Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Rate of HIV Seroconversion Among Seronegative Male Partners Living with HIV Positive Women in Addis Ababa, Ethiopia, 2019: A Retrospective Cohort Study
Authors Bantigen K, Kitaw L , Negeri H , Kebede M , Wassie A , Bishaw K , Tesema G
Received 9 September 2020
Accepted for publication 7 January 2021
Published 3 February 2021 Volume 2021:13 Pages 125—134
DOI https://doi.org/10.2147/HIV.S281281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Kerebih Bantigen,1 Leul Kitaw,1 Haweni Negeri,1 Mekonen Kebede,1 Addisu Wassie,2 Keralem Bishaw,3 Getaye Tesema4
1Nursing and Midwifery, Addis Ababa University, Addis Ababa, Ethiopia; 2Midwifery, Welayita Sodo University, Welayita Sodo, Ethiopia; 3Midwifery, Debre Markos University, Debre Markos, Ethiopia; 4Midwifery, Debreberhan University, Debreberhan, Ethiopia
Correspondence: Kerebih Bantigen
Nursing and Midwifery, Addis Ababa University, Addis Ababa, Ethiopia
Tel +251 991134859
Email [email protected]
Objective: This study aimed to assess rate of HIV seroconversion and predictors among seronegative male partners living with HIV-positive women in Addis Ababa, Ethiopia, 2019.
Methods: Institutional-based retrospective cohort was used to conduct the study. All eligible 227 sample medical records were used for the study. Kaplan–Meier analysis was used to estimate seroconversion time. Cox proportional-hazard regression was used to identify predictor variables.
Results: In this study, 227 seronegative male partners living with HIV-positive women were followed for 60 months retrospectively and 38 (16.7%) seroconversion was observed. The overall seroconversion rate was 6.4 (95%CI: 4.64– 8.76) per 100 person-year observation. Time of antiretroviral therapy (ART) initiation, CD4 level, condom use, and having history of pregnancy after being diagnosed as discordant were identified significant predictors of seroconversion.
Conclusion: The risk of HIV transmission from seropositive partner to seronegative partner in a discordant couple is poorly controlled. Seronegative partners in discordant a couple can be seropositive at any time with influence of predictors unless proper protective measures, counseling, and follow-up are given emphasis.
Keywords: HIV, discordant couple, seroconversion rate, Ethiopia
Introduction
HIV/AIDS epidemic become one of the greatest health challenge that the world had ever faced.1 In 2017, nearly seven million individuals were HIV infected and about 940,000 people died with AIDS-related illnesses globally.2 HIV is more prevalent in Sub-Saharan Africa and the annual HIV transmission from the infected partner to negative partner is high; this is because of high prevalence of a discordant couple.3 The prevalence of an HIV discordant result is high in Africa with HIV-1 discordant results ranging from 36 to 85% in eastern and western Africa.4
In Ethiopia HIV/AIDS is the major cause of death for the productive age population, estimated as 34% of all deaths in ages between 15 and 24 years, and 66% of all deaths in reproductive age groups.5 A cross-sectional study done in Dilla, Ethiopia shows 5.9% prevalence of HIV discordant results and it is more prevalent in young age groups.6
Couples HIV tests and counseling, and results disclosure have significant importance to reduce new infection in discordant results.7 The risk of HIV transmission from seropositive partner to negative partner is very high among discordant couples who do not take protective measures. Therefore, most discordant couples become concordant positive eventually. A study in China shows 2.6% annual seroconversion rate among HIV discordant couples, but a treatment follow-up cohort shows 26% relative decrement in HIV transmission among discordant couples.8 From a retrospective study in China, 125 HIV seroconversion were observed among 1854 discordant couples, 2.52/100 person-years seroconversion.9 A five-year follow-up observational study in China shows 6.3% and 40.14% annual and total seroconversion rate, respectively and 5.87% seroconversion rate observed from other studies.10,11 Among 4813 serodiscordant couples, 127 HIV seroconversion spouses were identified, with a total seroconversion rate of 0.63 per 100 person-years.12 A two-year follow-up cohort study in Masaka, Uganda found new HIV incidence rate of 4.3% per year among discordant couples.13 Other similar follow-up study done in Lusaka, Zambia indicates 17.96% seroconversion with in a two-year follow-up among 2388 discordant couples.14
Half of the new HIV cases in Africa resulted from seroconversion among stable discordant couples.15 In Rwanda and Zambia, about 95% of emerging HIV cases resulted from seroconversion of discordant couples.16 In Sub-Saharan Africa, contribution of outdoor gained HIV infections were less than 10% and most of the negative partners gained the HIV virus from their positive partner.17 The risk of HIV transmission varies according to the HIV and discordant prevalence in the country. The risk of seroconversion is high in countries that are more prevalent.18 Different studies done on this very important topic identify some predictors for seroconversion in discordant couples like poor couples testing and counseling service, inconsistent condom use, high viral load, late initiation of ART, CD4 number, having genital ulcer or sexually transmitted infection (STI) and being younger age were predictors of HIV seroconversion in discordant couples.19,20 However, most studies lack assessment of the risk of HIV seroconversion in the male discordant partner who lives with a seropositive woman, even if sex is considered as one covariate.
