American Indian21 and Alaskan Native populations.22,23 The American Indian population has not been characterized in the pivotal studies of bimatoprost implant and data on these patients can help inform treatment decisions. The present study aimed to assess the effectiveness and safety of bimatoprost intracameral implant in a real-world private practice with a significant American Indian subgroup of glaucoma patients.
Materials and Methods
Study Design
This was a retrospective study of patients with glaucoma who received a bimatoprost implant (Durysta, Allergan plc, Dublin, Ireland) between June 2020 and May 2022 at the Oklahoma Eye Surgeons, Oklahoma City, OK, United States. The Institutional Review Board (IRB) Committee ruled that approval was not required for this study. All patients were informed of the procedure and consented prior to implementation per standard clinic procedure. No patient safety was compromised throughout the study. This study complies with the Declaration of Helsinki.
Patients
Eligible patients were 18 years of age or older with open-angle glaucoma who had received a bimatoprost implant and had a follow-up of at least 12 months. Patients were also required to have available IOP measurements and topical IOP-lowering medication count at the pre-implant (baseline) appointment. Exclusion criteria were current participation or participation in an investigational drug or device clinical trial within 30 days of implant procedure. If both eyes of a patient meet the study inclusion criteria, data from both eyes were collected, and the eyes were paired in the electronic data capture (EDC) system using each eye’s unique identifier. Eyes were identified as “study eye” (the first eye to receive a bimatoprost implant) and “fellow eye”. Eyes were subgrouped by baseline IOP≥21 mmHg versus IOP<21 mmHg.
Data Collection
Retrospective data were collected from medical records at baseline, defined as the last IOP measurement before receiving a bimatoprost implant, and post-implantation time points at one month (± 30 days), six months (± 30 days), and 12 months (± 30 days). Baseline data included demographics, medical and ophthalmic history, and study eligibility. Ethnicity and race for each subject were self-reported to site staff. The following parameters were recorded at the baseline and each follow-up visit: number of IOP lowering medications, IOP and visual acuity (measured by Snellen eye chart). In addition, secondary glaucoma intervention was recorded after baseline, while adverse events were assessed after each visit. Adverse events were assessed based off of the clinical exam and Principal Investigator’s medical judgement. Visual field testing and OCT optic nerve head imaging were used to additionally judge cases of disease progression. Patients with missing data were excluded from the study.
Study Hypothesis and Endpoints
The study aimed to compare outcomes with a bimatoprost implant in the American Indian subgroup with that of the broader patient population in this practice. The hypothesis was that there is no difference in the IOP response in the subgroup of patients of American Indian descent compared with a matched general population.
The co-primary endpoints were changes in mean IOP from baseline to Month 6 and the number of topical IOP-lowering medications from baseline to Month 6. Secondary endpoints included changes in mean IOP and number of topical IOP-lowering medications from baseline at Month 12, the proportion of eyes achieving ≥20% mean IOP reduction from baseline at Month 12 and the proportion of patients on the same or lower number of topical IOP-lowering medications at all time points following baseline.
Statistical Methods
Baseline demographic and clinical characteristics were analyzed using descriptive statistics. For primary endpoints, results were tabulated with a 95% confidence interval (CI) calculated using the normal approximation method. A paired t–test was used for comparison of pre-implant and post-implant treatment values. Welch’s two-sample t-test was used for the comparison of outcomes from the overall and American Indian populations. The rate of complete success (defined by the proportion of patients being off IOP-lowering medications at the primary endpoint of 6 months) and qualified success (defined by the IOP being controlled according to the investigator) were calculated with normal approximation.
