Back to Journals » Drug, Healthcare and Patient Safety » Volume 13
Rational Drug Use Evaluation Based on World Health Organization Core Drug Use Indicators in Ethiopia: A Systematic Review
Authors Mekonnen BD , Ayalew MZ, Tegegn AA
Received 19 March 2021
Accepted for publication 9 July 2021
Published 27 July 2021 Volume 2021:13 Pages 159—170
DOI https://doi.org/10.2147/DHPS.S311926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Siew Siang Chua
Birye Dessalegn Mekonnen,1 Mekuanent Zemene Ayalew,2 Asnakew Asres Tegegn2
1Department of Nursing, Teda Health Science College, Gondar, Ethiopia; 2Department of Pharmacy, Teda Health Science College, Gondar, Ethiopia
Correspondence: Birye Dessalegn Mekonnen Email [email protected]
Background: Rational use of medicines plays a vital role in avoiding preventable adverse drug effects, maximizing therapeutic outcomes with promoting patient adherence, and minimizing the cost of drug therapy. Irrational use of drugs is often observed in countries with weak health care systems. No review has been done that systematically expresses rational drug use practice based on the three WHO core drug use indicators in Ethiopia. Thus, this study was aimed to review systematically the prescribing, health-facility, and patient-care indicators based on WHO core drug use indicators in Ethiopia.
Methods: A systematic article search was conducted in different electronic databases including PubMed/ MEDLINE, the Cochrane Library, EMBASE, Web of Science, POPLINE, the Global Health, and Google scholar. Quality assessment was conducted using Newcastle-Ottawa quality assessment scale. Studies were synthesized and grouped in to prescribing, patient care and health facility indicators.
Results: From a total of 6239 articles, 21 studies were found suitable for the review. The highest average number of drugs per encounter was 2.5 while the lowest was 0.98. The percentage of generic drug use was ranged from 70.5% to 100%. The highest percentage of encounters with an antibiotic was 85%. The lowest percentage of drugs prescribed from essential drugs list was 81.4%. The highest percentage of drugs actually dispensed and adequately labeled was 96.16% and 96.25%, respectively.
Conclusion: This study showed that the practice of rational drug use varied across region of the country. The average number of drugs per prescription, percentage of drugs encounter with antibiotics, drugs prescribed by their generic name, average consultation time, average dispensing time, percentage of drugs adequately labeled, and availability of essential drugs showed deviation from the standard recommended by WHO. Thus, provision of regular training for prescribers and pharmacists, and ensuring the availability of essential drugs should be encouraged.
Keywords: rational drug use, review, World Health Organization, Ethiopia
Background
Rational drug use is defined as the process of appropriate prescribing, and dispensing of drugs for the appropriate patient for diagnosis, prevention, and treatment of diseases.1 Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest possible cost to them and their community.2 The essential goal of rational drug use is to minimize the cost of drug therapy, avoid preventable adverse drug reactions and drug interactions, and enhance the quality of therapeutic care while promoting patient adherence.3,4
Rational use of essential medicines can prevent, treated, or alleviated most leading causes of death and disability in developing countries including Ethiopia.5,6 Drugs are considered an indicator of quality of health care globally as they play a vital role in saving lives.7 The World Health Organization (WHO) has developed prescribing, health-facility, and patient-care indicators to evaluate the practice of rational drug use in health facilities.3 However, the WHO estimates that more than half of all the medicines are prescribing and dispensing inappropriately, and more than half of the patients fail to take the prescribed drugs correctly.8 Evidence also indicates that multi-drug prescribing, overprescribing, misuse of drugs, overuse of antibiotics and injections, and use of unnecessary expensive drugs are most common problems of irrational drug use.9,10
Intervention strategies that can improve rational drug use such as use of clinical guidelines, health providers training, patient and public education, banning unsafe drugs, limiting the import of drugs on the market, availability of essential drug list, and establishment of functional drug and therapeutic committee should be employed.11,12 The state of different countries and other partner organizations have employed with restless efforts to strengthen rational drug use practice in health facilities and community.7,13,14