HIV seroconversion in discordant couples increases incidence of new HIV infection and this has multidimensional psychosocial impacts like discrimination at work place, loss of employment, fear of discrimination with children in school, bad marital relationship, lack of social support, hopelessness and depression.21 Maternal HIV infection is one cause for poor growth and development of children besides other socioeconomic impacts. Since being discordant increases the risk of HIV transmission to the negative partner.22 In many studies, the rate of HIV seroconversion is more prevalent in the younger age group and this has enormous socioeconomic impact in a country, since youths are an asset and backbone of a country in all socioeconomic aspects.14
HIV/AIDS epidemic has significant impact on country economic development by reduce income of a family, decreasing productivity, reducing labor-hours, and reducing the amount of land cultivated.23 The expense used for ART and opportunistic infection treatment has a negative impact on the development of a country. As of 2017, developing nations considered 21.3 billion US$ for HIV/AIDS reaction.2 Working on discordant couples is one critical measure to reduce HIV transmission from seropositive partner to negative partner among discordant couples.19
Different studies imply that effective discordant couple management approach is crucial to reduce new HIV incidence. The risk of HIV transmission enhanced or reduced by the influence of essential predictors. Despite high hazard of HIV transmission in a discordant couple, research, and follow-up interventions are minimal in Ethiopia. There are no previous studies about the rate of HIV seroconversion among discordant couples in Ethiopia and limited studies have been done on this very important topic. Some of seronegative discordant partners considered themselves as naturally immune or resistant to the virus and this leads them to ignore protective measures and follow-up. Some studies also indicate that the risk of HIV transmission from female to male is less in discordant partners; this misleads the male partner to take the wrong measures. Therefore, this study was designed to assess the rate of HIV seroconversion and predictors among seronegative male partners living with HIV-positive women in Addis Ababa, Ethiopia, 2019.
Methodology
Study Design, Setting, and Period
We conducted a facility based retrospective cohort study in Addis Ababa public health institutions, from February 20 to May 20, 2019. Addis Ababa is the capital city of Ethiopia and seat of the African Union and Economic Commission for Africa. Addis Ababa has a population of 7.178 million as estimated in 2018. There are 11 hospitals and 100 health centers in Addis Ababa. There are also over 34 private hospitals and over 700 private clinics in Addis Ababa city. All hospitals provide inpatient and outpatient service. Most of those institutions provide ART and PMTCT service.
Source and Study Population
The source population was all HIVdiscordant couples tested and who received service in the PMTCT unit of Addis Ababa public health institutions from September 1, 2013 to September 30, 2018. The study population was all eligible medical records of HIV-discordant couples tested and who received service in the PMTCT unit of selected health institutions from September 1, 2013 to September 30, 2018.
Sample Size Determination
All seronegative male partners of HIV-positive woman tested in selected health institutions from September 1, 2013 to September 30, 2018 were screened for inclusion criteria. Six hundred and twenty-eight (628) discordant couples registered in this period. Among them 401 (63.8%) medical charts did not fulfil the inclusion criteria. The majority of them 301 (75.1%) had no partner follow-up. Lost and incomplete medical charts were 42 (10.5%) and 49 (12.2%), respectively. The remaining 9 (2.2%) had less than three months follow-up. Finally, only 227 seronegative male partners living with HIV-positive female partners were eligible for the study. Then all eligible (227) participants included in the study were taken as the final sample size.
