Back to Journals » OncoTargets and Therapy » Volume 12
Radiotherapy in advanced glottic laryngeal carcinoma in a patient with Wegener’s granulomatosis: how much radiation dose is needed?
Authors Lazzari G , Briatico Vangosa A, De Cillis MA, Silvano G
Received 31 July 2018
Accepted for publication 23 November 2018
Published 23 January 2019 Volume 2019:12 Pages 753—757
DOI https://doi.org/10.2147/OTT.S182011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Grazia Lazzari,1 Alessandra Briatico Vangosa,2 Maria Assunta De Cillis,3 Giovanni Silvano1
1Radiation Oncology Unit, San Giuseppe Moscati Hospital, Taranto, Italy; 2Department of Radiology, San Giuseppe Moscati Hospital, Taranto, Italy; 3Otorinolaringoiatry Unit, San Giuseppe Moscati Hospital, Taranto, Italy
Abstract: Wegener’s granulomatosis (WG) is an autoimmune disorder characterized by necrotizing granulomas involving mainly the upper–lower respiratory and renal tracts, albeit a potentially life-threatening involvement of other body parts is not rare. Furthermore, there are several reports accounting for an increased risk of solid malignancies due to the autoimmune disease per se, or the immunosuppressive therapies. Among treatments, radiotherapy could be a therapeutic option as proven by its use in typical WG lesions such as solitary granulomas or subglottic stenosis, successfully treated with low radiation dose. Herein, we report a case of squamous cell carcinoma of the glottis–subglottic larynx, T3 N0 M0 stage, occurring in a patient with a long-standing WG, heavily treated in the past with cyclophosphamide and rituximab, who achieved a complete response of the tumor using a low-dose radiation therapy and no concurrent chemotherapy. The hypothesis is that this cancer probably arose from a subglottic stenosis as a late manifestation of WG and exhibited more radiosensitivity than a naïve tumor. If so, solid tumors occurring on granulomas within an autoimmune disease course should be treated with a lower radiation dose.
Keywords: Wegener’s granulomatosis, ANCA-associated vasculitis, squamous cell carcinoma, cyclophosphamide, rituximab
Introduction
Wegener’s granulomatosis (WG), most recently renamed as granulomatosis with polyangiitis (GPA), is an antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV)1 that usually affects the small and medium vessels mainly in the respiratory tract and kidneys. However, quite a number of various body parts can be interested as skin, eyes, digestive tract, and central nervous system.2,3 In particular, the upper respiratory tract is involved in 70%–100% of cases mainly with symptoms due to otitis, sinusitis, nasal erosions, and subglottic stenosis. Furthermore, solid and hematologic malignancies have been described in the disease’s course probably as a result of the underlying autoimmune disorder per se4 or a consequence of the suitable immunosuppressive therapies.5
In this regard, previous studies have recorded an increased incidence of malignancies among patients with AAV compared with the general population particularly for bladder cancers, malignant lymphomas, leukemia, and nonmelanoma skin cancers,6 while few cases of malignancies in the upper respiratory tract have been described. In particular, only two cases of nasal cavity squamous cell carcinoma (SCC) in WG patients treated with surgery or chemoradiation have been accounted.7,8
The novelty of this report is that such tumors arising from WG lesions could be more radiosensitive than naïve tumors requiring a low radiation dose as provided for solitary granulomas. Herein, we present a case of WG patient suffering from an advanced laryngeal SCC arising from a subglottic stenotic lesion, which resulted in a complete resolution with a low-dose radiation therapy (RT) without concurrent chemotherapy.
Case presentation
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review with the Editor in Chief of this journal. Ethical approval was obtained from the Perrino Hospital of Brindisi Ethic Committee for this report. In August 2011, a 61-year old Caucasian male patient came to our attention complaining of severe dysphonia. His medical history accounted for a WG diagnosed 10 years before on a lobectomy specimen of the left lung. The patient informed us about its course that had been characterized by recurrent episodes of otitis, sinusitis, febrile pneumonia with cough, and hemoptysis during a long chronic course associated with the ANCA titer positivity. As a therapy, the patient had been previously treated with steroids, cyclophosphamide (CYC), and rituximab (RTX). This last therapy had been prescribed as off-label modality between March 2007 and July 2011. As a result, the patient had achieved a clinical benefit and a reduction of the circulating ANCA (c-ANCA) titer.
