Back to Journals » International Journal of Nanomedicine » Volume 18
Reactive Oxygen Species Scavenging Nanozymes: Emerging Therapeutics for Acute Liver Injury Alleviation
Authors Sun T, Xiao S, Wang M, Xie Q, Zhang L , Gong M , Zhang D, Zhou C
Received 13 September 2023
Accepted for publication 5 December 2023
Published 22 December 2023 Volume 2023:18 Pages 7901—7922
DOI https://doi.org/10.2147/IJN.S435544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Tao Sun,1,2 Shilin Xiao,1 Miaomiao Wang,1 Qian Xie,1 Liang Zhang,1 Mingfu Gong,1 Dong Zhang,1 Chunyu Zhou1
1Department of Radiology, Xinqiao Hospital, Army Medical University, Chongqing, People’s Republic of China; 2Faculty of Materials Science and Chemistry, China University of Geosciences, Wuhan, People’s Republic of China
Correspondence: Dong Zhang; Chunyu Zhou, Department of Radiology, Xinqiao Hospital, Army Medical University, No. 183, Xinqiao Street, Shapingba District, Chongqing, People’s Republic of China, Tel +86 23 68763842, Email [email protected]; [email protected]
Abstract: Acute liver injury (AIL), a fatal clinical disease featured with a swift deterioration of hepatocyte functions in the short term, has emerged as a serious public health issues that warrants attention. However, the effectiveness of existing small molecular antioxidants and anti-inflammatory medications in alleviating AIL remains uncertain. The unique inherent structural characteristics of liver confer it a natural propensity for nanoparticle capture, which present an opportunity to exploit in the formulation of nanoscale therapeutic agents, enabling their selective accumulation in the liver and thereby facilitating targeted therapeutic interventions. Significantly increased reactive oxygen species (ROS) accumulation and inflammation response have been evidenced to play crucial roles in occurrence and development of AIL. Nanozymes with ROS-scavenging capacities have demonstrated considerable promise in ROS elimination and inflammation regulation, thereby offering an appealing therapeutic instrument for the management of acute liver injury. In this review, the mechanisms of different type of ALI were summarized. In addition, we provide a comprehensive summary and review of the available ROS-scavenging nanozymes, including transition metal-based nanozymes, noble metal nanozymes, carbon-based nanozymes, and some other nanozymes. Furthermore, the challenges still need to be solved in the field of ROS-scavenging nanozymes for ALI alleviation are also discussed.
Keywords: acute liver injury, nanozyme, ROS-scavenging, antioxidant, anti-inflammation
Introduction
Acute liver injury (ALI) is one of the main ongoing hot topics of medical research. Recently, the high incidence of acute liver injury triggered by coronavirus disease 2019 (COVID-19) infection and the overdose of antipyretic drugs have made ALI a serious clinical concern that warrants attention.1 As an essential organ of the human body, the liver assumes an indispensable function in various physiological processes.2 Accordingly, the liver exhibits a high susceptibility to numerous external factors and stimuli, and excessive transient stimulation or enduring chronic stimulation can readily induce acute liver injury or chronic liver diseases, such as hepatic fibrosis, liver cirrhosis, and liver cancer.3 ALI is distinguished by a swift deterioration of hepatocyte functions in the short term, especially jaundice and coagulation disorders, which generally arise from drug poisoning, alcohol abuse, virus infection, hepatic resection or transplantation surgery, etc.4–7 In the absence of prompt and efficacious intervention, ALI has the potential to advance into acute liver failure, ultimately culminating in a fatality.8 The prevalence of ALI exhibits a persistent upward trend, primarily attributed to substance abuse, unhealthy lifestyles, and environmental pollution, among others.9 The pathophysiological process of ALI is characterized by a multitude of intricate mechanisms, encompassing various intracellular and extracellular events.10 Recent studies have accumulated substantial evidence to support the pivotal involvement of oxidative stress and inflammation in various forms of liver injury.11 N-acetyl cysteine (NAC) and glutathione (GSH), well-known small molecular reactive oxygen species (ROS) scavengers, have been extensively used as clinical antidotes against ALI. Nevertheless, the narrow therapeutic window imposes limitations on their clinical application.12 Therefore, it is imperative to explore novel methodologies that can provide more comprehensive safeguarding against ALI.
Nanozymes, a kind of nanomaterials with enzyme-like catalytic characteristics, have garnered extensive interest in the scientific community due to their high catalytic activity, good physiological stability, and cost-effectiveness in terms of manufacturing and storage.13,14 Among them, nanozymes with ROS-scavenging capacities have attracted increasing attention in the field of ALI alleviation for their advantages in many aspects. First of all, ROS-scavenging nanozymes possess the ability to persistently eliminate excess ROS in a catalytic manner and regulate inflammation rather than a simple consumption of ROS in those small molecular scavengers.11,15 Subsequently, compared with small molecular ROS scavengers, the nanozymes possess the characteristics of controllable nanoparticle size and diversified surface modifications, which have been proven to have the effect of prolongating blood circulation time and enhancing hepatic accumulation.16 The liver possesses inherent structural characteristics that confer it with a natural propensity for nanoparticle capture, such as the presence of the mononuclear phagocyte system, which harbors a substantial population of macrophages responsible for the internalization of nanoparticles.17–20 Additionally, the utilization of specific target molecule on nanoscale delivery systems probably mitigate the undesired distribution of the therapeutic agents to other organs, thereby reducing off-target effects and systemic toxicity.21 For example, glycyrrhetinic acid is used as a hepatocyte-targeting ligand functionalized on the L-Se-methylselenocysteine nanoparticles. The further experiment showed that the resultant exhibit enhanced hepatocyte uptake and liver accumulation.22 These superior characteristics make ROS-scavenging nanozymes an excellent alternative for the treatment of ALI.
In this review, we focus on the developments of ROS-scavenging nanozymes and their applications in alleviation of acute liver injury in recent years (Figure 1). Firstly, we discuss the mechanisms of ROS-scavenging nanozymes to alleviate liver injury. Subsequently, we provide a comprehensive summary and review of the available ROS-scavenging nanozymes, including transition metal-based nanozymes, noble metal nanozymes, carbon-based nanozymes, and some other nanozymes. These antioxidant nanozymes exhibit a high level of efficacy and facilitate the alleviation of ALI. Finally, we critically examine the challenges and prospects in the field of ROS-scavenging nanozymes for ALI alleviation. We hope that this article will serve as a convenient reference for future endeavors in fundamental research and clinical application.
 |
Figure 1 Schematic illustration of the cause of acute liver injury and ROS-scavenging nanozymes for alleviation of acute liver injury. |
The Mechanisms of Different Type of Acute Liver Injury
When an acute liver injury occurs, excessive amounts of ROS are rapidly produced by the damaged mitochondria of hepatocytes, and inflammation-related immune cells. The representative ROS predominantly include hydrogen peroxide (H2O2), superoxide radical species (•O2−), hydroxyl radicals (•OH), and singlet oxygen (1O2). Oxidative stress, which arises from an excessive accumulation of ROS, plays a significant role in various biological processes including aging, inflammation, and apoptosis.23,24 The aforementioned stresses give rise to detrimental effects on diverse cellular constituents, encompassing proteins, lipids, and DNA, which play crucial roles in maintaining redox reactions and signal transduction for homeostasis. Consequently, these impairments contribute to cellular malfunction, demise, and ultimately the onset of pathological conditions. It is worth noting that although the critical role of oxide stress is similar, the mechanisms of different ALIs differ due to the inducer. Table 1 provides a concise overview of the pathogenesis of different types of acute liver injury, which will be further elaborated as follows.
 |
Table 1 Summary of Mechanisms for Different Types of Acute Liver Injury |
Pathogenesis of Various Forms of Acute Liver Injury
Drug-Induced Liver Injury
Drug-induced liver injury, a prevalent form of ALI, usually arises as a consequence of drug abuse or the undesirable side effects of a diverse range of pharmaceutical agents, encompassing anti-tumor chemotherapy drugs, anti-tuberculosis drugs, antipyretic and analgesic drugs, immunosuppressants, hypoglycemic and lipid-lowering drugs, as well as antibacterial, antifungal, and antiviral drugs, among others. Liver damage caused by acetaminophen (APAP) has become a leading cause of acute liver failure in many countries today because it is widely used as an antipyretic and analgesic.30–32 Take APAP-induced ALI as an example. Overdose of APAP produces an excessive amount of highly reactive intermediate metabolite N-acetyl-p-benzo-quinone imine (NAPQI) inside hepatocytes. Subsequently, the excessive NAPQI depletes glutathione in the cytosol and mitochondria, leading to oxidative stress, eg the overexpression of ROS and lipid peroxidation (LPO).25,26 The hepatocytes are prone to damage and necrosis due to the excessive accumulation of ROS and LPO in the liver, which is widely acknowledged as a primary causal factor.33,34 Therefore, therapeutic strategies focused on ROS scavenging are greatly demanded for drug-induced liver injury.
