Back to Journals » International Medical Case Reports Journal » Volume 15
Radiotherapy After Endoscopic Biopsy in an Adult with Pineocytoma, the Rare Brain Tumor in an Adult: A Case Report and Literature Review
Received 29 March 2022
Accepted for publication 14 June 2022
Published 18 June 2022 Volume 2022:15 Pages 307—311
DOI https://doi.org/10.2147/IMCRJ.S367293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Chi-Chih Hsieh, Jui-Sheng Chen
Department of Neurosurgery, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
Correspondence: Jui-Sheng Chen, Email [email protected]
Abstract: Tumors in the pineal region usually present with acute hydrocephalus. Histologic diagnosis is most important, as it greatly influences treatment, because over 17 tumor types occur in this area. Biopsies of these lesions play an important role in further management. Pineocytomas are benign and rare tumors that typically exhibit a slow progression. However, the appropriate treatment for pineocytoma varies. Surgical excision was considered for good long-term outcomes; however, this may not always be possible. Radiotherapy also appears to be effective in patients with residual pineocytomas. We report a case of pineocytoma with hemorrhagic transformation and complicated hydrocephalus. The patient refused to undergo aggressive tumor excision. Thus, the patient only underwent endoscopic biopsy and external ventricular drain (EVD) implantation, but the outcome was acceptable. In addition, we reviewed the current management strategies for pineocytomas in the literature.
Keywords: pineocytoma, pineal tumor, hydrocephalus, biopsy, subtotal resection
Introduction
Because of the anatomic location of these tumors and their mass effect, 60–90% of patients with pineal tumors have hydrocephalus when the lesion is presented.1,2 More than 17 tumor types arise in this area, which influences the treatment.3 The pineal region tumors could be heterogeneous; thus, initial biopsy samples might not represent the most aggressive portion of the tumor.
Pineal tumors belong to three major groups: germ cell tumors, pineal parenchymal tumors, and tumors originating from adjacent structures. Pineal parenchymal tumors, which can be classified into four categories (pineocytomas, pineoblastomas, papillary pineal tumors, and pineal parenchymal tumors with intermediate differentiation), account for less than 1% of all primary central nervous system tumors and 15–30% of pineal tumors.4 Pineocytomas are slow-growing tumors with relatively better behavior compared to the other three categories. This lesion may affect all age groups, but most commonly in patients aged 30–60 years old.4 The symptoms that are induced by pineal tumors usually cause a mass effect, including hydrocephalus, headache, and diabetes insipidus. Because of the rarity of this lesion, appropriate treatment for pineocytoma remains variable. The treatment of pineocytoma has been discussed in this article.
Case Report
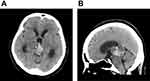
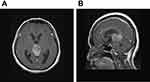
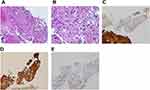
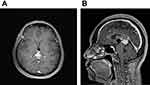
A 51-year-old woman had a history of bilateral benign breast tumors. She experienced sudden onset of headache, dizziness, nausea, double vision, and general weakness. She denied fever, chills, slurred speech, aura, or recent trauma history. On physical examination, clear consciousness, even pupil size with adequate light reflex bilaterally, full muscle power over four limbs, no visual field defect, no setting-sun sign, no obvious range of motion limitation, no proptosis, no nystagmus, no Collier’s sign, no ataxia, and no limb tremors were noted. Computed tomography revealed a heterogeneously enhanced tumor mass over the pineal region of approximately 3.8 cm with ventricular dilatation, a suspected pineal tumor with hemorrhagic transformation, and complicated hydrocephalus (Figure 1). Magnetic resonance imaging (MRI) revealed a lobulated heterogeneous enhancing tumor of the pineal region with foci of hemorrhage and calcifications, suggesting a pineal parenchymal tumor or metastasis. In addition, a mass effect with obstructive hydrocephalus and tectal plate compression were noted (Figure 2). The tumor marker alpha-fetoprotein and beta-human chorionic gonadotropin levels were within normal limits. After discussion with the patient, he did not want to accept the operation for total tumor resection or subtotal tumor resection, unless the tumor was highly malignant. Thus, the patient underwent an endoscopic biopsy and external ventricular drain implantation. In addition, the procedure of converting the external ventricular drain to a ventriculoperitoneal shunt was performed. Pathological examination revealed a hypervascular tumor composed of lobules of tumor cells with uniformly rounded nuclei. A hypercellular lesion but similar to normal pineal gland well differentiated cells was also noted. No necrosis or mitotic cells were observed. No definite papillary structures were observed. Immunohistochemically, the tumor cells were positive for synaptophysin but not GFAP. The Ki-67 proliferation index was very low (Figure 3). Thus, pineocytoma was diagnosed. After discussion with the patient, the patient agreed to receive radiotherapy (54 Gy in 30 fractions) under regular outpatient clinic follow-up. The MRI was followed up 6 months after the operation, and a mild decrease in tumor volume was noted (Figure 4). In addition, the clinical condition of double vision greatly improved during outpatient clinic follow-up. After 3 years of follow-up, the clinical condition was stable, and MRI showed no obvious change in the tumor volume (Figure 5).