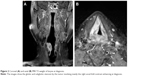
At presentation, a laryngoscopy was conducted and revealed a partial glottic-subglottic stenotic lesion due to a right vocal fold palsy. Moreover, a bilateral glottic thickening with edema and nodular spots in the mucosal surface mainly on the right vocal fold were found (Figure 1). The biopsy showed an infiltrating grade 2 (G2) squamous carcinoma of the glottis involving the right false vocal fold, the ventricular, and the subglottic space. The MRI sequences confirmed these endoscopic findings on the right vocal fold with the glottis–subglottic space reduction. No metastases in the neck lymph nodes were recorded (Figure 2). The total body computed tomography scan did not reveal distant metastases, so the disease was staged T3 N0 M0 according to the American Joint Committee on Cancer (AJCC) seventh edition.
Surgery was advised as a primary therapeutic approach, but the patient refused laryngectomy and accepted a definitive radiotherapy as a second option. The concomitant chemoradiation approach was excluded to minimize the risk of treatment-related acute toxicities. Radiotherapy consisted of a three-dimensional (3D) external beam conformal multiportal technique with multileaf collimator customized (MLC) fields of 6–10 MV photon beams. A first planning target volume included the larynx and bilateral lymph nodes neck levels II, III, and IV treated to a 50 Gy, 2 Gy/fr; then, a boost on larynx with two 6 MV MLC customized opposite fields was prescribed to deliver a 70 Gy total dose. Between the second and third week of RT, the patient began to complain of a worsening dysphagia with weight loss and recurrent febrile episodes; RT was temporarily discontinued with a first stop at 30 Gy delivered dose. An X-ray and CT scan of the chest showed pneumonia in the right lung; the blood count showed leukocytosis (WBC 9.000/mm3) with neutrophilia and severe lymphopenia (10%). The CD19/CD20 (cluster differentiation) mature B lymphocytes were absent. Furthermore, a hypogammaglobulinemia and an ANCA-negative titer were detected. Steroids, antifungal therapy, and antibiotic therapy were effective. Radiotherapy was resumed after an 8-day break.
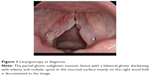
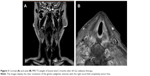
But dysphagia with swallowing impairment and weight loss inexplicably worsened. At 50 Gy delivered dose, the patient underwent laryngoscopy that revealed fixity of the right hemilarynx and mucosal edema with a fibrinous secretion on epiglottis. The swallowing tests showed a loss of oropharyngeal reflex due to nerve IX impairment. The palpatory laryngeal elevation reflex was absent due to nerves X and XI impairment. Palsy of the cranial nerves IX, X, and XI was diagnosed. These findings were suggestive of a neurological symptomatic relapse of the WG. A definitive enteral nutrition through percutaneous gastrostomy was required, and radiotherapy was definitively interrupted at 60 Gy delivered dose. Two months after radiotherapy interruption, an MRI scan of the neck was performed and as a result, it showed an edema of glottis and surprisingly a resolution of the glottic-subglottic cancer (Figure 3). This result was assessed by a control laryngoscopy (Figure 4) and confirmed by a new biopsy. In the suspect of a secondary tumor arising from a WG subglottic stenosis, a revision of the first biopsy specimen was conducted. The revised biopsy provided a diagnosis of an invasive SCC in the context of a fibrotic laryngeal tissue, which is a typical finding of the WG subglottic stenosis. Six months after the RT interruption, the patient developed aspiration pneumonia and an intestinal perforation with peritonitis and a thrombosis of portal veins. The patient died due to systemic fatal complications of an aggressive WG–ANCA-negative relapse.