Hepatic Ischemia-Reperfusion Injury
Hepatic ischemia-reperfusion injury (HIRI) is a significant complication that arises as a result of different liver surgical procedures, such as liver resection and transplantation, and causes of hepatic dysfunction and failure.35–38 HIRI is characterized by a phenomenon of inadequate oxygen and blood supply followed by reperfusion in the liver, resulting in a pronounced inflammatory response and an increased level of oxidative stress.39 The primary pathophysiological mechanism underlying hepatic ischemia-reperfusion injury entails the generation of ROS, which subsequently elicit endothelial dysfunction, DNA damage, and inflammatory reactions, ultimately culminating in cellular demise. The over-accumulation of ROS during HIRI induces oxidative damage of hepatocytes then apoptosis or necrosis and release of damage-associated molecular patterns, thereby promoting inflammatory response, which in turn increases the level of ROS.27 Despite the abundance of evidence regarding the efficacy of antioxidants in the management of HIRI, the number of drugs approved by the Food and Drug Administration for HIRI treatment remains limited. Therefore, it is crucial to develop novel efficient antioxidants to eliminate the excess ROS with the aim of mitigating and preventing HIRI.
Virus Infection-Induced Liver Injury
Viral hepatitis is a major public health problem which may change to chronic, and eventually lead to end-stage liver disease and even hepatocellular carcinoma.40–42 At present, viral hepatitis has been identified as A, B, C, D and E, among which hepatitis A and E usually present with a self-limited course followed by complete recovery,43,44 hepatitis B and C often result in chronic infection and responsible for the most adverse consequences of this disease.45,46 Apart from the above hepatitis virus, some non-hepatotropic viruses such as cytomegalovirus, Epstein-Barr virus and other infections can also cause liver damage.47–49
In recent years, with the outbreak of COVID-2019, virus infection-induced liver injury has attracted increasing attention.5 Multiple studies have substantiated the presence of liver injury in individuals afflicted with COVID-19,50 with a higher propensity for severe cases to exhibit pronounced liver impairment in comparison to milder cases.51 Moreover, the occurrence of an inflammatory cytokine storm has been observed in severe cases of COVID-19, but further investigation is required to determine if it is the underlying factor contributing to liver injury. Besides, the excessive utilization of medications in the treatment of COVID-19 presents a potential risk factor for liver injury.28 Therefore, it is imperative to not only address the primary disease resulting from coronavirus infection but also to closely monitor the incidence of liver injury and the administration of medications that have the potential to cause liver injury.52,53 Therefore, it is of great clinical significance to prevent liver injury through ROS-scavenging and inflammation regulation in coronavirus-infected patients.
Alcohol-Induced Liver Injury
Alcohol-induced liver injury is a common liver disease as a result of heavy drinking in many countries. Acute alcohol exposure can cause a “perfect storm” that favors inflammatory liver damage, including steatosis, dysregulated immunity response, and inflammation, thereby augmenting susceptibility to infection and increasing gastrointestinal tract permeability.54,55 Acetaldehyde, a metabolite of ethanol metabolism, exerts hepatotoxic effects by directly affecting liver cells through its actions on mitochondria, microtubules, and plasma membranes.29 The formation of complexes between acetaldehyde and proteins contributes to the degeneration and necrosis of liver cells. Furthermore, acetic acid, a metabolite of acetaldehyde, is converted into superoxide by xanthine oxidase, leading to LPO, damage to cell membrane lipids, and the promotion of liver injury. Both processes have the potential to increase the recruitment of pro-inflammatory macrophages in the liver, as well as augment the production of cytotoxic cytokines and ROS, which further promote the deterioration of the liver injury induced by acute alcohol exposure.
Mechanism of ALI Alleviation by Using ROS-Scavenging Nanozymes
Nanozymes are a category of nanomaterials that demonstrate enzyme-like characteristics by facilitating the transformation of substrates into products under physiological circumstances, although they may deviate from the catalytic pathway observed in natural enzymes.56 The past decade has witnessed the notable advancement of nanozymes, with the discovery of over 1200 distinct nanomaterials exhibiting various enzymatic activities. These nanomaterials encompass a wide range of compositions, such as noble-metal nanocrystals, transition metal-based nanomaterials, carbon-based nanomaterials, polymer-metal complexes, and metal–organic frameworks, etc.57 These nanozymes can be classified as mimics of oxidoreductases and hydrolases, including oxidase (OXD), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), nuclease, phosphatase, and others. Among them, nanomaterials with simulated CAT and SOD activity, and hydroxyl radical antioxidant capacity (HORAC) activity can eliminate ROS and generate oxygen to ameliorate the inflammatory microenvironment, which is beneficial for the alleviation of inflammatory responses. During the ALI process, there are abundant inflammatory macrophages and significantly increased ROS levels in the injured liver. The utilization of ROS-scavenging nanozymes in the management of ALI probably eliminate the excess ROS and improve the inflammatory microenvironment. Meanwhile, compared with small molecular ROS scavengers, ROS-scavenging nanozymes possess longer blood circulation time and preferable hepatic accumulation capacity,16 which reasonably enhance the therapy efficacy via improving the bio-availability of therapeutic agents and decreasing the possible non-specific organ distribution.
ROS-Scavenging Nanozymes for ALI Alleviation
In order to better study the mechanism and treatment of acute liver injury, different kinds of acute liver injury animal models have been established, including drug-induced liver injury (eg APAP-induced liver injury,58–60 anthracycline-induced liver injury61), chemical-induced liver injury (eg CCl4-induced liver injury62,63), immune-induced liver injury (eg lipopolysaccharide (LPS)-induced liver injury,64,65 D-galactosamine-induced liver injury,66 concanavalin A-induced liver injury67,68), alcohol-induced liver injury,69,70 and hepatic ischemia-reperfusion injury.7,35,71
Transition Metal-Based Nanozymes
Transition metal elements exhibit significant potential in the application of catalysis due to their variable valence state resulting from the incompletely filled valence d-orbitals.72,73 Many transition metal elements served as the active center of natural antioxidant enzymes, for instance, the active centers of SOD are mainly copper (Cu) and zinc (Zn) ions, or manganese (Mn) ions, or iron (Fe) ions.57,74 According to SOD with various metal centers were denoted as CuZn-SOD, Mn-SOD, and Fe-SOD, respectively. Transition metal elements-contained nanomaterials usually exhibit SOD-like activity due to their similar component with natural antioxidant enzymes. Transition metal-based nanozymes, such as transition metal-based oxides, sulfides, Prussian blue (PB), and Prussian blue analogues (PBA), as well as others, have been demonstrated to have significant ROS-scavenging capacity in a catalytic manner, showing promise for the alleviation of ROS-upregulated inflammation diseases, including ALI. A brief summary of transition metal-based antioxidant nanozymes for the alleviation of acute liver injury has been presented in Table 2, and a comprehensive examination of these nanozymes will be provided as follows.
 |
Table 2 Summary of ROS-Scavenging Nanozymes for the Alleviation of Acute Liver Injury |
Ce-Based Nanozymes
Ce-based nanoparticles, including CeO2, CeO2-x, and Ce2O3 nanoparticles, etc., exhibit multiple enzyme-like catalytic activity, such as SOD-like activity, CAT-like activity, and HORAC, which can efficiently convert •O2−, H2O2, and •OH to harmless H2O and O2. The superior ROS-scavenging enzyme-like activities of Ce-based nanozymes (CeNZs) could be attributed to the rapid transfer between the Ce3+ and Ce4+ ions on the surface of Ce-based nanoparticles. Due to their excellent antioxidant properties, CeNZs have been widely used to downregulate oxidative stress for the alleviation of ROS-related diseases including ALI.82 For instance, the study conducted by Ni et al demonstrated that ceria nanoparticles possess the capability to effectively mitigate the clinical manifestations of HIRI through the process of scavenging ROS, as well as inhibiting the activation of Kupffer cells and monocyte/macrophage cells (Figure 2A).35 The subsequent hepatic inflammatory response is mitigated by a substantial decrease in the release of pro-inflammatory cytokines and the limited recruitment and infiltration of neutrophils. Li et al developed CeO2 nanozymes for the detoxification and inflammatory regulation of drug-induced liver injury (DILI) (Figure 2B).58 CeNZs can effectively eliminate ROS within compromised hepatocytes, thereby facilitating the process of detoxification. Meanwhile, the CAT-like activity of CeNZs promotes the production of abundant oxygen, which exhibits a distinct inhibitory effect on pro-inflammatory macrophages, resulting in the alleviation of inflammation. The concurrent detoxification and inflammatory regulation capacities of CeNZs enable a DILI alleviation option with a comparatively extended therapeutic time window in contrast to NAC. Based on the excellent therapeutic efficacy of CeNZs on ALI, they further developed a ROS-sensitive nanozyme-augmented photoacoustic nanoprobe RSPN for the early diagnosis and therapy of acute liver failure.59 The CeNZs-mediated catalytic transformation of ROS into O2 bubbles was employed to augment the photoacoustic efficiency of photoacoustic imaging contrast agent ZnPc for the early diagnosis of ALI.
