Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Reactive Langerhans Cell Proliferation Mimicking Langerhans Cell Histiocytosis in Association with Sézary Syndrome: A Case Report and Literature Review
Authors Zhang J, Ma S, Yu J, Zheng S, Miao Y , Wang P, Yan X
Received 8 June 2021
Accepted for publication 15 August 2021
Published 24 August 2021 Volume 2021:14 Pages 1023—1028
DOI https://doi.org/10.2147/CCID.S323865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Jinjing Zhang,1 Shiyu Ma,1 Jinxiang Yu,1 Song Zheng,2 Yuan Miao,3 Pingping Wang,1 Xiaojing Yan1
1Department of Hematology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, 110001, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, 110001, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, 110001, People’s Republic of China
Correspondence: Xiaojing Yan
Department of Hematology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, 110001, People’s Republic of China
Tel +86-13889128302
Email [email protected]
Abstract: Sézary syndrome (SS) is a rare type of cutaneous T-cell lymphoma (CTCL) that is characterized by erythroderma, lymphadenopathy and circulating clonal T-cells (Sézary cells). However, to our knowledge, reactive Langerhans cell (LC) proliferation mimicking Langerhans cell histiocytosis (LCH) associated with SS has not been reported. In this report, we describe an unusual case of reactive LC proliferation mimicking LCH associated with SS in a 57-year-old female patient. With complaints of recurrent skin symptoms and enlarged lymph nodes (LNs), she was admitted to our center with a presumptive diagnosis of LCH as demonstrated by LN biopsy pathology. However, other than adenopathy, no lesions were noted in any organ system commonly involved in LCH. Typical Sézary cells were identified through morphology and further confirmed by flow cytometric immunophenotyping in peripheral blood (PB) and bone marrow (BM). In addition, T-cell receptor gene rearrangement was positive, whereas the BRAF V600E gene mutation was negative in skin, LN, PB and BM. The patient was ultimately diagnosed with SS with reactive LC proliferation. This case should remind clinicians to be wary of diagnosing LCH if LCH-like pathology occurs exclusively in LNs. Moreover, morphologic, immunologic, cytogenetic and molecular biologic studies should be performed to avoid misdiagnosis.
Keywords: reactive Langerhans cell, Langerhans cell histiocytosis, Sézary syndrome, TCR gene rearrangement, BRAF V600E
Introduction
Cutaneous T-cell lymphomas (CTCLs) encompass a heterogeneous group of rare non-Hodgkin lymphomas initially arising from malignant T lymphocytes localized in the skin. Sézary syndrome (SS), which is the leukemic variant of mycosis fungoides (MF), accounts for approximately 5% of CTCLs.1 SS is more aggressive than MF and characterized by erythroderma, lymphadenopathy and circulating clonal T-cells with cerebriform nuclei, so-called Sézary cells. Although the presence of large numbers of dendritic cells or Langerhans cells (LCs) mimicking Langerhans cell histiocytosis (LCH) has been described in some types of CTCLs, including MF, CD30-positive anaplastic large-cell lymphoma and lymphomatoid papulosis,2–4 reactive LC proliferation mimicking LCH associated with SS has not been previously reported. Herein, we present a diagnostically challenging case of SS, in which the lymph node (LN) biopsy revealed LC proliferation suspected for LCH.