Despite of efforts have been tried to promote rational drug use, hundreds of millions of people in both developed and the developing countries do not have access to essential medicines, which further leads to inappropriate use of medicines.6,15 Besides, medically inappropriate drugs, ineffective and inefficient use of medicines are commonly occurring in healthcare facilities.16 Inappropriate use of medicines substantially contributing to detrimental effects on health and economic burden.2 Irrational use of medicines is commonly practice due to failure to prescribe in accordance with clinical guidelines, shortage of essential drugs and guidelines, use of too many medicines per patient (polypharmacy), incorrect use of antimicrobials (inadequate dosage, and inappropriate duration, for non-bacterial infections), over-use of injections, inappropriate self-medication, and poor communication between health providers and patients.17,18 Consecutively, over-use of antimicrobials can lead to increase antibiotic resistance which causes hospitalization for a long period of time that results in acquiring a nosocomial infection and increased treatment costs.2,19
Different countries implemented different National Medicine Policy (NMP) to achieve population welfare after WHO has devised a framework to help policy-makers improve access to essential medicines for Universal Health Coverage (UHC) by 2030.20 Ethiopia has also implemented Essential Medicine List and essential drug policy (EDP), and revised in the adaption of the three levels of health care system.21 Many countries are monitoring their National Medicine Policy using different indicators to improve the availability of drugs and quality of labeling, and the efficiency of administration.22 There is limited evidence that show the drug delivery system in health facilities has evaluated regularly in Ethiopia.
In Ethiopia, several fragmented studies have been conducted to investigate the practice of rational drug use in health care facilities using WHO drug use indicators.23–28 However, the studies showed a difference in practice of rational drug use, and to the authors knowledge, the literatures have not been examined systematically. Therefore, this systematic review was aimed to investigate the practice of rational drug use in health care facilities using WHO drug use indicators in Ethiopia.
Methods
Study Design
A systematic review was conducted using Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist (Supplementary file 1).29 The PRISMA checklist was used to identify study articles, screen their titles and abstracts, and to evaluate the full texts for final inclusion.
Eligibility Criteria
Observational studies addressing the practice of rational drug use pattern based on WHO prescribing indicators and conducted in Ethiopia were considered. Both published and unpublished articles were included in the review. Articles written and reported in English language, and provided sufficient data for the review were also considered. Primary studies conducted and reported up to the 4th of December, 2020 were considered. No restriction based on publication status was applied. Conversely, commentary, editorial, letter to the editor, books and book chapter, lecture, qualitative studies, and systematic reviews were excluded in this review.
Information Sources and Search Strategy
A systematic article search was conducted in the following electronic databases: PubMed/ MEDLINE, the Cochrane Library, EMBASE, Web of Science, POPLINE, the Global Health, and Google scholar from 8th of November to the 4th of December, 2020. The search was conducted using the following MeSH and key terms: “medicine” OR “drug” OR “pharmaceutical product” OR “antibiotic” AND “rational” OR “ration” OR “proper” AND “use” OR “prescribe” OR “prescription” OR “dispense” OR “self-medication” OR “self-treatment” OR “polypharmacy” AND Ethiopia (Supplementary file 2). Grey literatures were searched from Google and related government websites. Moreover, the reference lists of identified studies were also reviewed.
Study Selection
Titles and abstracts of articles were independently screened for inclusion eligibility by two reviewers (MZA and AAT). Then, full-text articles were retrieved and screened to confirm eligibility. Differences between reviewers in this process were resolved through discussion with third reviewer (BDM) by full-text evaluation. EndNote X7 was used to manage article selection process, and to remove all duplicates.
Data Collection Process and Data Items
Data extraction was performed by two authors (MZA and AAT) independently using a standardized data extraction form. For each article, name of the first author, publication year, geographical region, study design, aim of study, study setting, average number of drugs per encounter, percentage of drugs prescribed by generic name, percentage of encounters with an antibiotics, percentage of encounters with an injections, percentage of drugs prescribed from essential drug list, average consultation time, average dispensing time, percentage of drugs actually prescribed, percentage of drugs adequately labeled, patients knowledge of correct dosage, availability of copy of essential drugs list or formulary, and availability of key drugs were extracted.