 |
Figure 2 Ce-based nanozymes (CeNZs) for ALI alleviation. (A) Schematic illustration of preventing hepatic ischemia-reperfusion injury by ceria NPs. Reprinted from Ni D, Wei H, Chen W, et al. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater. 2019;31:1902956. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.35 (B) Schematic mechanism of ceria nanozymes for drug-induced liver injury therapy by dual detoxification and inflammatory regulation. Reprinted from Nano Today, 35, Li F, Qiu Y, Xia F, et al. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. 100925, copyright 2020, with permission from Elsevier.58 (C) Schematic illustration of the preparation of mesoporous hollow manganese doped ceria nanoparticle and application for effectively prevention of hepatic ischemia reperfusion injury. Reprinted from Dove Medical Press, Si PR, Lei JX, Yang C, et al. Mesoporous hollow manganese doped ceria nanoparticle for effectively prevention of hepatic ischemia reperfusion injury. Int J Nanomedicine. 2023;18:2225–2238.71 |
The catalytic activity of CeNZs exhibits a strong positive correlation with both the specific surface area, the proportion of different valence states (Ce3+/Ce4+), and the concentration of oxygen vacancies (Ov). Doping is an effective strategy to increase the Ov concentration of Ce-based nanoparticles, which may lead to a substantial improvement in the catalytic efficiency of CeNZs. For example, Li et al designed Er3+-doped CeO2-x (Er-CeO2-x) NPs with multiple enzyme simulation activities for the treatment of LPS-induced acute liver injury.64 Si et al successfully synthesized manganese-doped mesoporous hollow ceria nanoparticles (MnOx-CeO2 NPs), and when the MnOx-CeO2 NPs with 4% Mn doping ratio exhibited the best anti-oxidant capability (Figure 2C). After doping with Mn, the oxygen vacancy concentration and specific surface area were significantly increased, which was directly related to the elevated anti-oxidant effect in the EPR measurement. These nanoparticles were further investigated for their potential in vivo application for inhibiting HIRI.71 The administration of MnOx-CeO2 NPs led to a notable decline in serum levels of alanine transaminase (ALT) and aspartate transaminase (AST), a reduction in malondialdehyde (MDA) level, an elevation in superoxide dismutase (SOD) level in the liver, and a mitigation of the pathological alterations induced by HIRI.
Fe-Based Nanozymes
Ferrum (Fe)-based nanozymes have attracted extensive attention in the field of biomedicine due to their excellent magnetic and catalytic properties.83–85 Prussian blue (PB) and its analogs possess multiple enzyme-like activities, including CAT, SOD, and POD, showing great prospects in the field of anti-oxidation and anti-inflammation.86 The antioxidant properties of PB nanozymes can be ascribed to the abundant variable valence states present within the structure of PB (such as Fe3+/Fe2+, [Fe(CN)6]3-/[Fe(CN)6]4-).87 PB nanozymes have been used for the alleviation of diseases associated with ROS, especially the ALI, e g, DILI and HIRI, etc. For example, Bai et al developed PB nanozymes to prevent anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation (Figure 3A).61 Huang et al used PB nanozymes to alleviate hepatic ischemia reperfusion injury by scavenging ROS in primary hepatocytes, reducing neutrophil infiltration and promoting macrophage polarization to the anti-inflammatory M2 type (Figure 3B).77 Sahu et al integrated PB nanozymes into the mesenchymal stem cells (MSCs) in order to enhance the viability of MSCs under conditions of high oxidative stress, while also amplifying their paracrine effect and anti-inflammatory properties (Figure 3C).76 This intervention led to a significant therapeutic outcome in the context of HIRI. Feng et al reports a simple and efficient one-step synthesis of Prussian blue (PB) nanozymes with multiple antioxidant enzymatic activities that effectively treat APAP-induced DILI (Figure 3D).60 According to in vivo experimental studies, the levels of serum biochemical indicators and histopathological examination of DILI mice livers showed that 12.5 mg/kg PB nanozymes could effectively inhibit liver necrosis and 25 mg/kg PB nanozymes achieved the same therapeutic effect as 300 mg/kg NAC. It is well-established that the substitution of iron with transition metals, including cobalt, nickel, manganese, copper, and zinc, enables the synthesis of diverse Prussian blue analogs (PBA) exhibiting distinct chemical compositions yet sharing similar crystal structures.88 These element substitutions probably facilitate the acquisition of novel traits in PBA, such as the alteration of multiple enzymatic activities. Moreover, Chen et al have successfully prepared multi-functional manganese Prussian blue nanozymes (MPBZs) with excellent ROS-scavenging capacity for the efficient treatment of APAP-induced liver injury by undergoing sequential processes ranging from antioxidation to anti-inflammation, including Nrf2 signaling pathway activation, inhibition of mitochondrial-induced oxidative stress and inflammation regulation.78
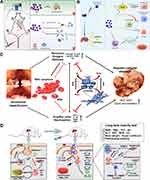 |
Figure 3 Fe-based nanozymes for ALI alleviation. (A) Schematic illustrations of PB nanozymes preventing anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation. Bai H, Kong F, Feng K, et al. Prussian blue nanozymes prevent anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation. ACS Appl Mater Interfaces. 2021;13:42382–42395. Copyright © 2021 American Chemical Society.61 (B) Schematic diagram of the mechanisms of PB scavengers protect against hepatic ischemia reperfusion injury. Huang YX, Xu QY, Zhang J, et al. Prussian blue scavenger ameliorates hepatic ischemia-reperfusion injury by inhibiting inflammation and reducing oxidative stress. Front Immunol. 2022;13:891351.77 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (C) Schematic illustrations of PB nanozyme impregnated mesenchymal stem cells for hepatic ischemia-reperfusion injury alleviation. Reprinted from Sahu A, Jeon J, Lee MS, et al. Nanozyme impregnated mesenchymal stem cells for hepatic ischemia-reperfusion injury alleviation. ACS Appl Mater Interfaces. 2021;13:25649–25662. Copyright © 2021 American Chemical Society.76 (D) Schematic illustrations of PB nanozymes preventing APAP-induced liver injury by scavenging ROS, relieving oxidative stress, and regulating the inflammatory response. Feng Q, Xu H, Pan X, et al. Antioxidation and anti-inflammatory activity of Prussian blue nanozymes to alleviate acetaminophen-induced acute liver injury. ACS Appl Nano Mater. 2023;6:8468–8481. Copyright © 2023 American Chemical Society.60 |
Cu-Based Nanozymes
Copper (Cu) plays a crucial role as a vital trace element within the human body, contributing significantly to both structural composition and physiological functions.89 Cu-dependent natural antioxidant enzymes, like Cu-Zn SOD, tyrosinase, and ceruloplasmin are organisms’ essential components to effectively eliminate ROS under oxidative stress.90 In the realm of ROS-scavenging and anti-inflammation, Cu-based nanozymes have garnered significant interest as of late. For example, Lin et al designed a copper-tannic acid coordination nanosheets (CuTA) nanozyme that combined copper ion and tannic acid.91 CuTA nanozyme exhibited superior SOD-like activity, CAT-like activity, and HORAC, serving as a powerful antioxidant for the regulation of inflammation. Deng et al developed ultrasmall Cu2O and Cu hybrid nanozymes (Cu5.4O nanozymes) with broader-spectrum enzymatic catalytic properties and antioxidant activities for inflammatory diseases therapeutic interventions and preventive measures, including ALI, acute kidney injury, and diabetic wound healing (Figure 4A).13 Jin et al further fabricated a synergistic therapeutic platform (HLCs/Cu NZs@fiber/dECM) consisting of Cu5.4O nanozymes-loaded PLGA nanofibers and decellularized extracellular matrix hydrogels for the alleviation of ALI and preventing the deterioration of hepatocytes necrosis (Figure 4B).62
 |
Figure 4 Cu-based nanozymes for ALI alleviation. (A) Schematic illustration of Cu5.4O ultrasmall nanoparticles (USNPs) as a broad-spectrum ROS scavenging agent in the treatment of ROS-related diseases, including ALI, acute kidney injury and diabetic wound healing. Liu T, Xiao B, Xiang F, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788.13 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (B) Schematic illustration of the HLCs/Cu NZs@fiber/dECM for CCl4-induced acute liver failure through the effects of ROS elimination, angiogenesis promotion, anti-inflammation, hepatocyte-related functions, and facilitating liver regeneration. Reprinted from Bioact Mater, 28, Jin Y, Zhang J, Xu Y, et al. Stem cell-derived hepatocyte therapy using versatile biomimetic nanozyme incorporated nanofiber-reinforced decellularized extracellular matrix hydrogels for the treatment of acute liver failure. 112–131, Copyright 2023.62 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Mn-Based Nanozymes
Manganese (Mn) is a multivalent transition metal element, the transformation of various valence states makes manganese exhibits superior catalytic activity, and thus various manganese-based nanozymes have emerged. Meanwhile, another essential trace element in the human body, manganese is the main active component of some metalloenzymes and participates in many physiological activities, which makes manganese-based nanomaterials get important applications in the biomedical field. For example, manganese is the active center of Mn-SOD, which is an important antioxidant enzyme in mitochondria and plays a critical role in the protection of cells from oxidative damage. Inspired by the natural Mn-SOD, some Mn-based nanozymes with antioxidant activities have been designed and used for ROS-scavenging and anti-inflammation. For example, Yao et al synthesized Mn3O4 nanoparticles with remarkable ROS-scavenging activities for ROS-induced ear-inflammation treatment.92 The Mn3O4 NPs could be used not only to eliminate •O2−, but also catalyze the scavenging of H2O2 and •OH. Owing to its superior antioxidant activities, Mn-based nanozymes have also been involved in the alleviation of ALI. Deng et al developed a platelet membrane (PM)-coated, Mn-doped, and tempol-grafted mesoporous silica nanoparticles (TMSN@PM) (Figure 5A and B) with excellent ROS-scavenging, oxygen production, and MRI capacity for the ameliorating and therapy monitoring of inflammation, including ALI and acute pancreatitis (Figure 5C).80 Manganese-based composite nanozymes, such as mesoporous hollow doped ceria nanoparticles (MnOx-CeO2 NPs) and BSA-functionalized CeO2 and MnO2 composite nanoparticles (CM NCs), also have been developed to alleviate the hepatic ischemia-reperfusion injury (Figures 2C).71,75 The CM NCs can scavenge and eliminate •O2−, H2O2, and •OH during the reperfusion process, subsequently suppressing the activation of Kupffer cells and neutrophils, and reducing the secretion of inflammatory factors.