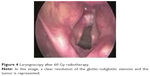  | Figure 4 Laryngoscopy after 60 Gy radiotherapy. |
Discussion
WG is a rare autoimmune disorder defined as a systemic vasculitis affecting small- and medium-sized vessels. It is characterized by inflammatory granulomas with necrotizing vasculitis and by the presence of c-ANCA.1,9 According to the Chapel Hill Consensus Conference 2012, this disease has been included in the group of small vessel vasculitis as AAV and renamed as GPA.10 This disease predominantly affects the upper–lower airways and kidneys. Nevertheless, other organs could be involved as the central nervous system. Malignancies have been proven too.11 It is unclear whether the rheumatic disease per se or the immunosuppressive suitable therapies as CYC and RTX play a role in the risk of cancer development.12,13 In particular, this risk has been accounted for bladder cancer, malignant lymphomas, and nonmelanoma skin cancers.
Up to now, few reports on upper airways cancer in WG patients have been accounted; in fact, two cases of nasal cavity SCC have been described and treated with surgery and radiotherapy.7,8
The novelty of our case consists of a complete resolution of the glottic-subglottic cancer after 60 Gy RT delivered dose and no concurrent cisplatin-based chemotherapy. The standard pattern of care in advanced laryngeal cancer like in our case consists of high-dose radiotherapy with concurrent chemotherapy. In fact, a lot of evidences have provided the curative role of radiotherapy and radiochemotherapy on the local control, survival, and organ sparing in subglottic primary cancer delivering to the gross tumors a total doses of 70–70.2 Gy in 35–39 fractions.14,15 In our case, a tumor staged as T3 N0 M0 was cured with 60 Gy radiation dose without chemotherapy, supporting the hypothesis of a very radiosensitive tumor probably arisen from a subglottic stenosis, which is a typical WG laryngeal lesion.
Subglottic stenosis occurs in 6%–23% of cases; it is considered as a late complication of this disease, mainly occurring in the ANCA-negative WG course as found in our patient. In fact, he was ANCA negative at that time.16 This lesion consists of a fibrosis texture of the mucosal, submucosal, or laryngeal cartilages and has been shown to be very sensitive to radiation. To this regard, Neviani et al described the complete resolution of a refractory WG subglottic stenosis using two courses of conventional fractionated RT at 1-month interval with 20 and 26 Gy, respectively.17 A previous report by Eagleton et al had just provided the effective use of RT with a mechanical dilation of endobronchial obstruction secondary to WG.18
In our case, the revision of the first diagnostic biopsy revealed a squamous cancer within a fibrotic tissue, suggesting a probable origin from the WG subglottic stenotic lesion very sensitive to radiation.
The radiosensitivity of the WG lesions is a well-known finding as described in WG solitary granulomas successfully treated with low-dose radiotherapy, ranging between 40 and 50 Gy with conventional fractionation.19 In this concern, Wygoda et al published a case report of facial solitary granuloma with ethmoidal involvement well responding to a 30 Gy 3D conformal radiotherapy.20
Taken collectively, all these information lead us to speculate the therapeutic response of our case as the effect of a low-dose radiotherapy on a radiosensitive tumor probably arising from a previous asymptomatic subglottic stenosis in a long survivor WG patient treated in the past with CYC and RTX.
Conclusion
We described a case of glottic-subglottic cancer in a WG patient treated with exclusive radiotherapy well responding to a 60 Gy total delivered dose and no concurrent chemotherapy. As an explanation, we expected that this tumor resulted from a previous subglottic stenosis, which have been found as very sensitive to low radiation doses. If so, tumors arising from WG lesions could be considered radiosensitive more than naïve cancers and should be successfully treated with lesser radiation dose to reduce the acute and late normal tissue toxicities.
Acknowledgment
We would like to thank the Radiation Oncology Institute of the San Giuseppe Moscati Hospital for the support.
Disclosure
The authors report no conflicts of interest in this work.
