Back to Journals » International Journal of Nanomedicine » Volume 15
Radiotherapy-Activated Hafnium Oxide Nanoparticles Produce Abscopal Effect in a Mouse Colorectal Cancer Model
Authors Zhang P, Darmon A, Marill J, Mohamed Anesary N, Paris S
Received 19 February 2020
Accepted for publication 21 April 2020
Published 29 May 2020 Volume 2020:15 Pages 3843—3850
DOI https://doi.org/10.2147/IJN.S250490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Ping Zhang,* Audrey Darmon,* Julie Marill, Naeemunnisa Mohamed Anesary, Sébastien Paris
Nanobiotix, Paris, France
*These authors contributed equally to this work
Correspondence: Sébastien Paris
Email [email protected]
Purpose: Despite tremendous results achieved by immune checkpoint inhibitors, most patients are not responders, mainly because of the lack of a pre-existing anti-tumor immune response. Thus, solutions to efficiently prime this immune response are currently under intensive investigations. Radiotherapy elicits cancer cell death, generating an antitumor-specific T cell response, turning tumors in personalized in situ vaccines, with potentially systemic effects (abscopal effect). Nonetheless, clinical evidence of sustained anti-tumor immunity as abscopal effect are rare.
Methods: Hafnium oxide nanoparticles (NBTXR3) have been designed to increase energy dose deposit within cancer cells. We examined the effect of radiotherapy-activated NBTXR3 on anti-tumor immune response activation and abscopal effect production using a mouse colorectal cancer model.
Results: We demonstrate that radiotherapy-activated NBTXR3 kill more cancer cells than radiotherapy alone, significantly increase immune cell infiltrates both in treated and in untreated distant tumors, generating an abscopal effect dependent on CD8+ lymphocyte T cells.
Conclusion: These data show that radiotherapy-activated NBTXR3 could increase local and distant tumor control through immune system priming. Our results may have important implications for immunotherapeutic agent combination with radiotherapy.
Keywords: CD8+ T cells, antitumor immune response, TILs, NBTXR3, radioenhancer
Introduction
Since decades, radiotherapy (RT) is one of the main treatments for many cancer types, employed to locally destroy cancerous cells and achieve substantial tumor debulking. About 60% of all cancer patients will receive irradiations as part of their treatment.1 Beyond the ability of RT to kill cancer cells by production of free radicals and generation of single and double-strand breaks in DNA,2 preclinical and clinical studies have demonstrated that RT can prime an immunomodulatory response.3,4 For example, RT can stimulate MHC class I expression on cancer cells,5 induce the immunogenic cell death6,7 important for CD8+ cytotoxic T lymphocyte activity,8,9 and also activate expression of various pro- and anti-inflammatory cytokines and adhesion molecules, allowing recruitment and activation of both innate and adaptive immune cells into the tumor.10 Unfortunately, RT rarely produces a sustained anti-tumor response as immune escape frequently occurs with tumor recurrence.11,12 Moreover, the so-called “abscopal effect” which corresponds to reduction of metastatic burden outside the irradiated area is rarely observed after radiotherapy.13–16 Finally, because of toxicity to surrounding healthy tissue, the maximum dose of irradiation will always be limited.
The high electron density of functionalized hafnium oxide nanoparticles (NBTXR3)17,18 allows a high probability of interaction with incoming ionizing radiation, increasing energy dose deposit within cells. Thanks to this primary physical mode of action, we have previously reported in preclinical studies the increased ability of RT-activated NBTXR3 (NBTXR3+RT) to induce cancer cell death and control tumor growth, compared to RT alone.17,19 We recently reported that NBTXR3+RT can enhance cGAS-STING pathway activation in human colorectal cancer cells.20 Further, NBTXR3+RT demonstrated clinically meaningful benefit for patients with locally advanced Soft Tissue Sarcoma compared to RT alone, in the randomized controlled Phase II/III Act.in.Sarc study (NCT02379845).21
In this article, we evaluated the impact of NBTXR3+RT on the anti-tumor immune response. We demonstrated the ability of NBTXR3 to kill more cancer cells and to induce an abscopal effect. Examination of immune cell infiltrates in treated and untreated tumors revealed that NBTXR3+RT can increase the infiltrates of CD8+ T cells in both tumors. We also show that the abscopal effect produced by NBTXR3+RT is dependent on CD8+ T-cells. Taken together, these results indicate that NBTXR3+RT can efficiently prime an anti-tumor immune response, which could have important implications for the use of NBTXR3+RT with immunotherapy.