Sampling Procedure
Four hospitals and ten health centers were selected randomly by lottery method among public health institutions to provide PMTCT and ART services. In Ethiopia Option B+ PMTCT, registration began in 2013.
All medical charts of discordant couples registered from September 1, 2013 to September 30, 2018 were collected in selected health institutions and screened for completeness, partner visit, and duration of follow-up. Finally, all eligible samples were selected, census sampling was used.
Data Collection Method
Structured checklist prepared from national ART follow-up chart and similar studies were used to review charts retrospectively.9,24 The data was collected from a medical recorded chart of discordant couples. Four BSc nurse data collectors and one MSc nurse supervisor were assigned to collect the data. One-day training on the process of data collection was organized for the data collectors and the assigned supervisor. First all medical charts of a discordant couple recorded from September 1, 2013 to September 30, 2018 were collected and only charts that met the inclusion criteria were selected and reviewed. Discordant partners advised to have a test every six months but the majority of them showed suspension to test with in their appointment. The median time to test the partner for the first time after diagnosis of index case was eight months. This adjournment might be due to delayed disclosure or partner declines the test.
Dependent and Independent Variables
The outcome variable is “Rate of HIV seroconversion” and the independent variables include sociodemographic characteristics, index partner treatments, health status of index partner, personal behavior, and fertility desire.
Conceptual and Operational Definition
Discordant Couple
Discordant couple is a couple in which one partner is HIV positive and other partner is HIV negative in couples HIV test.25
Index Partner
Index partner shows the HIV-positive partner in a discordant couple.
Time of ART Initiation
Early: ART started in CD4 level between 350 and 500 cells/µL or above and Late: ART started in CD4 count <350 cells/µL.26
ART Treatment Adherence
Good: if the adherence is >95% (missing ≤2 doses in 30 dose or ≤3 doses in 60 doses). Fair: 85–94% (missing 3–5 doses in 30 dose or 3–9 doses in 60 dose).Poor: <85% (missing ≥9 doses in 30 doses or >9 doses in 60 doses).24
Incomplete Recorded Charts
Chart that lacks major variables (time of diagnosis as discordant, time of seroconversion, partner's HIV status, CD count, condom use, and history of ART initiation.
Index Partner CD4 Count
Last CD4 count: CD4 number at the end of follow-up. CD4 count at diagnosis: CD4 count while the couple diagnosed as discordant.
Fertility Desire
A women who has an interest in having children in the future.
Having History of Pregnancy
Index partner having history of one or more pregnancies after being diagnosed as seropositive.
Censored
Censored are those seronegative male partners of HIV-positive women who did not develop the outcome of interest, seroconversion at the end of the follow-up period (death, divorced, transferred to other institutions, lost follow-up, and HIV-negative partner).
Event
Occurrence of seroconversion from follow-up period to the end of the study
HIV Seroconversion
“Seroconversion in a discordant couple is considered when a sufficient quantity of HIV antibodies is produced by an individual to become detectable on a given HIV antibody and/or antigen assay“ and diagnosed as HIV positive.27
Time of Seroconversion
The time that the HIV-negative partner was diagnosed as an HIV-positive patient after discordant diagnosis.
Rate of HIV Seroconversion
Number of negative partner who became HIV positive among exposed partners in 100 person-year follow-up.
Data Quality Control
The chart review checklist was adapted from the national ART follow-up chart to address all variables and relevant information. Pretest was done and correction was made on the checklist. Training was organized for data collectors and supervisor on the procedure of data collection and purpose of the study. At the time of data collection, the data was checked daily for completeness, accuracy, and consistency by the principal investigator and timely correction was made.