Quality Assessment
The quality of all the studies was evaluated prior to inclusion in the review by undertaking critical appraisal using Newcastle-Ottawa quality assessment scale adapted for cross-sectional studies.30 The assessment graded out of 10 stars (points). The assessment tool contains indicators which are clustered into three sections: The first sections focused on the methodological quality of each study and takes a maximum of five points. The second part of the tool assessed the comparability of the study and weighs a maximum of two points. The last section of the instrument evaluated the outcome measure of interest and weighs a maximum of three points. Finally, studies which has a score of five and above were included for the review. Three reviewers (BDM, AAT and MZA) checked the quality of studies separately. Disagreement among reviewers was resolved through discussion until agreement on all scores was met.
Data Synthesis
Data were managed by undertaking narrative synthesis, and patterns of rational use of drugs were summarized. Studies were tabulated based on all the variables considered likely to be grouped prescribing indicators, patient care indicators and health facility indicators. Rational use of medicine was assessed mainly using quantitative indicators. Data were abstracted in accordance with the key indicators of the WHO manual in their original form without any extra alternations or calculations from the reviewers. The indicators collected were prescribing indicators (percentage of number of drugs per encounter, percentage of drugs prescribed by generic name, percentage of encounters with an antibiotic, percentage of encounters with an injection prescribed, percentage of drugs prescribed from essential drugs list or formulary), patient care indicators (average consultation time, average dispensing time, percentage of drugs actually dispensed, percentage of drugs adequately labeled, patients’ knowledge of correct dosage), and health facility indicators (availability of copy of essential drugs list or formulary, and availability of key drugs). Any discrepancies were discoursed and finalized by the review team members.
Results
Search Results
Initially, a total of 6239 records were retrieved from several databases sources. Of these, 1189 articles were removed due to duplication. From the remaining 5050 articles, 4998 of studies were excluded after screened them using their titles and abstracts. Then, about 52 articles were included for full text screening based on the inclusion criteria. Consequently, 31 articles were excluded for the reason that the outcome of interest was found missing, conducted in other setting and had insufficient information. Finally, 21 articles were included in the final review (Figure 1).
 |
Figure 1 Flow chart of study selection for systematic review of rational drug use evaluation based on WHO drug-use indicators in Ethiopia, 2020. Note: Adatped Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.30 |
Study Characteristics
All studies included to this review used institution-based cross-sectional design, of which eleven were employed both prospective and retrospective cross-sectional study, nine were retrospective cross-sectional study, and one was prospective cross-sectional study. All primary studies reported the number of prescriptions they used for analysis. Hence, a total of 25,933 prescriptions in 21 studies were included for this systematic review. The review was conducted among studies with an estimated sample size ranged from 213 to 6429 prescriptions. The publication year of included studies was from 1998 to 2020. All studies included to this review used WHO drug use evaluation indicators to collect data. As well, eight studies used prescription review, nine studies used prescription review and face to face interview, two studies used observation and face to face interview, and two studies used prescription and drug list review. Concerning geographic distribution, seven studies were conducted from Amhara region,23,27,28,31–34 six were from Oromia region,24,26,35–38 four were from Southern Nations Nationalities and People’s Region (SNNPR),39–42 one was from Tigray region,43 one was from Somali region,25 one was from Harari region,44 and the remaining one was conducted from three regions of the country (Somali, Harari and Dire Dawa),45 There is no study that was conducted in Gambela, Benishangul and Afar regions, and Addis Ababa city administration (Table 1).