Case Presentation
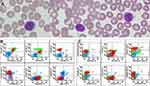
A 57-year-old female presented with longstanding skin symptoms and lesions, including a 6-year history of recurrent pruritus, a 4-year history of papules and painless inguinal lymphadenopathy, and a 2-year history of erythematous skin lesions and exfoliation that began on her legs, back, arms and eventually spread to erythroderma of the whole body, accompanied by keratoderma and fissures on her feet and hands and nail thickening with color change (Figure 1). She received some traditional Chinese medicines and halometasone cream discontinuously before hospitalization, and her skin lesions became more severe. Skin biopsy revealed non-specific dermatitis (Figure 1) without dermal lymphocytic atypia and epidermotropism. Immunohistochemical study revealed that superficial perivascular lymphocytes were mostly positive for CD3 and CD4, sparsely positive for CD8 and nearly negative for CD20. There were somewhat reduced number of CD5 or CD7 positive cells compared with the number of CD3 or CD4 positive cells. There were also a few CD1a positive cells in the superficial dermis. Intraepidermal CD3 or CD4 positive lymphocytes were absent. An inguinal LN biopsy showed extensive involvement of S-100, CD1a and CD207 positive and cyclinD1 negative Langerhans cells in the sinus and paracortical regions, presenting pale staining compared with residual follicles (Figure 2). Meanwhile, CD20 positive B cells in follicles and CD3 positive T cells in paracortex were observed (Figure 2). The patient denied polyuria, pulmonary symptoms, bone pain and fever. Physical examination revealed palpable inguinal and axillary lymphadenopathy. The laboratory tests revealed normal routine blood test results (white blood cell count, 5.42 ×109/L), liver and renal function, thyroid function, sex hormone levels, rheumatic antibody titers, immunoglobulin levels, and arterial blood gas results. Bone imaging of the skull, pelvis, thoracolumbar spine, and limbs and general skeletal ECT did not reveal any bone lesions. The lung computed tomography scan was normal (Supplementary Figure 1), and no hepatosplenomegaly was shown by abdominal ultrasound. LN ultrasound revealed lymphadenopathy involving the bilateral inguinal, axillary and neck LNs, and the largest LN was 3.01×1.37 cm. These findings did not support the initial diagnosis of LCH as evidence of systemic LCH evaluation were absent, except the lymphadenopathy. Given the patient’s erythroderma and lymphadenopathy, SS examinations were performed. A peripheral blood (PB) smear showed Sézary cells (Figure 3A), and flow cytometric immunophenotyping (FCI) of PB cells revealed abnormal mature T lymphocytes accounting for 21.9% (CD4: CD8=15.71), which mainly expressed CD3 (expressing to a lower degree), CD5, CD7, CD4, CD45RO, and TCRαβ; partially expressed CD2; and was negative for CD45RA, TCRγδ, CD25, CD161, CD38, CD16, CD57, HLA-DR, CD56, CD8, CD94, CD10, cKi67, CD34, CD117, or CD33 (Figure 3B). These abnormal mature T lymphocytes had infiltrated into the bone marrow (BM), as detected by FCI, accounting for 4.54% of all nucleated cells (Figure 3C). The CD26 expression was detected during the follow-up, the data showed CD4+CD26- cells accounting for 46.2% of total lymphocytes (Supplementary Figure 2). Moreover, T-cell receptor (TCR) gene rearrangement was found in PB, skin, LN and BM cells, whereas the BRAF V600E gene mutation was negative in any of these samples (Table 1, Supplementary Figure 3). Cytogenetic analysis of BM cells revealed a karyotype of 46, XX [20]. The level of β2-microglobulin (2.07 mg/L) and lactate dehydrogenase (281 U/L) was slightly elevated. Given the clinical presentation and pathological evaluation, FCI and molecular study results, the patient was diagnosed with SS (T4N1bM0B2, IVA1)1 with reactive LC proliferation. The patient received lenalidomide and dexamethasone for 10 months, and skin symptoms decreased.