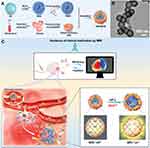 |
Figure 5 Mn-based nanozymes for ALI alleviation. (A) Schematic illustration of the preparation of TMSN@PM. (B) TEM image of TMSN@PM. (C) Schematic illustration of the in vivo MRI-guided real-time monitoring of the treatment process of inflammatory diseases using TMSN@PM. Reproduced with permission from Li X, Liu Y, Qi X, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34:e2109004. © 2022 Wiley-VCH GmbH.80 |
Other Transition Metal-Based Nanozymes
Besides Ce, Fe, Mn, and Cu-based nanozymes, other transition metal-based nanoparticles, such as zinc-based (Zn-based), nickel-based (Ni-based), molybdenum-based (Mo-based) and tungsten-based (W-based) nanoparticles, also show the enzyme catalytic activities of scavenging ROS due to their diversity of valence states and adjustability of crystal defects. These transition metal-based nanozymes have also emerged as novel antioxidants for the management of ALI. For example, Wu et al developed ZnO-NiO@COOH particles with increased surface area and oxygen vacancy active sites, which can efficiently adsorb and eliminate ROS to block the generation of inflammatory storms and promote the alleviation of ALI.8 Zhang et al prepared MoS2-PEG@BSA nanosheets that exhibit remarkable biocompatibility and enzymatic activity for the therapeutic intervention of ALI, showing promise in the therapy of ROS-related diseases.81 Xu et al synthesized a polyvinyl pyrrolidone modified tungsten disulfide (WS2-PVP) nanoflowers with CAT, SOD, and glutathione peroxidase (GPx) enzymes activities for the scavenging of ROS (Figure 6A and B).63 The WS2-PVP nanoflowers exhibited excellent cell protection and significantly improved treatment outcomes on ALI (Figure 6C).
 |
Figure 6 Other transition metal-based nanozymes for ALI alleviation. (A) FESEM image of WS2-PVP nanoflowers. (B) CAT-like activity, SOD-like activity and GPx-like activity of WS2-PVP nanoflowers. (C) Schematic diagram of ALI treatment with WS2-PVP nanoflowers. Reprinted from J Colloid Interf Sci, 625, Xu H, Zhang ZR, Zhang LY, et al. Tungsten disulfide nanoflowers with multi-nanoenzyme activities for the treatment of acute liver injury. 544–554, Copyright 2022, with permission from Elsevier.63 |
Noble Metal Nanozymes
Noble metal (Au, Ag, Pt, Pd, Ru, Rh, Os, and Ir) nanomaterials, including simple substances, alloys, and noble metal-containing composites, have been used extensively in the field of catalysis, electronics, and healthcare due to their unique optical, electrical, and catalytic properties. A great number of noble metal nanomaterials have been demonstrated with multi-enzymatic catalytic activities, such as OXD-like, POD-like, CAT-like, and SOD-like activity, etc.93–96 The enzyme-like catalytic activity of noble metal nanozymes could be regulated by altering their size, morphology, composition, surface modification and so on. Owing to their superior physicochemical properties and antioxidant enzyme-like activities, noble metal nanozymes have attracted increasing attention within the realm of biomedical applications, such as the therapy of malignant tumors, antibacterial treatment, and the alleviation of ROS-related inflammation diseases.74,97–100 For example, Lu et al developed a carvedilol-loaded gold star-like nanozyme with ROS-scavenging and autophagy-inhibiting capacity for the therapy of hepatic fibrosis.101 Xia et al prepared an ultrasmall oxidized ruthenium (sRuNP) nanozymes with improved antioxidant enzyme-like activity and regulatory T cells upregulation function for the highly efficient therapy of APAP-induced ALI (Figure 7A).102 Zhang et al developed Pt nanoparticles and carbon nanodots integrated nanozyme (Pt@CNDs) with boosted cascade SOD and CAT activities for antioxidant therapy of acute inflammation, including tetrachloromethane (CCl4)-induced ALI and phorbol 12-myristate 13-acetate (PMA)-induced ear inflammation (Figure 7B).103 Lu et al reported a MnO2-coated mesoporous PdPt alloy nanozyme with enhanced ROS-scavenging ability for the amelioration of APAP-induced ALI.104 With the rapid progress of nanotechnology, nanomedicines with self-propulsion capability have garnered increasing attention due to their active drug delivery and deeper penetration ability.105 However, most currently available ROS-scavenging nanozymes cannot actively eliminate ROS because they passively diffuse rather than self-charged propulsion. The exploration of actively ROS-scavenging nanozymes is highly desirable. In our recent work, we developed a self-propelled silica-supported ultrasmall AuNPs-tannic acid hybrid nanozyme (SAuPTB) to relieve and possibly even prevent APAP-induced ALI. In this work, SiO2 nanoparticles were used as the carrier for ultrasmall AuNPs loading, and which could produce large amount of O2 under H2O2, endowing the hybrid nanozyme with self-propelling properties. Moreover, tannic acid, a ubiquitous natural polyphenol with SOD and CAT-like activities was chelated on the surface of the synthesized nanoparticles to compensate for the lack of •OH scavenging properties of us-AuNPs and providing an approach to reduce nanomaterial biotoxicity and ensure biocompatibility (Figure 7C).106 The in vivo studies show that SAuPTB can accumulate at inflammatory sites in mouse liver, resulting in the decrease of alanine aminotransferase, aspartate aminotransferase, and ROS, reduction in pro-inflammatory cytokines and chemokines, hence reduced hepatocyte necrosis, liver injury, and mortality. Furthermore, SAuPTB activates the nuclear erythroid 2-related factor 2 pathway to upregulate antioxidative genes and reduce oxidative stress.