Results
Baseline Data
This study included 156 eyes that met eligibility criteria and underwent bimatoprost implantation. The baseline demographic and clinical characteristics of the total population and the American Indian population are presented in Tables 1 and 2, respectively. In total, 53 eyes had a baseline IOP of 21 mmHg or higher, and 103 eyes had an IOP lower than 21 mmHg. On average, the age of the study eye was 74.99 years, and most eyes were from White patients (66.67%). In the American Indian cohort, 23 eyes (14.74%) were included (IOP≥21: n=7; IOP<21: n=16), with a mean eye age of 74.74 years. For baseline characteristics, 93% of patients had a grade IV angle. Seventeen percent of patients were phakic, and 83% were pseudophakic.
 |
Table 1 Demographics and Baseline Characteristics of Eyes in the Total Population |
 |
Table 2 Demographics and Baseline Characteristics of Eyes in the Subgroup of American Indian Patients |
There was no significant difference in baseline clinical characteristics between the overall and American Indian populations. The mean IOP before the operation was 19.32 mmHg overall and 17.09 mmHg among patients of American Indian descent (p=0.097), with a mean number of IOP-lowering medications of 1.74 and 2.00, respectively (p=0.438). Within the IOP≥21 subgroup, the mean IOP before implantation was 26.25 mmHg in the overall population and 23.43 mmHg in the American Indian population (p=0.052) and the mean number of IOP-lowering medications was 1.38 and 1.71, respectively (p=0.649). In the two study populations, comparable baseline IOP (mean, 15.76 mmHg versus 14.31 mmHg; p=0.212) and number of medications (mean, 1.93 versus 2.12; p=0.599) were reported among those with IOP<21 mmHg.
Outcomes in the Overall Population
Overall mean IOP and number of medications at baseline and follow-up visits at 1, 6 and 12 months for the two subgroups are presented in Figures 1 and 2. Reductions in mean IOP from baseline were statistically significant at all follow-up time points in eyes with a mean baseline IOP of 21 mmHg or greater. In this subset, eyes had a significantly lower mean IOP by 6.40 mmHg (p<0.0001) and the mean number of IOP-lowering medications (−0.34; p=0.048) at Month 6 post-implant. Among eyes with baseline IOP below 21 mmHg, the mean number of IOP-lowering medicines at 6 months decreased significantly by 1.32 from baseline (p<0.0001), with a change in IOP of +0.93 mmHg. At 6 months, the complete success rate was 61.15% with a median of 0.0 IOP lowering medications and a mean of 0.8 IOP lowering medications. The qualified success rate was 85.98%.
Data further demonstrated that bimatoprost implant was associated with improvements in secondary endpoints. Compared with baseline, there was a significant IOP reduction of 8.23 mmHg at Month 12 versus baseline (p<0.05) within the IOP≥21 subgroup, with a mean change in the number of IOP-lowering medications of −0.04 (p=0.858). For eyes in the IOP<21 subgroup, the mean number of medications was significantly lower at one year compared with baseline (−0.93; p<0.0001). We observed no significant difference between the mean baseline and 12-month IOP in this subset. Figures 1 and 2 show data for these two subgroups of eyes.
The percentage of eyes with a decrease in IOP for at least 20% one year after a bimatoprost implant was 73.58% in the IOP≥21 subgroup and 24.27% in the IOP<21 subgroup (Table 3). Among eyes with a baseline IOP of 21 mmHg or greater, 41.51% did not require any medications and 30.19% received at least one fewer medication at Month 12 than before a bimatoprost implant (Table 3). In the IOP<21 subgroup, nearly half of the eyes (43.69%) were medication-free at 12 months and 63.11% had a reduction of at least one in their medication count compared with baseline. In both IOP subgroups, most patients were on the same or fewer hypotensive medications at all follow-ups (Table 3). At 6 and 12 months, respectively, an increase in medications was reported only in 3.2% and 30.2% of patients with baseline IOP≥21 mmHg, and 4.6% and 11.7% of patients with baseline IOP<21 mmHg.
 |
Table 3 IOP and IOP-Lowering Medication Outcomes at Baseline and Each Post-Implant Time Point in the Total Population and Subgroups Differentiated by Baseline IOP of 21 mmHg |
Outcomes in the American Indian Population
Results of a subgroup analysis in the American Indian population showed comparable results to those observed in the broad study population. In the IOP≥21 subgroup, the 6-month IOP was significantly lower by 7.00 mmHg compared with the baseline IOP (p<0.001). The mean number of IOP-lowering medications remained the same at 1.71. The benefit of bimatoprost implant with respect to IOP-lowering medication count was observed in the eyes with baseline IOP below 21 mmHg, with a significant reduction in the mean number of medicines by 1.24 at 6 months (p<0.001). There were no IOP improvements in this subset of eyes between baseline and Month 6. These results are presented in Figures 3 and 4.