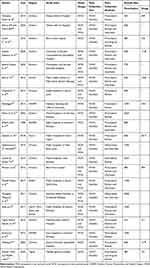 |
Table 1 Characteristics of Primary Studies Included in the Systematic Review of Rational Drug Use Evaluation Based on WHO Drug-Use Indicators in Ethiopia, 2020 |
Assessing Risk of Bias
The risk of bias of each study concerning methodological quality, the comparability of the study, and the outcomes measure of interest was evaluated using the Newcastle-Ottawa assessment scale. Accordingly, the quality scores of each study ranged from six to nine (Table 2).
 |
Table 2 Quality Assessment of Primary Studies Included in the Systematic Review of Rational Drug Use Evaluation Based on WHO Drug-Use Indicators in Ethiopia, 2020 |
Prescribing Indicators
The highest average number of drugs per encounter (2.5) was found in Dessie referral hospital, followed by Debremarkose referral hospital (2.4), while the lowest (0.98) was in Gondar hospital, Northwest Ethiopia. Of the 21 reviewed studies, 18 have reported percentage of drugs prescribed by generic name. Accordingly, the highest percentage of generic drug use was noted in Public hospitals in Gamo Gofa Zone (100%) whereas the lowest percentage of generic drug use was observed in Bahir Dar (70.5%) and Gondar (72.6%) hospitals. The highest percentage of encounters with an antibiotic was reported in Tercha Zonal Hospital (85%). Furthermore, the highest percentage of encounters with an injection prescribed was documented in Sodo Christian Hospital (57.2%) while the lowest was noticed in Jimma University specialized Hospital (2.9%). Fifteen studies have been reported the percentage of drugs prescribed from essential drugs list or formulary with the lowest (81.4%) percentage reported in Bahir Dar hospital (Table 3).
 |
Table 3 Prescribing Indicators in Ethiopia, 2020 |
Patient-Care and Health-Facility Indicators
Of the 21 reviewed primary studies, 12 studies have evaluated patient care indicators. In view of that, the longest average consultation time (10.46 minutes) was recorded in public health centers in Fafen Zone, Eastern Ethiopia. The longest average dispensing time (6.74 minutes) was observed in Health facilities in Jimma zone, whereas the shortest average dispensing time (0.7 minutes) was noted in Debremarkose referral Hospital. The highest percentage of drugs actually dispensed (96.16%) was observed in Debremarkose referral Hospital. Furthermore, the highest percentage of drugs adequately labeled (96.25%) was noted in Asirade Zewudie hospital. The percentage of patients who understand the correct dosage of drugs was highest in a study conducted at selected Health Facilities in Southwest Ethiopia.
From a total of 21 primary studies reviewed, eleven studies have evaluated health facility indicators. Ten of the studies revealed that availability of copy of essential drugs list or formulary was 100%. The availability of key drugs in Bule Hora hospital is 100%, whereas there were no available essential drugs in University of Gondar Comprehensive Specialized Hospital (Table 4).
 |
Table 4 Patient Care and Health Facility Indicators in Ethiopia, 2020 |
Discussion
Irrational prescribing practices and Unwise use of drugs exist all over the globe, predominantly in developing countries including Ethiopia, adversely affecting treatment outcomes.46 Also, poor prescribing practices lead to unsafe and ineffective treatment; increase the length of the disease or getting worse, and danger for the patient; and add more expensive costs.39
The results of this systematic review showed that the average number of drugs per encounter in more than half of the studies is optimal with the levels recommended by the WHO.47 However, the average number of drugs per encounter in nine studies was higher than the levels recommended by WHO. This indicates that poly pharmacy could be a problem in many parts of the country. Similarly, review article done in 11 African countries primary health care centers showed that the average number of drugs per encounter varied across study settings.48
In this review, the highest percentage of drugs that were prescribed by their generic name was noted in a study done in public hospitals of Gamo Gofa Zone (100%). This was consistent with the value recommended by the WHO (100%).47 The proportion of drugs prescribed by generic name in this review were higher than other studies conducted in Saudi Arabia (61.2%),49 United Arab Emirates (19.4%),50 and Palestine (5.5%).51 However, other studies included in this review reported lower percentage of drugs that were prescribed by their generic name than that recommended by the WHO. On the other hand, the lowest percentage of drugs that were prescribed by their generic name was observed in Bahir Dar (70.5%) hospital which is relatively similar with a review article done in 11 African countries primary health care centers that showed the percentage of drugs prescribed by their generic name 68.0%.48 This review suggests the need for improving a better generic prescribing practices. Furthermore, the lower percentage of prescribing drugs by generic name could be attributed to preferences of prescribers to prescribe drugs by brand name, that might result in customer dissatisfaction as dispensing pattern by brand name results in exponentially higher costs for both the health facility and patients.52
In this review, the highest percentage of encounters with antibiotic was reported in Tercha Zonal Hospital (85%) which extremely exceeds the optimum value recommended by WHO (<30%).47 Moreover, only two studies have reported percentage of drugs encounter with antibiotics within the WHO standard. The reason for higher antibiotic prescribing practice could indicate that the prevalence of diseases that require antibiotic for its treatment is higher in Ethiopia. Moreover, this higher antibiotic prescribing rate may indicate a higher likelihood of the development of antibiotic resistance.