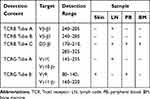 |
Table 1 Results of TCR Gene Rearrangement in Skin, LN, PB and BM Cells |
Discussion
SS, an aggressive and leukemic CTCL, is marked by erythroderma, lymphadenopathy and circulating clonal T-cells (Sézary cells) with marked exfoliation and intense pruritus. The recommended criteria for the diagnosis of SS include one or more of the following: an absolute Sézary cell count of at least 1000 cells/mm3; demonstration of immunophenotypical abnormalities (an expanded CD4+ T-cell population resulting in a CD4:CD8 ratio >10, loss of any or all of the T-cell antigens CD2, CD3, CD4, and CD5, or both); and demonstration of a T-cell clone in the PB by molecular or cytogenetic methods.5 Clinically, the symptoms of the patient presented here included erythroderma and lymphadenopathy accompanied by intense pruritus, exfoliation, keratoderma and fissures on her feet and hands and nail thickening with color change, which are in line with the clinical features of SS. FCI of PB and BM cells revealed an immunophenotype consistent with abnormal mature T lymphocytes, and the absolute Sézary cell count was 1187/mm3. From a molecular perspective, the T-cell clones in the PB, skin, LNs and BM cells showed positive TCR gene rearrangement. Therefore, the patient met all 3 recommended diagnostic criteria for SS and was eventually diagnosed with SS. In addition, the same TCR gene rearrangement was discovered in different samples, which further confirmed the diagnosis of SS and predicted advanced stage disease.6
Though a diagnosis of SS was eventually made, the patient was initially diagnosed with LCH based on the obvious LC proliferation noted in the LN biopsy immunohistochemistry results. LCH is currently regarded as an inflammatory myeloid neoplasia due to the clonal expansion of myeloid precursors and features involvement of one or multiple organs, such as the bone, skin, lung, LNs, thyroid, liver, spleen, BM, and central nervous system.7 However, the skin manifestations of our patient were not consistent with those of skin LCH lesions, which commonly present as papules, intertrigo, nodules or ulcerating lesions with scaling or crusting in the skin folds or anogenital areas.7 We wondered whether the BRAF V600E mutation which was the most common molecular abnormality of LCH was present in our patient. However, the BRAF V600E mutation was not present in LN, PB, skin or BM cells. Previous studies have indicated that cyclinD1 is a useful immunohistochemical marker for differentiating LCH and reactive LC proliferation.8,9 Negative expression of cyclinD1 can also provide evidence for the diagnosis of reactive LC proliferation associated with SS but not for the diagnosis of LCH associated with SS.
There have been only limited reports describing reactive LC proliferation associated with CTCL, and these cases have not included SS.2–4 Reactive LC proliferation mimicking LCH associated with SS has not been reported before. The common clinical features of the four reported CTCL cases with reactive LC proliferation included a variety of long-term skin manifestations, most lasting for several years, which is similar to what was seen in our patient. However, in the previous cases, numerous CD1a-positive LCs were accompanied by malignant T lymphocytes in the same biopsy sample, which was not for our patient.2–4 The atypical histologic features in the skin and numerous LCs infiltrating the LNs of our patient posed a diagnostic challenge for both the dermatologist and hematologist. Although there was no typical malignant T lymphocyte infiltration into the skin and LNs, the presence of TCR gene rearrangement suggested a clonal disease, and consideration of the clinical manifestations was important for achieving the correct diagnosis. The morphology of the cells seen on the PB smear may have been ignored initially due to the lack of abnormal findings on routine blood examination, but the morphology of these cells (a small population of lymphocytes with grooved, lobulated nuclei) ultimately informed the correct diagnosis. FCI, which is a recommended method to detect tumor cells, further confirmed that the abnormal monoclonal T lymphocytes.10 Although CD4+CD7- and CD4+CD26- are the most common immunophenotypes used to identify neoplastic populations in MF/SS, strong expression of CD7 can also be seen in SS.10 Although we did not detect the CD26 expression at diagnosis due to lack of antibody, the follow-up data indicated that the abnormal mature T cells at diagnosis were MF/SS cells. T-cell clonality is commonly detected through polymerase chain reaction amplification of the rearranged TCR gene, followed by confirmation of TCR gene rearrangement with Biomed GeneScan analysis, which is helpful for the diagnosis of SS.11 Therefore, morphologic, immunological, cytogenetic and molecular biologic studies should be performed and the results should be analyzed comprehensively to avoid misdiagnosis.
Interestingly, the same TCR-γ gene rearrangement was detected in the skin and PB cells, whereas the same TCR-β gene rearrangement was identified in the PB, BM and LN cells in our case. However, the same TCR gene rearrangement was not detected in skin, LN, PB and BM cells. Although a lack of identical TCR gene rearrangement in different samples has been reported in previous studies,12 there has been no study comparing the TCR sequence in cells from four different tissues, as we did. However, the importance of differences in TCR sequence remains unknown, although several hypotheses can be considered. First, the differences may be due to the heterogeneity of different tissues, as single-cell profiling can offer insight into the underlying heterogeneity of SS recently.13,14 Second, the differences may be the result of clonal evolution, as the four samples were derived from the same patient at different times during the course of disease.