References
Bacon PA. The spectrum of Wegener’s granulomatosis and disease relapse. N Engl J Med Overseas Ed. 2005;352(4):330–332. | ||
Fauci AS, Wolff SM. Wegener’s granulomatosis: studies in eighteen patients and a review of the literature. Medicine. 1973;52(6):535–561. | ||
Kim SH, Park J, Bae JH, Cho MS, Park KD, Jeong JH. ANCA-negative Wegener’s granulomatosis with multiple lower cranial nerve palsies. J Korean Med Sci. 2013;28(11):1690–1696. | ||
Pankhurst T, Savage CO, Gordon C, Harper L. Malignancy is increased in ANCA-associated vasculitis. Rheumatology. 2004;43(12):1532–1535. | ||
Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35(1):100–105. | ||
Knight A, Askling J, Ekbom A. Cancer incidence in a population-based cohort of patients with Wegener’s granulomatosis. Int J Cancer. 2002;100(1):82–85. | ||
Stein J, Sridharan ST, Eliachar I, Niv A, Wood B, Hoffman GS. Nasal cavity squamous cell carcinoma in Wegener’s granulomatosis. Arch Otolaryngol Head Neck Surg. 2001;127(6):709–713. | ||
Kuan EC, Peng KA, Gonzalez LO, Sercarz JA. A case of squamous cell carcinoma of the nasal cavity in a patient with granulomatosis with polyangiitis (Wegener granulomatosis). Ear Nose Throat J. 2018;97(1–2):E37–E41. | ||
Russell KA, Fass DN, Speks U. Clinical and prognostic value of antineutrophil cytoplasmic antibodies in Wegener’s granulomatosis and microscopic polyangiitis: comment on the article by Russell, et al. Reply. Arthritis Rheum. 2002;46(81):279–280. | ||
Jennette JC, Falk RJ, Bacon PA. Revised international Chepel Hill Consensus Conference Nomenclature of vasculitides. Arthritis Rheum. 2012;2013(65):1–11. | ||
Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol. 2016;3(3):122–133. | ||
Rahmattulla C, Berden AE, Wakker SC, et al. Incidence of malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis diagnosed between 1991 and 2013. Arthritis Rheumatol. 2015;67(12):3270–3278. | ||
van Daalen EE, Rizzo R, Kronbichler A, et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2017;76(6):1064–1069. | ||
Lévy A, Blanchard P, Janot F, et al. Résultats de la radiothérapie dans les carcinomes épidermoides du larynx avec atteinte sous-glottique. [Results of definitive radiotherapy for squamous cell carcinomas of the larynx patients with subglottic extension]. Cancer Radiother. 2014;18(1):1–6. French. | ||
Hata M, Taguchi T, Koike I, et al. Efficacy and toxicity of (chemo)radiotherapy for primary subglottic cancer. Strahlenther Onkol. 2013;189(1):26–32. | ||
Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum. 1996;39(10):1754–1760. | ||
Neviani CB, Carvalho HA, Hossamu C, Aisen S, Nadalin W. Radiation therapy as an option for upper airway obstruction due to Wegener’s granulomatosis. Otolaryngol Head Neck Surg. 2002;126(2):195–196. | ||
Eagleton LE, Rosher RB, Hawe A, Bilinsky RT. Radiation therapy and mechanical dilation of endobronchial obstruction secondary to Wegener’s granulomatosis. Chest. 1979;76(5):609–610. | ||
Ziótkowska E, Pietrusi | ||
Wygoda A, Rutwouski T, Skladowski K, Hejduk B. Low dose radiotherapy as an effective treatment in a patient with solitary Wegener’s granulomatosis resistant to systemic treatment – case report. Wspolczesna Onkol. 2013;17(1):107–111. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

 ska E, Biedka E, Makarewicz R. Efeekt radioterapii u chorego na ziarniniaka Wegenera-opis przypadku. Wspolczesna Onkol. 2008;12:406–409.
ska E, Biedka E, Makarewicz R. Efeekt radioterapii u chorego na ziarniniaka Wegenera-opis przypadku. Wspolczesna Onkol. 2008;12:406–409.