In this review, five studies have reported the percentage of drugs encounter with an injection in accordance with the recommended value of WHO standard (13.4–24.1%).47 Based on the study setting, the highest percentage of encounters with an injection was documented in Sodo Christian Hospital, SNNP region (57.2%) followed by Debremarkose referral Hospital, Amhara region of 48.36%. This higher an injection prescribing rate could play a vital role in the transmission of highly serious blood-borne infections, which leads to disability and death.53
The percentage of drugs prescribed from essential drugs list or formulary in two studies was found to be 100% which is similar to the recommended reference value of WHO.47 In this review, the lowest percentage of drugs prescribed from essential drugs list or formulary (81.4%) was reported in Bahir Dar hospital, Amhara region which is slightly lower than a review article done in 11 African countries primary health care centers showed that the proportion of drugs prescribed from the essential drug list was 88.0%.48 The result indicated that the need for improving better adherence to essential drugs list or formulary during prescribing in Ethiopia.
In this review, the longest average consultation time (10.46 minutes) was recorded in public health centers in Fafen Zone, Eastern Ethiopia. However, almost all studies which have evaluated patient care indicators, and documented very shorter than the recommended average consultation time (10 minutes) of WHO.47 Studies included in this review have reported the average dispensing time in minutes. Based on region, the longest average dispensing time (6.74 minutes) was observed in Health facilities in Jimma zone, Oromia region, whereas the shortest average dispensing time (0.7 minutes) was noted in Debremarkose referral hospital, Amhara region. This lower consultation and dispensing times are contributing factors to treatment failure, poor treatment adherence, poor satisfaction of patients with the services, and subsequent drug adverse events. Thus, health care workers have to be made efforts for further improvement. Consecutively, patients’ knowledge of the correct dosage of drugs reviewed in this study were lower than the WHO standard (100%).47 Patient’s knowledge on the dosage of medicine, duration and frequency is essential to prevent underuse, overuse, and misuse of drugs.44
In this review, the percentage of drugs actually dispensed in each study was lower than the WHO standard (100%). The highest percentage of drugs actually dispensed (96.16%) was observed in Debremarkose referral hospital, Amhara region. Furthermore, the highest percentage of drugs adequately labeled (96.25%) was noted in Asirade Zewudie hospital, Amhara region. This proportion was lower than the WHO standard (100%). This attributed to short dispensing times which absolutely affect appropriate labeling and provision of drug information. In addition, this inadequate labeling of medicines may result in failure of treatment and drug toxicity.
In this study, ten of the studies revealed that availability of copy of essential drugs list or formulary was 100% within the WHO standard (100%). This could be indicated better use of available copy of essential drugs list or formulary in Ethiopia. The availability of key drugs in Bule Hora hospital is 100%, whereas there the availability of essential drugs in University of Gondar Comprehensive Specialized hospital was reported as 0%. The lack of essential drugs at the health facilities is detrimental to the patient as the physician or clinician cannot prescribe the right medication. Moreover, the lack of key drugs in the hospital is an indicator of weak pharmaceutical services in health facilities and disrupts the overall provision of health services.
Limitations
Though this review has its own strengths, such as critically appraising the included studies, it has several limitations. Some pertinent data were not reported in some of the studies. The practice of rational drug use pattern based on WHO prescribing indicators varied across the studies, which require further assessment of its pooled estimate. The results of this systematic review may not be representative of all regions of the country because studies were not included all regions of the country. Besides, the differences in prescribers’ wisdom or skill and patient characteristics also influence.