Data Analysis Method
Data entry and analysis was done by using EpiData version 4.2 and SPSS version 25, respectively. Descriptive statics was used to summarize the result. Kaplan–Meier was used to estimate HIV seroconversion time. Finally, bivariable and multivariable Cox regression analyses were computed for predictor variables. Both crude hazard ratio (CHR) and adjusted hazard ratio (AHR) were used to determine predictor variables with 95%CI and, p-value <0.05 was considered as significant. Time changing covariates: the value of covariates like ART adherence, condom use and CD4 number changed over time. In the counting process the data changing status of those time-varying covariates were minimal. So, the value of the time-varying covariates fixes to a certain time. The baseline data and the most recent value was used. For censored: the baseline data and the last follow-up value was used. For event: the baseline data and the recent value before seroconversion was used. Finally, the value that showed higher significant association was taken.
Results
Among 227 eligible medical records, 84 (37%) were from four hospitals and the remaining 143 (63%) were from ten selected health centers. The median time to test the partner for the first time after diagnosis of index case was eight months.
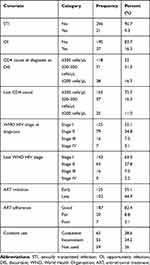
The mean age of participants at the time of diagnosis as discordant was 26.85 years (SD ±4.205). Only 40.9% of seropositive female partners had an educational level of secondary school and above and 26% had no formal education. Regarding employment status, around 95 (41.9%) of the participants were unemployed at the time of diagnosis (Table 1). About 102 (44.9%) of the index partners had late ART initiation. Seven (3.1%) of the participants had poor ART adherence.
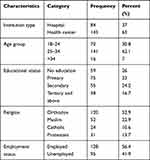 |
Table 1 Sociodemographic Characteristics of Index Partners Tested in the PMTCT Unit of Addis Ababa Selected Public Health Institutions, from September 2013 to September 2018 (n=227) |
Regarding to condom use, the number of a discordant couple who had consistent condom use, inconsistent condom use, and those did not use condoms were nearly similar in frequency.
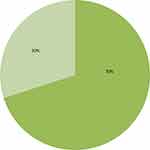
Nearly half of the participants 118 (52%) had CD4 count of ≥350 cells/µL. Regarding the last WHO clinical staging of HIV, more than half 143 (63.0%) of the index partners were stage I. Stage III and Stage IV accounts for 16 (7.0%) and five (2.2%), respectively (Table 2). Among HIV-positive women, nearly onethird 68 (30.0%) of them had history of one or more pregnancies while they were in a discordant relationship (Figure 1).
Seroconversion Status of Seronegative Male Partners
A five-year retrospective cohort with median follow-up time of 32 months was undertaken among 227 seronegative male partners living with HIV-positive female partners. In the overall five-year cohort, 38 (16.7%) seroconversions were observed (Figure 2). The event of seroconversion occurred in all five-year follow-ups. However, the highest number of seroconversion occurs during the first two years of a discordant relationship (Table 3). The overall seroconversion rate was 6.4 (95%CI: 4.64–8.76) per 100 person-year observation. The Kaplan–Meier analysis indicated substantial evidence of differences in survival times. Time of ART initiation, CD4 count, condom use, and having history of pregnancy after being diagnosed as discordant were the major variables that showed significant seroconversion time differences in the Kaplan–Meier estimation. The risk of seroconversion increased though time (Figure 3).
 |
Table 3 Number of Discordant Partners Had Seroconversion in the Five-year Follow-up Cohort |
Predictors of HIV Seroconversion in Male Seronegative Partners
Eighteen independent variables were analyzed in the Cox regression analysis with the dependent variable. Nine variables, which have a P-value of <0.25 in the bivariate Cox, were entered to a multivariate Cox proportional hazard regression analysis. However, only four variables such as time of ART initiation, condom use, last CD4 count, and presence of pregnancy after being diagnosed as discordant were independent predictors.
Delay in ART treatment initiation among HIV-positive woman increases the risk of HIV transmission by 2.5 times to their seronegative partner than early ART treatment initiation (AHR=2.551, 95%CI: 1.009–6.451). Regarding condom use, inconsistent condom use and not using condoms totally were 4.6 and 4.3 times more risk for seroconversion in seronegative male partners than consistent condom use (AHR=4.665, 95%CI: 1.593–13.659) and (AHR=4.346, 95%CI: 1.633–11.571), respectively. Considering CD4 count, last CD4 count of index partner ≤200 cells/µL had 3.1 times more risk for seroconversion in seronegative partner than last CD4 level of >350 cells/µL (AHR=3.121, 95%CI: 1.204–8.091). The risk of HIV seroconversion among seronegative male partners was four times higher when the positive partner had history of one or more pregnancies in their discordant relationship compared to positive partner who had no report of pregnancy throughout their discordant relationship (AHR=4.061, 95%CI: 1.696–9.722) (Table 4).