Regarding secondary outcomes, the IOP significantly decreased from baseline through Month 12 (−5.86 mmHg; p<0.05) in the IOP≥21 subgroup (Figure 3), with a mean change in the number of IOP-lowering medications of −0.42 (p=0.407). In the IOP<21 subgroup, the mean number of topical IOP-lowering medications was significantly lower at 12-month follow-up compared with baseline (−0.87; p<0.05).
The proportion of eyes that achieved 20% or greater reduction in IOP at 12 months after bimatoprost implant was 71.43% in the IOP≥21 subgroup, with 42.86% of eyes being medication-free and 57.14% receiving at least one medication fewer (Table 4). Among eyes with IOP less than 21 mmHg at baseline, 25.00% had at least a 20% reduction in IOP one year after operation. One-quarter of the eyes were medication-free, while 68.75% of eyes had at least one fewer hypotensive medication. At all time points following baseline, most patients maintained or reduced their medication burden; only 28.6% and 14.3% in the IOP≥21 group (n=7) and 6.25% and 12.5% of patients with baseline IOP<21 mmHg (n=16) increased the number of IOP-lowering medications at 6 and 12 months, respectively.
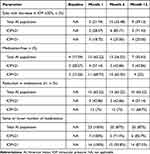 |
Table 4 IOP and IOP-Lowering Medication Outcomes at Baseline and Each Post-Implant Time Point in the American Indian Population and Subgroups Differentiated by Baseline IOP of 21 mmHg |
Comparison Between the Overall and American Indian Populations
We used Welch’s two-sample t–test to compare the clinical outcomes with bimatoprost implant between the overall population and the subset of patients of American Indian origin. The results showed no statistically significant differences in the efficacy of bimatoprost implant treatment between the two patient groups. These included changes in the mean IOP from baseline to Month 6 (p=0.643) and Month 12 (p=0.685), as well as the number of medications from baseline to Month 6 (p=0.300) and Month 12 (p=0.627) in the specific total populations.
Comparable were also results in the two patient populations when stratified by the baseline IOP. No significant differences were reported between the overall and American Indian populations for changes in the mean IOP from baseline to Month 6 (IOP≥21 group: p=0.057; IOP<21 group: p=0.567) and Month 12 (IOP≥21 group: p=0.856; IOP<21 group: p=0.774). Data also showed no statistically significant difference between the two populations in the number of medications from baseline to Month 6 (IOP≥21 group: p=0.371; IOP<21 group: p=0.518) and Month 12 (IOP≥21 group: p=0.931; IOP<21 group: p=0.478).
Safety
We observed no safety concerns with bimatoprost implant. Throughout the study, no patient required removal of the implant and there were no cases of low-grade iritis, corneal edema, and inflammation related to the treatment.
Due to the inability to insert a second bimatoprost implant and progressing glaucoma, 22 patients developed an IOP above target and required further surgical intervention. This included OMNI procedure in seven patients; cyclophotocoagulation (CPC), Ahmed glaucoma valve implantation, and XEN Gel Stent insertion in four patients each; Ex-Press shunt insertion, goniotomy and iStent inject insertion in one patient each.