Conclusion
This systematic review showed that the practice of rational drug use varied across region of the country. The average number of drugs per prescription, percentage of drugs encounter with antibiotics, percentage of drugs encounter with injection, drugs prescribed by their generic name, average consultation time, average dispensing time, percentage of drugs actually dispensed, percentage of drugs adequately labeled, and availability of essential drugs showed deviation from the standard recommended by WHO. Thus, this study suggests the need to design strategies to change the trend of drug use patterns, ranging from education, managerial approaches, and regulations and policies. Furthermore, interventions such as provision of regular training for prescribers and pharmacists, establish a drug and therapeutic committee, ensure the availability of essential drugs should be encouraged. Moreover, it is necessary to applicable develop measurement criteria to determine each indicator at the level of health facilities that can be used by health facilities itself to improve health services.
Abbreviations
EDL, Essential Drug List; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; SNNPR, Southern Nations Nationalities and People’s Region; WHO, World Health Organization.
Data Sharing Statement
All relevant data generated and analyzed in the analysis process is included in this article.
Acknowledgments
The authors would like to thank the authors of the included primary studies, which used as source of information to conduct this systematic review and meta-analysis.
Author Contributions
All authors (BDM, MZA and AAT) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Organization WH. Promoting Rational Use of Medicines: Core Components. World Health Organization; 2002.
2. Ofori-Asenso R, Agyeman AA. Irrational use of medicines—a summary of key concepts. Pharmacy. 2016;4(4):35. doi:10.3390/pharmacy4040035
3. Kar SS, Pradhan HS, Mohanta GP. Concept of essential medicines and rational use in public health. Indian J Community Med. 2010;35(1):10. doi:10.4103/0970-0218.62546
4. Koyuncuoglu CZ, Aydin M, Kirmizi NI, et al. Rational use of medicine in dentistry: do dentists prescribe antibiotics in appropriate indications? Eur J Clin Pharmacol. 2017;73(8):1027–1032. doi:10.1007/s00228-017-2258-7
5. Selection WECot, Medicines UoE, Organization WH. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013 (Including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children). World Health Organization; 2014.
6. Bbosa GS, Wong G, Kyegombe DB, Ogwal-Okeng J. Effects of intervention measures on irrational antibiotics/antibacterial drug use in developing countries: a systematic review. Health. 2014;06(02):171–187. doi:10.4236/health.2014.62027
7. Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children’s fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. Am J Trop Med Hyg. 2012;87(5_Suppl):6–10. doi:10.4269/ajtmh.2012.12-0221
8. Almarsdóttir AB, Traulsen JM. Rational use of medicines – an important issue in pharmaceutical policy. Pharm World Sci. 2005;27(2):76–80. doi:10.1007/s11096-005-3303-7
9. Jain S, Upadhyaya P, Goyal J, et al. A systematic review of prescription pattern monitoring studies and their effectiveness in promoting rational use of medicines. Perspect Clin Res. 2015;6(2):86. doi:10.4103/2229-3485.154005
10. Organization WH. WHO Biennial Report: Rwanda Country Office 2012–2013. 2014.
11. Chauhan I, Yasir M, Kumari M, Verma M. The pursuit of rational drug use: understanding factors and interventions. Pharmaspire. 2018;10(2):48.
12. Pronovost PJ. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310(23):2501–2502. doi:10.1001/jama.2013.281334
13. Kamarudin G, Penm J, Chaar B, Moles R. Educational interventions to improve prescribing competency: a systematic review. BMJ Open. 2013;3(8):e003291. doi:10.1136/bmjopen-2013-003291
14. Burrone E, Gotham D, Gray A, et al. Patent pooling to increase access to essential medicines. Bull World Health Organ. 2019;97(8):575. doi:10.2471/BLT.18.229179
15. Organization WH. Promoting Access, Quality and Innovation to Save and Improve Lives: Essential Medicines and Health Products. World Health Organization; 2016.