 |
Figure 7 Noble metal nanozymes for ALI alleviation. (A) Schematic illustration of tuning the size of RuNPs to boost their antioxidant activity and the application for sRuNP for highly efficient ALI therapy. Reprinted from Xia F, Hu X, Zhang B, et al. Ultrasmall ruthenium nanoparticles with boosted antioxidant activity upregulate regulatory T cells for highly efficient liver injury therapy. Small. 2022;18:2201558. © 2022 Wiley-VCH GmbH.102 (B) Schematic illustration of synthesis of Pt@CNDs with cascade superoxide dismutase-catalase activities and applications in eliminating intracellular ROS. Reprinted from Nano Today, 49, Zhang Y, Gao W, Ma Y, et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. 101768, Copyright 2023, with permission from Elsevier.103 (C) Schematic representation of SAuPTB nanozymes relieves APAP-induced ALI by attenuating ROS and regulating inflammation. Reprinted from Zhou C, Zhang L, Xu Z, et al. Self-Propelled Ultrasmall AuNPs-tannic acid hybrid nanozyme with ROS-scavenging and anti-inflammatory activity for drug-induced liver injury alleviation. Small. 2023;19:2206408. Copyright © 2023 Wiley-VCH GmbH.106 |
Carbon-Based Nanozymes
The aforementioned ROS-scavenging nanozymes were almost developed through the utilization of the variable valence states of metal centers or the metal-mediated specific adsorption/desorption. These metal-contained nanozymes probably suffer from in vivo biosafety concerns, such the undesirable metal ions leakage or metabolic residues. Differ from metal-containing nanozymes, carbon-based nanomaterials, such as carbon dots, graphene, graphene quantum dots, fullerenes, carbon nanotubes, and carbon nanospheres et al, have been widely developed to mimic the enzyme-like activity due to their metal-free characteristics, good biocompatibility, and well-defined electronic and geometric structures.107,108 Previous studies have demonstrated that carbon-based nanozymes exhibit the OXD-, CAT-, POD-, SOD-, and GPx-like catalytic activities stemming from their unique catalytic centers, eg, C=O groups, etc.109 Carbon-based nanozymes, being a group of highly advanced nanomaterials, have exhibited significant promise in various biomedical domains such as biosensing, disease detection, and therapeutic interventions. Based on the CAT- or SOD-like catalytic activities, carbon-based nanozymes can be used to eliminate excess ROS for inflammation alleviation. In recent years, carbon-based nanomaterials also have attracted great research interest in the alleviation of ALI as a result of their excellent catalytic ROS-scavenging capacities and liver target ability. For example, Umezaki et al prepared hydrophilic C60(OH)10 nanoparticles with ROS-scavenging properties for the treatment of an APAP-induced liver injury.110 Zhou et al developed biocompatible [60]/[70] fullerenols with superior ROS-scavenging ability for the alleviation of hepatotoxicity induced by doxorubicin chemotherapy (Figure 8B).111 Long et al developed hydrophilic carbohydrate-derived nanoparticles with good colloidal stability and blood circulation lifetime, effective liver delivery ability, and excellent ROS scavenging capability to prevent the mice from hepatic ischemia-reperfusion injury (Figure 8E).112 Xu et al developed an esterase-responsive carbon quantum dot-dexamethasone (CD-Dex) nanodrug for liver fibrosis therapy to simultaneously target pathological microstructures, scavenge ROS, and suppress inflammation (Figure 8A).113 Composition adjusting via element doping or surface integrating is an effective strategy to improve the catalytic performance of carbon-based nanozymes. For instance, Chen et al prepared nitrogen-doped carbon dots for HIRI alleviation (Figure 8D).114 Bai et al fabricated a lecithin-encapsulated selenium-doped carbon dots nanoparticles that exhibit a significant propensity for hepatic accumulation and effective scavenging capacity of ROS and inhibition of the release of inflammatory cytokines, thereby manifesting a favorable therapeutic effect beneficial therapeutic efficacy on HIRI (Figure 8C).115 Zhang et al constructed a Pt@CDs nanocomposite by integrating carbon dots with Pt NPs, which demonstrates promising potential as a highly efficient cascade antioxidant nanozyme, capable of safeguarding biological systems against damages caused by ROS, such as the ALI and acute ear inflammation (Figure 7B).103
 |
Figure 8 Carbon-based nanozymes for ALI alleviation. (A) Schematic illustration of esterase-responsive CD-Dex with ROS elimination and inflammation suppression capabilities for liver fibrosis therapy. Reprinted from Xu YC, Chen J, Jiang W, et al. Multiplexing nanodrug ameliorates liver fibrosis via ROS elimination and inflammation suppression. Small. 2022;18:2102848. Copyright © 2021 Wiley-VCH GmbH.113 (B) Schematic illustration of [70]/[60] fullerenols against oxidative injury induced by reduplicative chemotherapy. Reprinted from Zhou Y, Li J, Ma HJ, et al. Biocompatible [60] / [70] fullerenols: potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mater Interfaces. 2017;9:35539–35547. Copyright © 2017 American Chemical Society.111 (C) Schematic illustration of the mechanism of self-assembled selenium-doped carbon quantum dots as antioxidants for HIRI management. Bai B, Qi S, Yang K, et al. Self-assembly of selenium-doped carbon quantum dots as antioxidants for hepatic ischemia-reperfusion injury management. Small. 2023;19:2300217. Copyright © 2023 Wiley-VCH GmbH.115 (D) Schematic illustration of nitrogen-doped carbon dots (CNDs) for reactive oxygen species scavenging on HIRI. Reprinted from Chen D, Wang CQ, Yu HJ, et al. Nitrogen-doped carbon dots with oxidation stress protective effects for reactive oxygen species scavenging on hepatic ischemia-reperfusion injury. ACS Appl Nano Mater. 2023. Copyright © 2023 American Chemical Society.114 (E) Schematic of carbohydrate-derived nanoparticles (C-NPs) for Hepatic ischemia-reperfusion injury treatment. Reprinted from Long Y, Wei H, Li J, et al. Prevention of hepatic ischemia-reperfusion injury by carbohydrate-derived nanoantioxidants. Nano Lett. 2020;20:6510–6519. Copyright © 2020 American Chemical Society.112 |
Other Nanozymes
Apart from the above-mentioned transmit-metal nanozymes, noble metal nanozymes, and carbon-based nanozymes, there are some other nanozymes with similar antioxidant enzymatic activities that have been used for the therapeutic intervention of various ALI, such as phosphorus-based nanoparticles, selenium-based nanoparticles, and some natural antioxidant enzyme-containing nanoparticles. For example, Ge et al developed black phosphorus quantum dots (BPQDs) with second near-infrared window (NIR-II) fluorescence imaging and ROS-scavenging capacity (Figure 9A).116 BPQDs have been found to be effective in providing protection to tissues against damage caused by ROS in cases of acute kidney and liver injury, which could be monitored by the responsive NIR-II FI. Lee et al have developed natural SOD-containing nanoparticles (d-HA/SOD/USCaP), which consist of multiple Cu-Zn SOD molecules embedded in a hydrophilic hyaluronic acid (HA) network decorated with ultrasmall calcium phosphate (USCaP).117 The d-HA/SOD/USCaP nanoparticles can effectively deliver the Cu-Zn SOD to hepatocytes, escape from the endosome after the cellular uptake, and retain its catalytic function both in the bloodstream and within the cytoplasm. The d-HA/SOD/USCaP exhibits superior therapeutic effects on the APAP-induced ALI via eliminating the excess ROS inside the liver (Figure 9B). Besides nanozymes, other nanoparticles with ROS-scavenging capacity also can be used as the therapeutic agents for the alleviation of ALI, such as polydopamine, melanin nanoparticles, etc.22,118,119 Although there has developed numerous antioxidants for the alleviation of ALI, the unclear long-term in vivo biosafety and degradation mechanism may hinder their translational application. Therefore, it still remains challenging to develop more efficient antioxidant nanozymes with excellent ROS scavenging capacity and negligible toxicity.