Discussion
This study showed that bimatoprost SR intracameral implant effectively lowered IOP in eyes with high, uncontrolled baseline IOP (≥21 mmHg). We observed a significant decrease in IOP as early as one month after the operation, with IOP remaining reduced through all subsequent follow-ups. The mean IOP declined from 26.25 ± 4.84 mmHg to 19.85 ± 8.01 mmHg at 6 months and further to 18.02 ± 6.74 mmHg at 12 months. Notably, almost three-quarters of the eyes achieved a reduction in IOP by at least 20% and over 40% were medication-free by the end of one year. In eyes with a controlled baseline IOP (<21 mmHg), bimatoprost implant significantly reduced the number of hypotensive medications while maintaining near-baseline IOP levels throughout the study. More specifically, the mean number of IOP-lowering medications dropped from 1.93 ± 1.21 at baseline to 0.61 ± 1.03 at 6 months and 1.00 ± 1.24 by 12 months. Through assessments at 6 and 12 months, approximately 73% and 63% of eyes, respectively, had a reduced number of medications by at least one compared with baseline. The percentage of eyes requiring no medications was also considerably higher than before implantation. Notably, most patients from the two study subgroups were on the same or fewer IOP-lowering medications at all time points.
In our study, we observed sustained IOP-lowering effects through 12 months following the administration of a single bimatoprost implant, which is consistent with recently reported results from a study of 46 eyes with open-angle glaucoma or ocular hypertension.24 In this retrospective analysis, 67% of the eyes did not require reinstating IOP-lowering eyedrops or undergoing a surgical procedure during a mean period of 260 days after a single bimatoprost implant. Together, these data suggest that the efficacy of the bimatoprost intracameral implant on IOP persists well beyond the anticipated complete bimatoprost release, which typically occurs within 3–4 months of administration, as demonstrated in preclinical and early clinical studies.14 The mechanism underlying the prolonged IOP reduction has been recently proposed and involves enhanced activation of metalloproteinases in the presence of bimatoprost at high concentrations, resulting in increased turnover of the extracellular matrix and durable remodeling of the aqueous outflow pathways.25 New results from the ARTEMIS studies further support these findings, indicating that the IOP-lowering effect of the bimatoprost implant was maintained after complete biodegradation of the implant.26 By 12 months, 82% of implants were absent or reduced to 25% or less of initial size, and 95% of implants were completely biodegraded or less than 25% of initial size by 20 months.
Further analysis of our study demonstrated that the efficacy of bimatoprost remained consistent in a subset of patients of American Indian descent, with no statistically significant differences in the primary endpoints of the study. This is a remarkable observation given that this population was not represented in the pivotal trials of bimatoprost implant.14,15 To our knowledge, this is also the first analysis of this population in a real-world setting.
Adherence to topical IOP-lowering medication is key for effective glaucoma management. Studies have shown an association between noncompliance and more severe glaucomatous visual field loss.27,28 Several factors can hamper adherence, including forgetfulness, difficulties in application due to reduced dexterity in elderly patients, medication cost, and the number of medications.13 Introducing more convenient treatment alternatives such as bimatoprost SR glaucoma implant may overcome compliance barriers.
Our study presented several limitations including retrospective, nonrandomized design and a relatively low number of eyes included in the American Indian subgroup. Moreover, the number of patients that had IOP>21 in the subgroup was n=7, which may be inadequate to demonstrate statistical significance. However, no statistical significance was found between the general population and American Indian groups among those with IOP<21. In addition, there was an issue with follow-up data collection as some patients fell outside the designated follow-up window and data retrieval from referring physicians was problematic. Despite these limitations, this study provides real-world insights into the effectiveness of bimatoprost intracameral implant in treating glaucoma patients, including a subgroup of those of American Indian origin.
Conclusion
This study showed that a bimatoprost implant is a useful minimally invasive option for providing IOP control in patients with high, uncontrolled baseline IOP. The implant may also reduce the burden on patients with controlled baseline IOP by lowering the number of medications.
Abbreviations
ADAGES, African Descent and Glaucoma Evaluation Study Groups; AI, American Indian; CI, confidence interval; EDC, electronic data capture; IOP, intraocular pressure; LALES, Los Angeles Latino Eye Study; PACG, primary angle-closure glaucoma; POAG, primary open-angle glaucoma; SD, standard deviation; SR, sustained release.
Acknowledgments
Data entry support was provided by Alyssa Beadle, BS, COA, OSC and Hannah Reynolds, BS, OSC. Medical writing support was provided by Scientific Writers Ltd., UK for manuscript preparation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
AbbVie Inc. This study was supported by Investigator Initiated grant from AbbVie.