16. Ocan M, Obuku EA, Bwanga F, et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15(1):1–11. doi:10.1186/s12889-015-2109-3
17. Mao W, Tang S, Chen W. Does perverse economic incentive lead to the irrational uses of medicines? Expert Rev Pharmacoecon Outcomes Res. 2013;13(6):693–696. doi:10.1586/14737167.2013.856266
18. Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: challenges for the internist of the third millennium. J Comorb. 2011;1(1):28–44. doi:10.15256/joc.2011.1.4
19. Olivier C, Williams-Jones B, Doize B, Ozdemir V. Containing global antibiotic resistance: ethical drug promotion in the developing world. In: Sosa AJ, Byarugaba DK, Amábile-Cuevas CF, Hsueh P, Kariuki S, Okeke IN, editors. Antimicrobial Resistance in Developing Countries. Springer: New York; 2010:505–524.
20. Atif M, Malik I, Dawoud D, Gilani A, Ahmed N, Babar Z-U-D. Essential medicine list, policies, and the World Health Organization. In: Babar ZUD, editor. Encyclopedia of Pharmacy Practice and Clinical Pharmacy. Oxford, UK: Elsevier; 2019:239–249.
21. Organization WH. Primary Health Care Systems ( Primasys): Case Study from Ethiopia. World Health Organization; 2017.
22. Hettihewa LM, Isuru A, Kalana J. Prospective encounter study of the degree of adherence to patient care indicators related to drug dispensing in health care facilities: a Sri Lankan perspective. J Pharm Bioallied Sci. 2011;3(2):298. doi:10.4103/0975-7406.80769
23. al. TADe. Assessment of drug use pattern using World Health Organization’s core drug use indicators at Debremarkose referal hospital, Northwest Ethiopia. IJIPSR. 2014;2(7):1270–1288.
24. Angamo MT, Wabe NT, Raju N. Assessment of patterns of drug use by using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in Southwest Ethiopia. J Appl Pharm Sci. 2011;1(7):62.
25. Bilal AI, Osman ED, Mulugeta A. Assessment of medicines use pattern using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in eastern Ethiopia. BMC Health Serv Res. 2016;16(1):1–8. doi:10.1186/s12913-016-1414-6
26. Gebramariam E, Ahmed M. Evaluation of rational medicine use based on WHO core drug use indicators in public hospitals in West Shoa Zone, Oromia, Ethiopia. Adv Pharmacoepidemiol Drug Saf. 2019;8(1):2167. doi:10.35248/2167-1052.19.8.225
27. Getahun KA, Redia AS, Aragaw TJ. Evaluation of medicine-use pattern using World Health Organization’s core drug-use indicators and completeness of prescription at university of Gondar comprehensive specialized hospital, Gondar, Ethiopia: Cross-Sectional Study. Integr Pharm Res Pract. 2020;9:219. doi:10.2147/IPRP.S261320
28. Mamo DB, Alemu BK. Rational drug-use evaluation based on World Health Organization core drug-use indicators in a tertiary referral hospital, Northeast Ethiopia: a cross-sectional Study. Drug Healthc Patient Saf. 2020;12:15. doi:10.2147/DHPS.S237021
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
30. Wells G, Shea B, O′ Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013.
31. Assen A, Abrha S. Assessment of drug prescribing pattern in dessie referral hospital, dessie. Int J Pharm Sci Res. 2014;5(11):777–781.
32. Assen M, Oumer S. Assessment of prescribing pattern in Bourumeda Hospital Northeast Ethiopia. Int J Pharm Sci Res. 2015;6(9):1214–1219.
33. Dessie B, Atalaye G, Diress E, Getahun A. Practice towards rational drug use at finotselam and asirade zewudie hospitals based on WHO core drug use indicators, Northwest Ethiopia. Sci World J. 2020;2020:1–5. doi:10.1155/2020/1634294
34. Desta Z, Abula T, Gebre-Yohannes A, Worku A. Drug prescribing patterns for outpatients in three hospitals in North-West Ethiopia. Ethiop J Health Dev. 2002;16(2):183–189. doi:10.4314/ejhd.v16i2.9809
35. Lenjisa JL, Fereja TH. A retrospective analysis of prescribing practices through WHO prescribing indicators at four selected hospitals of West Ethiopia. J Bioanal Biomed. 2014;6(2):29.