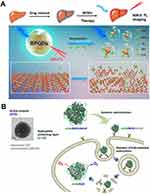 |
Figure 9 Carbon-based nanozymes for ALI alleviation. (A) Schematic illustration of NIR-II fluorescent biodegradable black phosphorus quantum dots (BPQDs) for precise acute liver injury imaging and therapy. Ge X, Su L, Yang L, et al. NIR-II fluorescent biodegradable nanoprobes for precise acute kidney/liver injury imaging and therapy. Anal Chem. 2021;93:13893–13903. Copyright © 2021 American Chemical Society.116 (B) Schematic illustration of targeted cellular delivery of robust d-HA/SOD/USCaP enzyme nanoparticles for the treatment of drug-induced liver injury. Reprinted from Acta Biomater, 81, Lee MS, Kim NW, Lee JE, et al. Targeted cellular delivery of robust enzyme nanoparticles for the treatment of drug-induced hepatotoxicity and liver injury. 231–241, Copyright 2018, with permission from Elsevier.117 |
Conclusion and Outlook
With the rapid development nanoscience and nanotechnology, its application in biomedicine has opened up a wide range of research interests. As one of the emerging research frontiers, nanozymes exhibit great prospects for disease therapy. Herein, we have summarized recent advancements in ROS-scavenging nanozymes and their applications in acute liver injury alleviation in recent years. We provide a comprehensive review of the available ROS-scavenging nanozymes, including transition metal-based nanozymes, noble metal nanozymes, carbon-based nanozymes, and some other nanozymes. The advancements presented provide compelling evidence to support the application of ROS-scavenging nanozymes in the therapeutic intervention of ROS-related inflammation, especially confirming the feasibility of constructing antioxidant nanozymes for mitigating liver injury. Although nanozymes have significant advantages in the application of biomaterials, there are still some key issues and challenges that need to be considered. (1) The ROS-scavenging efficiency of most nanozymes should be further improved. At present, most ROS-nanozymes employed in liver injury treatment required relatively high doses, which may cause long-term biotoxicity. Developing ROS-scavenging nanozymes with more efficient ROS-scavenging capacity possibly reduces the dosage of therapeutic nanozymes and improves biosafety. Incorporation of natural enzyme structural properties into the rational design of artificial antioxidant nanozymes probably be a highly promising strategy for enhancing catalytic performance. In addition, the introduction of the single metal atoms and rational design of cascade nanozymes may also provide promising approaches to improve the catalytic efficiency. (2) The stability and consistency of performance in vitro and in vivo should be verified. Nanozymes cannot avoid interactions with biomolecules after entering the blood circulation and cells, the adsorbed biomolecules certainly affect the active sites’ exposure and substances affinity, which probably alter their catalytic performance. Investigation of the stability and consistency of ROS-scavenging activities of nanozymes shows an important impact. (3) The long-term in vivo metabolic pathway of the ROS-scavenging nanozymes should be further investigated. As an endogenous agent, ROS-scavenging nanozymes probably possess long-term toxicity on undesired tissues due to their nonspecific distribution and cellular uptake. The absence of toxicity studies investigating systemic biodistribution, tolerance threshold, degradation, and clearance rate hinders the determination of long-term effects of potential toxicity on animals. Consequently, there is an urgent need to undertake more extensive assessments encompassing physical and chemical properties, nanoscience, and biosafety toxicity in order to evaluate the potential risks associated with ROS-scavenging nanozymes, so that to promote its clinical transformation. (4) The natural targeting or the targeted modification of nanozymes should be further explored. As we known, there are many special biomarkers in the injury site. Such biomarkers could be used to identify liver injury, or exclude injury, early in the disease process. Moreover, they would allow therapy to be targeted to patients at high risk of adverse outcomes or allow early, safe discharge of well patients. Ai et al recently revealed that negatively charged melanin nanozymes could naturally targeted to inflammation sites in the colon through electrostatic interactions and bind at the lesion site for more than 72 h after oral administration.120 The long-lasting characteristics of melanin nanozymes show super therapeutic effect on inflammatory bowel disease. Therefore, the natural targeting or the targeted modification of nanozymes could further improve and enhance the diagnosis and treatment of nanozymes.
Abbreviations
ALI, acute liver injury; ROS, reactive oxygen species; NAC, N-acetyl cysteine; GSH, glutathione; H2O2, hydrogen peroxide; •O2−, superoxide radical; •OH, hydroxyl radical; 1O2, singlet oxygen; APAP, acetaminophen; NAPQI, N-acetyl-p-benzo-quinone imine; LPO, lipid peroxidation; HIRI, hepatic ischemia-reperfusion injury; DAMPs, damage-associated molecular patterns; OXD, oxidase; POD, peroxidase; CAT, catalase; SOD, superoxide dismutase; HORAC, hydroxyl radical antioxidant capacity; PB, Prussian blue; PBA, Prussian blue analogues; CeNZs, Ce-based nanozymes; DILI, drug-induced liver injury; Ov, oxygen vacancies; LPS, lipopolysaccharide; USNPs, ultrasmall nanoparticles; Plt, platelets; Mn, Manganese; MSCs, mesenchymal stem cells; Zn-based, zinc-based; Ni-based, nickel-based; Mo-based, molybdenum-based; W-based, tungsten-based; WS2-PVP, polyvinyl pyrrolidone modified tungsten disulfide; CCl4, tetrachloromethane; PMA, phorbol 12-myristate 13-acetate; SAuPTB, ultrasmall AuNPs-tannic acid hybrid nanozyme; PPM, MnO2 coated mesoporous PdPt alloy nanozymes; BPQDs, black phosphorus quantum dots; NIR-II, second near-infrared window; HA, hyaluronic acid; USCaP, ultrasmall calcium phosphate.
Consent for Publication
The authors confirm that the details of any images can be published.
Acknowledgments
This work was funded by Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0763, CSTB2023NSCQ-MSX0174), Army Medical University Foundation (2022YQB090, 2021XQN17 and Red Medical Miaopu Talents of Chunyu Zhou), and Talent Project of Chongqing (Dong Zhang, CQYC202103075).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu Y, Mao Y, Chen C, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221–241. doi:10.1007/s12072-017-9793-2
2. Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–277. doi:10.1146/annurev-immunol-051116-052415
3. Weaver R, Blomme E, Chadwick A, et al. Managing the challenge of drug-induced liver injury: a roadmap for the development and deployment of preclinical predictive models. Nat Rev Drug Discov. 2020;19:131–148. doi:10.1038/s41573-019-0048-x
4. Garcia-Cortes M, Robles-Diaz M, Stephens C, et al. Drug induced liver injury: an update. Arch Toxicol. 2020;94:3381–3407. doi:10.1007/s00204-020-02885-1
5. Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi:10.1111/liv.14435
6. Gao L, Zhou Y, Zhong W, et al. Caveolin-1 is essential for protecting against binge drinking-induced liver damage through inhibiting reactive nitrogen species. Hepatology. 2014;60:687–699. doi:10.1002/hep.27162
7. Gobut H, Kucuk A, Sengel N, et al. Effects of cerium oxide (CeO2) on liver tissue in liver ischemia-reperfusion injury in rats undergoing desflurane anesthesia. BMC Anesthesiol. 2023;23:40. doi:10.1186/s12871-023-01999-0
8. Wu X, Liu S, Zhu H, et al. Scavenging ROS to alleviate acute liver injury by ZnO-NiO@COOH. Adv Sci. 2022;9. doi:10.1002/advs.202103982
9. Stravitz R, Lee W. Acute liver failure. Lancet. 2019;394:869–881. doi:10.1016/S0140-6736(19)31894-X
10. Yan M, Huo Y, Yin S, et al. Mechanisms of Acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–283. doi:10.1016/j.redox.2018.04.019
11. Liu M, Huang Q, Zhu Y, et al. Harnessing reactive oxygen/nitrogen species and inflammation: nanodrugs for liver injury. Mater Today Bio. 2022;13:100215. doi:10.1016/j.mtbio.2022.100215
12. Jaeschke H, Akakpo J, Umbaugh D, et al. Novel therapeutic approaches against acetaminophen-induced liver injury and acute liver failure. Toxicol Sci. 2020;174:159–167. doi:10.1093/toxsci/kfaa002
13. Liu T, Xiao B, Xiang F, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788. doi:10.1038/s41467-020-16544-7
14. Zhao S, Duan H, Yang Y, et al. Fenozyme protects the integrity of the blood–brain barrier against experimental cerebral malaria. Nano Lett. 2019;19:8887–8895. doi:10.1021/acs.nanolett.9b03774
15. Huang X, He D, Pan Z, et al. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater Today Bio. 2021;11. doi:10.1016/j.mtbio.2021.100124
16. Wang B, He X, Zhang Z, et al. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 2013;46:761–769. doi:10.1021/ar2003336