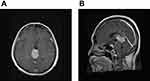 |
Figure 4 (A) T1 weighted with contrast-enhancement axial view. (B) T1 weighted with contrast-enhancement sagittal view. After 6 months of operation, a mild decrease of tumor volume was noted. |
Discussion
Tumors in the pineal region are more common in children (3–8% of pediatric brain tumor) than in adults (0.4–1%).3 Over 17 tumor types occur in the region.3 The appropriate treatment is critically dependent on diagnosis, and precision is paramount. Due to the anatomic location of these lesions and their mass effect, 60–90% of patients with pineal tumors have hydrocephalus at the time of presentation.1,2 The three histologic tumors, that most neoplasms arising within the pineal gland, are germ cell tumors, pineal parenchymal tumors, and gliomas. In one research from the SEER (the surveillance, epidemiology, and end results) database, these showed 59%, 30%, and 5% of patients, respectively.5 The World Health Organization classification of central nervous system tumors divides pineal parenchymal tumors into four groups, which includes pineocytoma (grade I), pineal parenchymal tumors of intermediate differentiation (grade II or III), papillary tumor of the pineal region (grade II or III), and pineoblastoma (grade IV).6 The biopsy for tissue proof played a very important role for further treatment strategy.
Biopsy is performed using two methods: endoscopic and stereotactic procedures. In Balossier’s research,7 stereotactic biopsies showed a better diagnosis rate than endoscopic procedures (93.7% versus 81.8%). However, 60–90% of patients with pineal tumors have hydrocephalus. Compared with endoscopic procedures, stereotactic biopsies might not be suitably performed in hydrocephalic patients because of the risk of postoperative swelling, leading to worse conditions of obstruction and brain herniation. Thus, the stereotactic procedure was a better choice for tissue diagnosis, but endoscopic procedures were more suitable for simultaneous combined biopsy and hydrocephalus management concurrently. In addition, external ventricular drainage may be performed endoscopically simultaneously to manage the hydrocephalus.
Subtotal resection, gross total resection, and radiation therapy are options for pineocytoma management. In Aaron’s research,8 they compared the 1- and 5-year overall survival rates in patients with pineocytoma who underwent biopsy plus radiotherapy, subtotal resection, subtotal resection plus radiotherapy, and gross total resection. Gross total resection showed significantly better outcomes in the 1- and 5-year overall survival rates compared with subtotal resection plus radiotherapy (91% versus 88% and 84% versus 17%, respectively).8 No significant difference was noted between surgical resection and biopsy plus radiotherapy, and the same result was also noted between subtotal resection and subtotal resection plus radiotherapy.8 If gross total resection was possible, this may be a better strategy compared with subtotal resection plus radiation therapy. If gross total resection is not possible or a high risk of mortality and morbidity is considered, biopsy plus radiotherapy or stereotactic radiosurgery might also be the choice of treatment. In Wilson’s research,9 patients with pineocytoma after surgical subtotal resection and adjuvant stereotactic radiosurgery showed significantly longer progression-free survival compared than those who did not undergo adjuvant stereotactic radiosurgery. In Stoiber’s research,10 for long term outcomes, local radiotherapy (biopsy plus radiotherapy, median total dose of 54 Gy) also seems to be effective in patients with pineocytoma. Thus, we administered the radiotherapy dose to our patient.
Conclusion
We present a case of pineocytoma that underwent endoscopic biopsy and ventriculoperitoneal shunt plus radiotherapy. A good clinical outcome was noted after three years of follow-up. The endoscopic approach is the best tool for managing hydrocephalus and biopsy simultaneously. Gross total resection is the most appropriate treatment for pineocytoma. However, for patients who did not want to accept the possibility of aggressive surgery, as in our case, radiotherapy still has a role after the diagnosis was made.
The Patient Consent
The patient provided informed consent for the publication of her case details and accompanying images.
The Institutional Consent
The study have been approved by the institutional review board of the E-Da hospital.
Funding
This study was not supported by any grants.
Disclosure
The authors declare no conflict of interest.
References
1. Robinson S, Cohen AR. The role of neuroendoscopy in the treatment of pineal region tumors. Surg Neurol. 1997;48:360–365. doi:10.1016/S0090-3019(97)00018-9
2. Yamini B, Refai D, Rubin CM, Frim DM. Initial endoscopic management of pineal region tumors and associated hydrocephalus: clinical series and literature review. J Neurosurg. 2004;100:437–441. doi:10.3171/ped.2004.100.5.0437
3. Edwards MS, Hudgins RJ, Wilson CB, Levin VA, Wara WM. Pineal region tumors in children. J Neurosurg. 1988;68:689–697. doi:10.3171/jns.1988.68.5.0689
4. Favero G, Bonomini F, Rezzani R. Pineal gland tumors: a review. Cancers. 2021;13:1547. doi:10.3390/cancers13071547
5. Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors: experience from the SEER database. J Neurooncol. 2009;94:351–358. doi:10.1007/s11060-009-9881-9
6. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi:10.1007/s00401-016-1545-1
7. Balossier A, Blond S, Touzet G, et al. Endoscopic versus stereotactic procedure for pineal tumour biopsies: comparative review of the literature and learning from a 25-year experience. Neurochirurgie. 2015;61:146–154. doi:10.1016/j.neuchi.2014.06.002
8. Clark AJ, Sughrue ME, Ivan ME, et al. Factors influencing overall survival rates for patients with pineocytoma. J Neurooncol. 2010;100:255–260. doi:10.1007/s11060-010-0189-6
9. Wilson DA, Awad AW, Brachman D, et al. Long-term radiosurgical control of subtotally resected adult pineocytomas. J Neurosurg. 2012;117:212–217. doi:10.3171/2012.5.JNS1251
10. Stoiber EM, Schaible B, Herfarth K, et al. Long term outcome of adolescent and adult patients with pineal parenchymal tumors treated with fractionated radiotherapy between 1982 and 2003–a single institution’s experience. Radiat Oncol. 2010;5:122. doi:10.1186/1748-717X-5-122
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.