Materials and Methods
Cells and Reagents
CT26.WT cells were purchased from the ATCC (#CRL-2638), cultivated according to the provider’s recommendations and screened for mycoplasma (CleanCells). NBTXR3 (Nanobiotix) is a sterile suspension of functionalized HfO2 spherical nanoparticles with a size centered on 50 nm, bearing a marked negative surface charge (–50 mV) in aqueous solution at pH 6–8.17
Mice
Six-week-old BALB/c female mice (Janvier Labs) were maintained under pathogen-free conditions in the animal facility at Gustave Roussy Institute (Villejuif, France). All animal experiments were carried out in compliance with French and European laws and regulations (European Directive 2010/63 EU). The local institutional animal ethics board and French Ministère de la Recherche approved all mouse experiments (permission numbers: 2016_031_4340 and 2016_129_8344).
Irradiation
For in vitro experiments, X-ray irradiations were delivered using an X-Ray generator at 125 kV (CellRad, Faxitron). For in vivo assays, tumor irradiations were performed with a 200 kV Varian NDI 226 X-ray irradiator. Selective irradiation of the tumor on mice was performed by the interposition of a lead shield, allowing full protection of the rest of the body, including proximal lymph nodes.
Cell Death Analysis
CT26.WT cells were seeded in duplicate in 6-well plates incubated overnight with or without 400 µM NBTXR3 nanoparticle suspension, then irradiated with a single fraction of 2, 3, 4, 5 or 6 Gy. After 48 h, cells were harvested, centrifuged then resuspended in 100 µL of 1× Binding Buffer per 106 cells, containing 10 µL Annexin V Conjugated to FITC (Annexin V-FITC Kit, Miltenyi Biotec, Germany). Cells were incubated in the dark at room temperature for 15 min; then, 1 µg/mL propidium iodide was added to the cell suspension, followed by flow cytometry analysis on Accuri C6 (BD Biosciences).
Intratumoral Bioavailability and Persistence of NBTXR3
Mice were subcutaneously injected into the flank with 3.105 CT26.WT cells to generate a single tumor. Once tumor volume was comprised between 50 and 120 mm3, NBTXR3 suspension equivalent to 25% of the baseline tumor volume was delivered by intratumoral injection. The next day, intratumoral NBTXR3 bioavailability was confirmed by µCT-scan (Voxcan, France). One-week post-i.t., a second CT-scan was performed to confirm NBTXR3 intratumoral persistence.
In vivo Experiments
For the abscopal assay, 3.105 CT26.WT cells were subcutaneously injected into both flanks of mice on the same day. Once the tumor had grown (50 to 120 mm3), mice were randomized to the different groups. A volume of NBTXR3 suspension (or vehicle) corresponding to 25% of the baseline tumor volume was delivered intratumorally into the right flank tumor only (ie, the left flank tumor was untreated). After 24 h, the right tumors were irradiated with 4Gy per fraction for 3 consecutive days. Length (L) and width (W) of tumors were measured 2–3 times per week using a digital caliper. Tumor volumes were calculated using the formula (L×W2/2). To evaluate the role of CD8+ T cell, an abscopal assay was implemented as previously described. The CD8+ T cells were depleted in some mice treated with NBTXR3+RT, by intraperitoneal injections of anti-CD8 (Ref. BP0004-1, clone 53-6.7, BioXcell) (100 µg/mice) at D-1, D1, D4, D7, D11 and D14. For both experiments, mice were sacrificed when one tumor reached 800mm3.