17. Wilhelm S, Tavares A, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:1–12. doi:10.1038/natrevmats.2016.14
18. Jenne C, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi:10.1038/ni.2691
19. Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7:99–104. doi:10.1097/00075198-200104000-00008
20. Hu M, Huang L. Nanomaterial manipulation of immune microenvironment in the diseased liver. Adv Funct Mater. 2019;29:1805760. doi:10.1002/adfm.201805760
21. de Almeida M, Susnik E, Drasler B, et al. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc Rev. 2021;50:5397–5434. doi:10.1039/d0cs01127d
22. Yuan X, Zhou Y, Sun J, et al. Preventing acute liver injury via hepatocyte-targeting nano-antioxidants. Cell Proliferat. 2023;56. doi:10.1111/cpr.13494
23. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi:10.1038/nri3423
24. Schieber M, Chandel N. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi:10.1016/j.cub.2014.03.034
25. Ali ES, Rychkov GY, Barritt GJ. TRPM2 non-selective cation channels in liver injury mediated by reactive oxygen species. Antioxidants. 2021;10:1243. doi:10.3390/antiox10081243
26. Wang Y, Zhao Y, Wang Z, et al. Peroxiredoxin 3 inhibits Acetaminophen-induced liver pyroptosis through the regulation of mitochondrial ROS. Front Immunol. 2021;12:652782. doi:10.3389/fimmu.2021.652782
27. Bhogal RH, Curbishley SM, Weston CJ, et al. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transplant. 2010;16:1303–1313. doi:10.1002/lt.22157
28. Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United Eur Gastroent. 2020;8:509–519. doi:10.1177/2050640620924157
29. Geng X, Du X, Wang W, et al. Confined cascade metabolic reprogramming nanoreactor for targeted alcohol detoxification and alcoholic liver injury management. ACS Nano. 2023;17:7443–7455. doi:10.1021/acsnano.2c12075
30. Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplant. 2004;10:1018–1023. doi:10.1002/lt.20204
31. Björnsson E, Jerlstad P, Bergqvist A, et al. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroentero. 2005;40:1095–1101. doi:10.1080/00365520510023846
32. Habib S, Shaikh OS. Drug-induced acute liver failure. Clin Liver Dis. 2017;21:151–162. doi:10.1016/j.cld.2016.08.003
33. Lei Y, Wang K, Deng L, et al. Redox regulation of inflammation: old elements, a new story. Med Res Rev. 2015;35:306–340. doi:10.1002/med.21330
34. Zhou Z, Song J, Nie L, et al. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597–6626. doi:10.1039/C6CS00271D
35. Ni D, Wei H, Chen W, et al. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater. 2019;31:1902956. doi:10.1002/adma.201902956
36. Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi:10.1038/nm.2507
37. Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia–reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastro Hepat. 2013;10:79–89. doi:10.1038/nrgastro.2012.225
38. Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–1106. doi:10.1016/j.jhep.2013.06.017
39. Galaris D, Barbouti A, Korantzopoulos P. Oxidative stress in hepatic ischemia-reperfusion injury: the role of antioxidants and iron chelating compounds. Curr Pharm Des. 2006;12:2875–2890. doi:10.2174/138161206777947614
40. Almeida PH, Matielo CEL, Curvelo LA, et al. Update on the management and treatment of viral hepatitis. World J Gastroenterol. 2021;27:3249–3261. doi:10.3748/wjg.v27.i23.3249
41. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos T R Soc B. 2017;372:20160274. doi:10.1098/rstb.2016.0274
42. Ari ZB, Weitzman E, Safran M. Oncogenic viruses and hepatocellular carcinoma. Clin Liver Dis. 2015;19:341–360. doi:10.1016/j.cld.2015.01.006
43. Shin E-C, Jeong S-H. Natural history, clinical manifestations, and pathogenesis of hepatitis A. CSH Perspect Med. 2018;8. doi:10.1101/cshperspect.a031708
44. Kamar N, Dalton HR, Abravanel F, et al. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi:10.1038/nrdp.2017.87
45. Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis. 2016;20:607–628. doi:10.1016/j.cld.2016.06.006
46. El‐Serag HB, Christie IC, Puenpatom A, et al. The effects of sustained virological response to direct‐acting anti‐viral therapy on the risk of extrahepatic manifestations of hepatitis C infection. Aliment Pharmacol Ther. 2019;49:1442–1447. doi:10.1111/apt.15240
47. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin N. 2020;49:331–346. doi:10.1016/j.gtc.2020.01.008
48. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein–Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroentero. 2017;52:893–897. doi:10.1080/00365521.2017.1319972
49. Horwitz CA, Burke MD, Grimes P, et al. Hepatic function in mononucleosis induced by Epstein-Barr virus and cytomegalovirus. Clin Chem. 1980;26:243–246. doi:10.1093/clinchem/26.2.243
50. Rowaiye AB, Okpalefe OA, Adejoke OO, et al. Attenuating the effects of novel COVID-19 (SARS-CoV-2) infection-induced cytokine storm and the implications. J Inflamm Res. 2021;14:1487–1510. doi:10.2147/jir.S301784
51. Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. 2020;5:428–430. doi:10.1016/s2468-1253(20)30057-1
52. Ibrahim H, Perl A, Smith D, et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. doi:10.1016/j.clim.2020.108544
53. Fu Y, Chen T, Weng L, et al. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed Pharmacother. 2021;141:111888. doi:10.1016/j.biopha.2021.111888
54. Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastro Hepat. 2015;12:231–242. doi:10.1038/nrgastro.2015.35
55. Massey VL, Arteel GE. Acute alcohol-induced liver injury. Front Physiol. 2012;3:193. doi:10.3389/fphys.2012.00193
56. Wei H, Gao L, Fan K, et al. Nanozymes: a clear definition with fuzzy edges. Nano Today. 2021;40:101269. doi:10.1016/j.nantod.2021.101269
57. Chen Z, Yu Y, Gao Y, et al. Rational design strategies for nanozymes. ACS Nano. 2023;17:13062–13080. doi:10.1021/acsnano.3c04378
58. Li F, Qiu Y, Xia F, et al. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. Nano Today. 2020;35:100925. doi:10.1016/j.nantod.2020.100925
59. Wu H, Xia F, Zhang L, et al. A ROS-Sensitive nanozyme-augmented photoacoustic nanoprobe for early diagnosis and therapy of acute liver failure. Adv Mater. 2022;34:2108348. doi:10.1002/adma.202108348
60. Feng Q, Xu H, Pan X, et al. Antioxidation and anti-inflammatory activity of Prussian blue nanozymes to alleviate acetaminophen-induced acute liver injury. ACS Appl Nano Mater. 2023;6:8468–8481. doi:10.1021/acsanm.3c00763
61. Bai H, Kong F, Feng K, et al. Prussian blue nanozymes prevent anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation. ACS Appl Mater Interfaces. 2021;13:42382–42395. doi:10.1021/acsami.1c09838
62. Jin Y, Zhang J, Xu Y, et al. Stem cell-derived hepatocyte therapy using versatile biomimetic nanozyme incorporated nanofiber-reinforced decellularized extracellular matrix hydrogels for the treatment of acute liver failure. Bioact Mater. 2023;28:112–131. doi:10.1016/j.bioactmat.2023.05.001
63. Xu H, Zhang ZR, Zhang LY, et al. Tungsten disulfide nanoflowers with multi-nanoenzyme activities for the treatment of acute liver injury. J Colloid Interf Sci. 2022;625:544–554. doi:10.1016/j.jcis.2022.06.043
64. Li Y, Li Y, Bai Y, et al. High catalytic efficiency from Er 3+ -doped CeO 2−x nanoprobes for in vivo acute oxidative damage and inflammation therapy. J Mater Chem B. 2020;8:8634–8643. doi:10.1039/D0TB01463J
65. Shao L, Xiong X, Zhang Y, et al. IL-22 ameliorates LPS-induced acute liver injury by autophagy activation through ATF4-ATG7 signaling. Cell Death Dis. 2020;11:970. doi:10.1038/s41419-020-03176-4
66. Yoshida T, Abe K, Ikeda T, et al. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur J Pharmacol. 2007;576:136–142. doi:10.1016/j.ejphar.2007.08.012
67. Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. doi:10.4049/jimmunol.159.3.1537
68. Ren J, Meng S, Yan B, et al. Protectin D1 reduces concanavalin A-induced liver injury by inhibiting NF-κB-mediated CX3CL1/CX3CR1 axis and NLR family, pyrin domain containing 3 inflammasome activation. Mol Med Rep. 2016;13:3627–3638. doi:10.3892/mmr.2016.4980
69. Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi:10.1007/s00204-009-0432-0
70. Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi:10.1053/gast.2003.50087
71. Si PR, Lei JX, Yang C, et al. Mesoporous hollow manganese doped ceria nanoparticle for effectively prevention of hepatic ischemia reperfusion injury. Int J Nanomedicine. 2023;18:2225–2238. doi:10.2147/ijn.S400467
72. Long X, Qiu W, Wang Z, et al. Recent advances in transition metal–based catalysts with heterointerfaces for energy conversion and storage. Mater Today Chem. 2019;11:16–28. doi:10.1016/j.mtchem.2018.09.003
73. Liu L, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem Rev. 2018;118:4981–5079. doi:10.1021/acs.chemrev.7b00776