Discussion
This study aimed to assess the rate of HIV seroconversion and predictors among seronegative male partners living with HIV-positive women. The overall HIV seroconversion rate among seronegative male partners was 6.4per100 person-year follow-up. This study finding showed that discordant partners are at risk of seroconversion unless appropriate measures are be taken. This finding is in line with a study done in Lusaka, Zambia14 and Masaka, Uganda.13 In contrast, the seroconversion rate of this study was lower than the studies done in China.10,28 This difference might be because of sample size difference and ignorance of sex variable in this study. However, this might be due to HIV discordant prevalence and socioeconomic differences between China and the research area of this study. Inconsistent condom use and not using a condom during sex showed a high risk of HIV transmission from seropositive women to their seronegative partners. The finding showed similarity with a study done in China.29 Condoms block entrance of a viral particle to the negative partner and they also prevent STIs, which further increase the risk of HIV transmission by making genital lacerations.30 This evidence showed similarity with a qualitative study done in Jimma, Ethiopia,31 and Addis Ababa, Ethiopia.32
Regarding CD4 count, index partners with a CD4 count of ≤200 cells/µL showed high risk of HIV transmission from seropositive women to their seronegative partners compared to CD4 count of ≥350 cells/µL. CD4 count gets lower when the viral load increases. Index partner with low viral load showed less risk of HIV transmission to their negative partners. This finding shows similarity with studies done in China.9,29 Discontinuation of ART drugs increase the viral load in the index partner and this further increases the risk of HIV transmission to the negative partner.30,33 Index partner who had late ART treatment initiation, CD4 count <350 cells/µL showed higher risk of HIV transmission to their negative partners than those had early initiation of ART treatment. Early initiation of ART treatment controls the virus replication and enhances the positive partner CD4 level. Therefore, the survival time of seronegative partners was increased by early initiation of ART treatment.8,30,33
The finding showed that a woman who had a history of pregnancy after being diagnosed as discordant increases the risk of HIV transmission to their seronegative partner. This is mainly because desire of pregnancy leads to sexual intercourse without a condom. Scientific reports support that pregnancy desire in a discordant couple must be considered under medical advice by considering the viral load. The study showed similarity with studies done in Kenya,34 and Zambia.35
The risk of HIV transmission from seropositive partner to seronegative partner is poorly controlled. Seronegative partners in discordant couples can be seropositive at any time with the influence of predictors unless proper protective measures, counseling and follow-up are taken. Last CD4 count, condom use, time of ART treatment initiation, and occurrence of pregnancy after couple diagnosed as discordant were significantly associated predictors and should get emphasis in the health-care system to reduce the risk of viral transmission. The majority of discordant partners had no regular follow-up; this will increase the risk of seroconversion and requires public health emphasis. However, this study has its own limitation. The majority of participants were excluded from the study by exclusion criteria. Comparison of the result with other findings done in the study area was difficult; there was no similar literature in the study area. It was difficult to categorize participants as cohort and control group; prospective close follow-up under the category will provide better understanding about seroconversion in discordant couples.
Ethical Clearance and Informed Consent
Letter of ethical clearance was taken from Institutional Review Board of Addis Ababa University College of Health Science. Letters of cooperation were written to selected health institutions and permission for data collection was obtained from selected hospitals and health centers. The study was conducted through a review of medical records; the individual participants were not subjected to any harm. The study participants informed consent was waived due to the retrospective nature of the review. The Institutional Review Board of Addis Ababa University, College of Health Science, School of Nursing and Midwifery approved and gave ethical clearance in reference of institutional review number/IRB 047/19/SNM. The data was locked in private computer and accessed by only authors and very essential individuals participated in the study. A coding system was used to maintain anonymity and confidentiality. The authors confirm that this study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable for this study.
Acknowledgment
First, authors gratefully acknowledge Addis Ababa University, College of Health Science, School of Nursing and Midwifery for offering such an opportunity to undertake this study.