In summary, we describe an unusual case of reactive LC proliferation mimicking LCH. The atypical histologic features in the skin and numerous LCs infiltrating the LNs of our patient may mask underlying SS and posed a diagnostic challenge for both the dermatologist and hematologist. LCH should be diagnosed with caution if LCH-like pathology occurs only in LNs and not in other common LCH sites, and more importantly, MICM studies should be conducted as an important additional step in combination with clinical and pathologic assessments to avoid misdiagnosis.
Ethics and Consent Statement
This study was approved by Ethics Committee of the First Affiliated Hospital of China Medical University.
Consent for Publication
Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Funding
This work was supported by the National Youth Top-notch Talent of Ten Thousand Program (2014-253). The funding body had no role in the design of the study or collection, analysis, or interpretation of data, nor in writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Whittaker S, Hoppe R, Prince HM. How I treat mycosis fungoides and sezary syndrome. Blood. 2016;127:3142–3153. doi:10.1182/blood-2015-12-611830
2. Christie LJ, Evans AT, Bray SE, et al. Lesions resembling Langerhans cell histiocytosis in association with other lymphoproliferative disorders: a reactive or neoplastic phenomenon? Hum Pathol. 2006;37(1):32–39. doi:10.1016/j.humpath.2005.08.024
3. Ezra N, Van Dyke GS, Binder SW. CD30 positive anaplastic large-cell lymphoma mimicking Langerhans cell histiocytosis. J Cutan Pathol. 2010;37(7):787–792. doi:10.1111/j.1600-0560.2009.01430.x
4. Jokinen CH, Wolgamot GM, Wood BL, Olerud J, Argenyi ZB. Lymphomatoid papulosis with CD1a+ dendritic cell hyperplasia, mimicking Langerhans cell histiocytosis. J Cutan Pathol. 2007;34(7):584–587. doi:10.1111/j.1600-0560.2006.00659.x
5. Vonderheid EC, Bernengo MG, Burg G, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46(1):95–106. doi:10.1067/mjd.2002.118538
6. Vega F, Luthra R, Medeiros LJ, et al. Clonal heterogeneity in mycosis fungoides and its relationship to clinical course. Blood. 2002;100(9):3369–3373. doi:10.1182/blood.V100.9.3369
7. Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood. 2020;135(16):1319–1331. doi:10.1182/blood.2019000934
8. Shanmugam V, Craig JW, Hornick JL, Morgan EA, Pinkus GS, Pozdnyakova O. Cyclin D1 is expressed in neoplastic cells of Langerhans cell histiocytosis but not reactive Langerhans cell proliferations. Am J Surg Pathol. 2017;41(10):1390–1396. doi:10.1097/PAS.0000000000000897
9. Chatterjee D, Vishwajeet V, Saikia UN, Radotra B, De D, Bansal D. CyclinD1 is useful to differentiate Langerhans cell histiocytosis from reactive Langerhans cells. Am J Dermatopathol. 2019;41(3):188–192. doi:10.1097/DAD.0000000000001250
10. Hristov AC, Vonderheid EC, Borowitz MJ. Simplified flow cytometric assessment in mycosis fungoides and Sézary syndrome. Am J Clin Pathol. 2011;136(6):944–953. doi:10.1309/AJCP09OTJOYAVZZK
11. Walia R, Yeung CCS. An update on molecular biology of cutaneous T cell lymphoma. Front Oncol. 2019;9:1558. doi:10.3389/fonc.2019.01558
12. Delfau-Larue MH, Laroche L, Wechsler J, et al. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood. 2000;96(9):2987–2992. doi:10.1182/blood.V96.9.2987
13. Borcherding N, Voigt AP, Liu V, Link BK, Zhang W, Jabbari A. Single-cell profiling of cutaneous T-cell Lymphoma reveals underlying heterogeneity associated with disease progression. Clin Cancer Res. 2019;25(10):2996–3005. doi:10.1158/1078-0432.CCR-18-3309
14. Buus TB, Willerslev-Olsen A, Fredholm S, et al. Single-cell heterogeneity in Sézary syndrome. Blood Adv. 2018;2(16):2115–2126. doi:10.1182/bloodadvances.2018022608
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.