74. Liu Y, Cheng Y, Zhang H, et al. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci Adv. 2020;6:eabb2695. doi:10.1126/sciadv.abb2695
75. Zhang S, Cao Y, Xu B, et al. An antioxidant nanodrug protects against hepatic ischemia–reperfusion injury by attenuating oxidative stress and inflammation. J Mater Chem B. 2022;10:7563–7569. doi:10.1039/D1TB02689E
76. Sahu A, Jeon J, Lee MS, et al. Nanozyme impregnated mesenchymal stem cells for hepatic ischemia-reperfusion injury alleviation. ACS Appl Mater Interfaces. 2021;13:25649–25662. doi:10.1021/acsami.1c03027
77. Huang YX, Xu QY, Zhang J, et al. Prussian blue scavenger ameliorates hepatic ischemia-reperfusion injury by inhibiting inflammation and reducing oxidative stress. Front Immunol. 2022;13:891351. doi:10.3389/fimmu.2022.891351
78. Chen C, Wu H, Li Q, et al. Manganese Prussian blue nanozymes with antioxidant capacity prevent Acetaminophen-induced acute liver injury. Biomater Sci. 2023;11:2348–2358. doi:10.1039/d2bm01968j
79. Zhang B, Chen G, Wu X, et al. Biomimetic Prussian blue nanozymes with enhanced bone marrow-targeting for treatment of radiation-induced hematopoietic injury. Biomaterials. 2023;293:121980. doi:10.1016/j.biomaterials.2022.121980
80. Li X, Liu Y, Qi X, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34:e2109004. doi:10.1002/adma.202109004
81. Zhang ZR, Zhao JL, Chen Z, et al. A molybdenum-based nanoplatform with multienzyme mimicking capacities for oxidative stress-induced acute liver injury treatment. Inorg Chem Front. 2023;10:1305–1314. doi:10.1039/d2qi02318k
82. Oró D, Yudina T, Fernández-Varo G, et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol. 2016;64:691–698. doi:10.1016/j.jhep.2015.10.020
83. Sun T, Liu Y, Zhou C, et al. Fluorine-mediated synthesis of anisotropic iron oxide nanostructures for efficient T2-weighted magnetic resonance imaging. Nanoscale. 2021;13:7638–7647. doi:10.1039/D1NR00338K
84. Chen Z, Yin -J-J, Zhou Y-T, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6:4001–4012. doi:10.1021/nn300291r
85. Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi:10.1038/nnano.2016.168
86. Feng K, Zhang J, Dong H, et al. Prussian blue nanoparticles having various sizes and crystallinities for multienzyme catalysis and magnetic resonance imaging. ACS Appl Nano Mater. 2021;4:5176–5186. doi:10.1021/acsanm.1c00617
87. Zhang W, Hu S, Yin -J-J, et al. Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J Am Chem Soc. 2016;138:5860–5865. doi:10.1021/jacs.5b12070
88. Lu K, Zhu X-Y, Li Y, et al. Progress in the preparation of Prussian blue-based nanomaterials for biomedical applications. J Mater Chem B. 2023;11:5272–5300. doi:10.1039/D2TB02617A
89. Dong C, Feng W, Xu W, et al. The coppery age: copper (Cu)-involved nanotheranostics. Adv Sci. 2020;7:2001549. doi:10.1002/advs.202001549
90. Cao Z, Wang H, Chen J, et al. Silk-based hydrogel incorporated with metal-organic framework nanozymes for enhanced osteochondral regeneration. Bioact Mater. 2023;20:221–242. doi:10.1016/j.bioactmat.2022.05.025
91. Lin S, Cheng Y, Zhang H, et al. Copper tannic acid coordination nanosheet: a potent nanozyme for scavenging ROS from cigarette smoke. Small. 2020;16:1902123. doi:10.1002/smll.201902123
92. Yao J, Cheng Y, Zhou M, et al. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci. 2018;9:2927–2933. doi:10.1039/C7SC05476A
93. He W, Zhou Y-T, Wamer WG, et al. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials. 2013;34:765–773. doi:10.1016/j.biomaterials.2012.10.010
94. Ge C, Fang G, Shen X, et al. Facet energy versus enzyme-like activities: the unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano. 2016;10:10436–10445. doi:10.1021/acsnano.6b06297
95. Comotti M, Della Pina C, Falletta E, et al. Aerobic oxidation of glucose with gold catalyst: hydrogen peroxide as intermediate and reagent. Adv Synth Catal. 2006;348:313–316. doi:10.1002/adsc.200505389
96. Nomura M, Yoshimura Y, Kikuiri T, et al. Platinum nanoparticles suppress osteoclastogenesis through scavenging of reactive oxygen species produced in RAW264.7 Cells. J Pharmacol Sci. 2011;117:243–252. doi:10.1254/jphs.11099FP
97. Dai Y, Ding Y, Li L. Nanozymes for regulation of reactive oxygen species and disease therapy. Chinese Chem Lett. 2021;32:2715–2728. doi:10.1016/j.cclet.2021.03.036
98. Zhang DY, Tu T, Younis MR, et al. Clinically translatable gold nanozymes with broad spectrum antioxidant and anti-inflammatory activity for alleviating acute kidney injury. Theranostics. 2021;11:9904–9917. doi:10.7150/thno.66518
99. Cai Rui L. Research progress of noble metal⁃based nanozymes. Chem J Chinese U. 2020;42:1188–1201. doi:10.7503/cjcu20200591
100. Zhou C, Zhang L, Sun T, et al. Activatable NIR-II plasmonic nanotheranostics for efficient photoacoustic imaging and photothermal cancer therapy. Adv Mater. 2021;33:2006532. doi:10.1002/adma.202006532
101. Lu Q, Zhou Y, Xu M, et al. Sequential delivery for hepatic fibrosis treatment based on carvedilol loaded star-like nanozyme. J Control Release. 2022;341:247–260. doi:10.1016/j.jconrel.2021.11.033
102. Xia F, Hu X, Zhang B, et al. Ultrasmall ruthenium nanoparticles with boosted antioxidant activity upregulate regulatory T cells for highly efficient liver injury therapy. Small. 2022;18:2201558. doi:10.1002/smll.202201558
103. Zhang Y, Gao W, Ma Y, et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. Nano Today. 2023;49:101768. doi:10.1016/j.nantod.2023.101768
104. Lu Y, Pan X, Cao C, et al. MnO2 coated mesoporous pdpt nanoprobes for scavenging reactive oxygen species and solving acetaminophen-induced liver injury. Adv Healthc Mater. 2023;12:2300163. doi:10.1002/adhm.202300163
105. Bechinger C, Di Leonardo R, Lowen H, et al. Active particles in complex and crowded environments. Rev Mod Phys. 2016;88. doi:10.1103/RevModPhys.88.045006
106. Zhou C, Zhang L, Xu Z, et al. Self-Propelled Ultrasmall AuNPs-tannic acid hybrid nanozyme with ROS-scavenging and anti-inflammatory activity for drug-induced liver injury alleviation. Small. 2023;19:2206408. doi:10.1002/smll.202206408
107. Ding H, Hu B, Zhang B, et al. Carbon-based nanozymes for biomedical applications. Nano Res. 2021;14:570–583. doi:10.1007/s12274-020-3053-9
108. Iohara D. Preparation and evaluation of fullerene based nanomedicine. Yakugaku Zasshi. 2019;139:1539–1546. doi:10.1248/yakushi.19-00172
109. Kong B, Yang T, Cheng F, et al. Carbon dots as nanocatalytic medicine for anti-inflammation therapy. J Colloid Interf Sci. 2022;611:545–553. doi:10.1016/j.jcis.2021.12.107
110. Umezaki Y, Iohara D, Anraku M, et al. Preparation of hydrophilic C-60(OH)(10)/2-hydroxypropyl-beta-cyclodextrin nanoparticles for the treatment of a liver injury induced by an overdose of Acetaminophen. Biomaterials. 2015;45:115–123. doi:10.1016/j.biomaterials.2014.12.032
111. Zhou Y, Li J, Ma HJ, et al. Biocompatible [60] / [70] fullerenols: potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mater Interfaces. 2017;9:35539–35547. doi:10.1021/acsami.7b08348
112. Long Y, Wei H, Li J, et al. Prevention of hepatic ischemia-reperfusion injury by carbohydrate-derived nanoantioxidants. Nano Lett. 2020;20:6510–6519. doi:10.1021/acs.nanolett.0c02248
113. Xu YC, Chen J, Jiang W, et al. Multiplexing nanodrug ameliorates liver fibrosis via ROS elimination and inflammation suppression. Small. 2022;18:2102848. doi:10.1002/smll.202102848
114. Chen D, Wang CQ, Yu HJ, et al. Nitrogen-doped carbon dots with oxidation stress protective effects for reactive oxygen species scavenging on hepatic ischemia-reperfusion injury. ACS Appl Nano Mater. 2023. doi:10.1021/acsanm.3c01885
115. Bai B, Qi S, Yang K, et al. Self-assembly of selenium-doped carbon quantum dots as antioxidants for hepatic ischemia-reperfusion injury management. Small. 2023;19:2300217. doi:10.1002/smll.202300217
116. Ge X, Su L, Yang L, et al. NIR-II fluorescent biodegradable nanoprobes for precise acute kidney/liver injury imaging and therapy. Anal Chem. 2021;93:13893–13903. doi:10.1021/acs.analchem.1c02742
117. Lee MS, Kim NW, Lee JE, et al. Targeted cellular delivery of robust enzyme nanoparticles for the treatment of drug-induced hepatotoxicity and liver injury. Acta Biomater. 2018;81:231–241. doi:10.1016/j.actbio.2018.09.023
118. Zhao CY, Li Z, Chen JX, et al. Site-specific biomimicry of antioxidative melanin formation and its application for acute liver injury therapy and imaging. Adv Mater. 2021;33(34). doi:10.1002/adma.202102391
119. Yang P, Wang TY, Zhang JH, et al. Manipulating the antioxidative capacity of melanin-like nanoparticles by involving condensation polymerization. Sci China Chem. 2023. doi:10.1007/s11426-023-1542-8
120. Huang Q, Yang Y, Zhu Y, et al. Oral metal-free melanin nanozymes for natural and durable targeted treatment of inflammatory bowel disease (IBD). Small. 2023;19:e2207350. doi:10.1002/smll.202207350
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