Second, we would like to express our heartfelt gratitude to Addis Ababa Health Bureau for their cooperation by providing necessary information to support us to undertake this study.
We are also grateful to all clinicians working on ART adherence office and ART Clinic of selected health institutions. Our thanks also go to my colleagues especially Mr. Grum Sebsibie, Mr. Wudma Alemu, Mr. Tefera Mulugeta, Mr. Ketema Bizuwork, and Mr. Jembere Tesfaye for their commitment to support us throughout our work. Finally, we would like to acknowledge Addis Ababa University institutional repository that store the thesis and allow as to retrieve this article from the thesis. The source of this article is a thesis found in Addis Ababa University Institutional repository. URI: http://etd.aau.edu.et/handle/123456789/21384)
Author Contributions
All authors made significant contribution for this paper and they fulfill the criteria of authorship based on your guideline. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Addis Ababa University, Ethiopia. The funder had no role in the study design, data collection, and analysis, interpretation of data, the decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. World Health Organization: World Health Statistics 2014. WHO; 2014.
2. Moges NA, Kassa GM, Boneya DJ. Rate of HIV transmission and associated factors among HIV-exposed infants in selected health facilities of East and West Gojjam Zones, Northwest Ethiopia; retrospective cohort study. BMC Infect Dis. 2017;17(1):475. doi:10.1186/s12879-017-2578-3
3. Awad SF, Chemaitelly H, Abu-Raddad LJ. Estimating the annual risk of HIV transmission within HIV sero-discordant couples in sub-Saharan Africa. Int J Infect Dis. 2018;66:131–134. doi:10.1016/j.ijid.2017.10.022
4. Lingappa J, Lambdin B, Bukusi E, et al. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS One. 2008;3(1).
5. Central Statistical Authority and ORC Macro. Ethiopia Demographic and Health Survey (2011). Addis Ababa, Ethiopia, and Calverton, MD: Central Statistical Authority and ORC Macro; 2011.
6. Tadesse M. Assessment of HIV discordance and associated risk factors among couples receiving HIV test in Dilla, Ethiopia. BMC Res Notes. 2014;7(1):893. doi:10.1186/1756-0500-7-893
7. Meles H, Wolday D, Fontanet A, et al. Indeterminate human immunodeficiency virus Western blot profiles in ethiopians with discordant screening-assay results. Clin Diagn Lab Immunol. 2002;9(1):160–163. doi:10.1128/cdli.9.1.160-163.2002
8. Jia ZP, Mao YP, Zhang FP, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013;382(9899):1195–1203. doi:10.1016/S0140-6736(12)61898-4
9. Zhang YJ, Feng XX, Fan YG, et al. HIV transmission and related risk factors among serodiscordant couples in Liuzhou, China. J Med Virol. 2015;87(4):553–556. doi:10.1002/jmv.24093
10. Yang RR, Gui X, Xiong Y, Gao SC, Yan YJ. Five-year follow-up observation of HIV prevalence in serodiscordant couples. Int J Infect Dis. 2015;33:179–184. doi:10.1016/j.ijid.2015.02.007
11. Smith MK, Westreich D, Liu H, et al. Treatment to prevent HIV transmission in serodiscordant couples in Henan, China, 2006 to 2012. Clin Infect Dis. 2015;61(1):111–119. doi:10.1093/cid/civ200
12. Chen FF, Wang L, Han J, et al. HIV sero-conversion rate and risk factors among HIV discordant couples in Zhumadian city, Henan province. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(1):10–14.