Disclosure
Steven R. Sarkisian Jr., MD is a consultant on the speakers’ bureau for Allergan/AbbVie and reports grants, personal fees from Allergan, during the conduct of the study; grants, personal fees from Alcon, personal fees from Aerie, grants from AbbVie, grants from Allysta, personal fees from Bausch & Lomb, personal fees from BVI, personal fees from Carl Zeiss, grants from Elios, grants, personal fees from Glaukos, grants, personal fees from IStar, personal fees from Katena, personal fees from MST, personal fees from Novartis, grants from Ocular Science, grants, personal fees from Ocular Therapeutix, personal fees from Santen, grants, personal fees from Sight Sciences, personal fees from TearLab Corp, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Tham YC, Li X, Wong TY., et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686. doi:10.7759/cureus.11686
3. Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. doi:10.1016/S0140-6736(14)62111-5
4. Boland MV, Ervin A-M, Friedman DS, et al. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the u.s. Preventive services task force. Ann Intern Med. 2013;158(4):271–279. doi:10.7326/0003-4819-158-4-201302190-00008
5. Kass MA. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi:10.1001/archopht.120.6.701
6. Group CN. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. doi:10.1016/S0002-9394(98)00223-2
7. Heijl A. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
8. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology. 2021;128(1):1.
9. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi:10.1097/ICO.0b013e3181c325b2
10. Patterson E. Open-angle glaucoma 365.10. In: Roy and Fraunfelder’s Current Ocular Therapy. Elsevier; 2008:504–508.
11. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(6):S57–S68. doi:10.1016/j.survophthal.2008.08.002
12. Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):
13. Olthoff C, Schouten J, Vandeborne B, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961.e7. doi:10.1016/j.ophtha.2004.12.035
14. Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–1641. doi:10.1016/j.ophtha.2020.06.018
15. Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017–2033. doi:10.1007/s40265-021-01624-9
16. Varma R. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1132–1140. doi:10.1016/j.ophtha.2004.02.002
17. Sommer A. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore eye survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi:10.1001/archopht.1991.01080080050026
18. Tielsch JM. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore eye survey. JAMA. 1991;266(3):369–374. doi:10.1001/jama.1991.03470030069026
19. Kapetanakis VV, Chan MPY, Foster PJ, et al. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100(1):86–93. doi:10.1136/bjophthalmol-2015-307223
20. Girkin CA. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128(5):541–550. doi:10.1001/archophthalmol.2010.49
21. Flanagin A, Frey T, Christiansen SL. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621–627. doi:10.1001/jama.2021.13304
22. Mansberger SL, Romero FC, Smith NH, et al. Causes of visual impairment and common eye problems in northwest American Indians and Alaska natives. Am J Public Health. 2005;95(5):881–886. doi:10.2105/AJPH.2004.054221
23. Woodward MA, Hughes K, Ballouz D, et al. Assessing eye health and eye care needs among north American native individuals. JAMA Ophthalmol. 2022;140(2):134–142. doi:10.1001/jamaophthalmol.2021.5507
24. Xu W, Zhou P, Kansara ND, Frankfort BJ, Blieden LS, Chang PT. Intraocular pressure and eyedrop usage reduction with intracameral bimatoprost implant. J Ocul Pharmacol Ther. 2023;39(6):398–403. doi:10.1089/jop.2023.0013
25. Stamer WD, Perkumas KM, Kang MH, Dibas M, Robinson MR, Rhee DJ. Proposed mechanism of long-term intraocular pressure lowering with the bimatoprost implant. Invest Opthalmol Vis Sci. 2023;64(3):15. doi:10.1167/iovs.64.3.15
26. Weinreb RN, Bacharach J, Brubaker JW, et al. Bimatoprost implant biodegradation in the phase 3, randomized, 20-month ARTEMIS studies. J Ocul Pharmacol Ther. 2023;39(1):55–62. doi:10.1089/jop.2022.0137
27. Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. doi:10.1016/j.ophtha.2011.05.013
28. Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2020;127(4):477–483. doi:10.1016/j.ophtha.2019.10.022
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