36. Ayen W. Base line survey on drug prescribing indicators for outpatient in Jimma University specialized hospital in South west Ethiopia. Ethiop J Health Sci. 2005;15(2):147–156.
37. Mariam AH, Raghavendra Y, Bobasa EM. Evaluating rational drug use with the help of World Health Organization’s core indicators in Bule Hora Hospital, Southern Ethiopia. encounters. 2015;7(8):11.
38. Teshale C, Hussein J, Mussa S. Assessment of the quality of pharmaceutical service in Jimma Zone, Oromia regional state, South West Ethiopia. Int J Pharm Teach Pract. 2014;5(2):1–6.
39. Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University teaching and referral hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res. 2013;13(1):1–6. doi:10.1186/1472-6963-13-170
40. Dilbato DD, Kuma ZG, Tekle-Mariam S. A baseline survey on prescribing indicators and the underlying factors influencing prescribing in Southern Ethiopia. Ethiop J Health Dev. 1998;12(2).
41. Mensa M, Tadesse T, Ayele A. Assessment of drug use pattern by using WHO core drug use indicators at public hospitals in Ethiopia. J Community Med Health Educ. 2017;7(05):2161–2711. doi:10.4172/2161-0711.1000559
42. Summoro TS, Gidebo KD, Kanche ZZ, Woticha EW. Evaluation of trends of drug-prescribing patterns based on WHO prescribing indicators at outpatient departments of four hospitals in southern Ethiopia. Drug Des Devel Ther. 2015;9:4551. doi:10.2147/DDDT.S83588
43. Yilma Z, Liben M. Assessment of drug prescription pattern in Mekelle general hospital, Mekelle, Ethiopia, using World Health Organization prescribing indicators. Biomed Res Int. 2020;2020:1–6. doi:10.1155/2020/3809157
44. Gashaw T, Sisay M, Mengistu G, Amare F. Investigation of prescribing behavior at outpatient settings of governmental hospitals in eastern Ethiopia: an overall evaluation beyond World Health Organization core prescribing indicators. J Pharm Policy Pract. 2018;11(1):1–11. doi:10.1186/s40545-018-0152-z
45. Sisay M, Mengistu G, Molla B, Amare F, Gabriel T. Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross sectional study. BMC Health Serv Res. 2017;17(1):1–9. doi:10.1186/s12913-017-2097-3
46. Singh T, Banerjee B, Garg S, Sharma S. A prescription audit using the World Health Organization-recommended core drug use indicators in a rural hospital of Delhi. J Educ Health Promot. 2019;8:37.
47. Organization WH. Using Indicators to Measure Country Pharmaceutical Situations: Fact Book on WHO Level I and Level II Monitoring Indicators. Using Indicators to Measure Country Pharmaceutical Situations: Fact Book on WHO Level I and Level II Monitoring Indicators. 2006:xviii, 84–xviii.
48. Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015). BMC Public Health. 2016;16(1):1–14. doi:10.1186/s12889-016-3428-8
49. El Mahalli A. WHO/INRUD drug prescribing indicators at primary health care centres in Eastern province, Saudi Arabia. East Mediterr Health J. 2012;18(11):1091–1096. doi:10.26719/2012.18.11.1091
50. Sharif SI, Alabdouli AH, Sharif RS. Drug prescribing trends in a general hospital in Sharjah, United Arab Emirates. Am J Pharmacol Sci. 2013;1(1):6–9. doi:10.12691/ajps-1-1-2
51. Fattouh R, Abu Hamad B. Impact of using essential drug list: analysis of drug use indicators in Gaza Strip. East Mediterr Health J. 2010;16(8):886–892. doi:10.26719/2010.16.8.886
52. Alam MM, Sikdar P, Kumar A, Mittal A. Assessing adherence and patient satisfaction with medication. Int J Pharm Healthc Mark. 2018;12(4):409–432. doi:10.1108/IJPHM-10-2016-0053
53. Farrell M, Martin NK, Stockings E, et al. Responding to global stimulant use: challenges and opportunities. Lancet. 2019;394(10209):1652–1667. doi:10.1016/S0140-6736(19)32230-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