13. Ruzagira E, Wandiembe S, Abaasa A, et al. HIV incidence and risk factors for acquisition in HIV discordant couples in Masaka, Uganda: an HIV vaccine preparedness study. PLoS One. 2011;6(8):e24037. doi:10.1371/journal.pone.0024037
14. Sullivan PS, Fideli U, Wall KM, et al. Prevalence of seroconversion symptoms and relationship to set-point viral load: findings from a subtype C epidemic, 1995–2009. AIDS (London, England). 2012;26(2):175–184. doi:10.1097/QAD.0b013e32834ed8c8
15. Curran K, Baeten JM, Coates TJ, Kurth A, Mugo NR, Celum C. HIV-1prevention for HIV-1 serodiscordant couples. Curr HIV/AIDS Rep. 2012;9(2):160–170. doi:10.1007/s11904-012-0114-z
16. Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–2191. doi:10.1016/S0140-6736(08)60953-8
17. Chemaitelly H, Abu-Raddad LJ. External infections contribute minimally to HIV incidence among HIV sero-discordant couples in sub-Saharan Africa. Sex Transm Infect. 2013;89(2):138–141. doi:10.1136/sextrans-2012-050651
18. Chemaitelly H, Awad SF, Abu-Raddad LJ. The risk of HIV transmission within HIV-1 sero-discordant couples appears to vary across sub-Saharan Africa. Epidemics. 2014;6:1–9. doi:10.1016/j.epidem.2013.11.001
19. Chen L, Pan X, Yang J, et al. [Incidence rate of HIV transmission in HIV discordant couples in Zhejiang province, 2009–2013]. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36(8):857–861. Chinese.
20. Wall KM, Kilembe W, Vwalika B, et al. Risk of heterosexual HIV transmission attributable to sexually transmitted infections and non-specific genital inflammation in Zambian discordant couples, 1994–2012. Int J Epidemiol. 2017;46(5):1593–1606. doi:10.1093/ije/dyx045
21. Tayal N Poverty and Illness: double edged sword facing people living with HIV/AIDS (Plwha) and HIV discordant couple 2015. 257–263
22. König Walles J, Balcha TT, Winqvist N, Björkman P. Growth pattern in Ethiopian infants - the impact of exposure to maternal HIV infection in relation to socio-economic factors. Glob Health Action. 2017;10(1):1296726. doi:10.1080/16549716.2017.1296726
23. Wondimagegnhu BA An assessment of the socio-economic impact of HIV/AIDS on agricultural production in Ethiopia: the case of Ada’a district in Eastern Showa province in Ethiopia. 2008.
24. FMOH. Ethiopian federal ministry of health, HIV care ART follow-up and intake form
25. WHO. World Health Organization: guidance on couple HIV testing and counseling, including antiretroviral therapy for treatment and prevention in serodiscordant couples, recommendations for a public health approach; 2012.
26. Endalamaw A, Demsie A, Eshetie S, Habtewold TD. A systematic review and meta-analysis of vertical transmission route of HIV in Ethiopia. BMC Infect Dis. 2018;18(1):283. doi:10.1186/s12879-018-3189-3
27. WHO. World Health Organization: Delivering HIV Test Results and Messages for Re-Testing and Counseling in Adults. WHO; 2010.
28. Zheng Z, Li Y, Jiang Y, Liang X, Qin S, Nehl EJ. Population HIV transmission risk for serodiscordant couples in Guangxi, Southern China: a cohort study. Medicine (Baltimore). 2018;97(36):e12077. doi:10.1097/MD.0000000000012077
29. Fang-fang WL CHEN, Juan HAN, Li-yan WANG, Wen-sheng HE, Wei GUO, Jianping ZHOU. HIV sero-conversion rate and risk factors among HIV discordant couples in Zhumadian city, Henan province. Chin J Epidemiol. 2013;34:01.
30. Loutfy MR, Wu W, Letchumanan M, et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS One. 2013;8(2):e55747. doi:10.1371/journal.pone.0055747
31. Nega Jibat M, Berihanu Nigussie M, Selamawit Tesfaye M. Original article socioeconomic challenges and coping mechanisms of HIV serodiscordant couples in Jimma Town, Oromia/Ethiopia. Eur Sci J. 2014;10.
32. Getachew T A struggle to maintain relationship” - sexual life and fertility desire in long-term HIV Sero-discordant couples: a grounded theory study. 2011.
33. Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi:10.1016/S0140-6736(10)60705-2
34. Brubaker SG, Bukusi EA, Odoyo J, Achando J, Okumu A, Cohen CR. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med. 2011;12(5):316–321. doi:10.1111/j.1468-1293.2010.00884.x
35. Wall KM, Kilembe W, Vwalika B, et al. Sustained effect of couples’ HIV counselling and testing on risk reduction among Zambian HIV serodiscordant couples. Sex Transm Infect. 2017;93(4):259–266. doi:10.1136/sextrans-2016-052743
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.