Immunohistochemistry and Digital Pathology Analysis
For immunohistochemistry analyses, the same schedule than for abscopal assays was followed, except that animals were sacrificed 72 h after the last dose of irradiation. Treated and untreated tumors were immediately excised then fixed. For each tumor, 3 slices of 4 µm from FFPE blocks (first third, middle and last third of the tumor) were put on the same slide and slices were stained using specific antibodies raised against CD4 (#50134-R0001, Interchim), CD8 (#AB203035, Abcam) or CD68 (#AB125212, Abcam) on Ventana Discovery XT autostainer and stained by haematoxylin/eosin/safran on Leica ST5020 multistainer. For digital pathology analysis, each stained slide was scanned with Aperio AT Turbo x40 (Excilone, France).
Statistical Analysis
In vitro studies have been independently repeated at least three times. Results are expressed as mean ± SEM. Normality distribution of values was assessed by Shapiro–Wilk normality test. Experiments with normal distribution were analyzed by two-tailed t-test. Experiments with non-normal distribution were analyzed by Mann–Whitney test. A p value <0.05 was considered statistically significant. For in vivo studies, mean tumor volume was calculated for each group and used for drawing growth curves. The overall survival has been determined by the analysis of Kaplan–Meier curves and calculation of the median survival. Statistical analyses of mean tumor growth curves and Kaplan–Meier curves were performed using Two-Way ANOVA test and Log-rank (Mantel-Cox) test, respectively. Data are reported as means ± SEM. The software GraphPad Prism 7® v.7.04 was used for graph plotting and biostatistics.
Results
NBTXR3+RT Efficiently Kill Cancer Cells
We first examined the ability of NBTXR3+RT to kill cancer cells, compared to RT alone. The different cellular physiological states (eg, viability, early apoptosis, early necrosis and late apoptosis/necrosis) were measured 48h after treatment by Annexin V-FITC/propidium iodide staining and flow cytometry (Figure 1). For cells treated with NBTXR3 alone, viability (98.1%±0.65) was the same than for untreated control cells (98.1%±0.37), showing the non-toxicity of the nanoparticles. Compared to RT alone, treatment with NBTXR3+RT led to a significant decrease in cell viability due to enhancement of early apoptosis, early necrosis and late apoptosis/necrosis (Figure 1A–D). The effect of NBTXR3+RT was particularly marked for early apoptosis from 2Gy (Figure 1C). Interestingly, the decrease of cell viability achieved with NBTXR3+4Gy was greater than 6Gy alone, highlighting the radioenhancement ability of NBTXR3. These results are in good accordance with our previously published results of clonogenic assay with various human cancer cells.19
NBTXR3 Remains in the Tumor
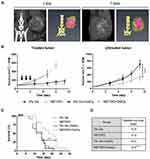
In patients, NBTXR3 is delivered via intratumoral injection. To evaluate the dispersion and persistence of these nanoparticles in our in vivo tumor model, mice bearing a CT26.WT subcutaneous tumor received an intratumoral injection of NBTXR3. Due to the high density of hafnium that composes NBTXR3, the nanoparticles are easily detectable by Micro Computed Tomography (µ-CT) scan, within tissues (Figure 2A). The day after injection, µ-CT scan showed that nanoparticles were distributed in a large part of the tumor. One week after the injection, these nanoparticles remained in the tumor of the same animal, demonstrating the good the persistence of the nanoparticles in the tumor.
NBTXR3+RT Produces an Abscopal Effect
To evaluate the ability of NBTXR3+RT to produce an abscopal response, we used mice bearing subcutaneous tumor on both flanks. The right tumors were treated with or without NBTXR3 then irradiated (or not), whereas the left tumor remained untreated. The treated tumor growth curves for RT and NBTXR3+RT groups were similar, and clearly showed the effect of the RT (Figure 2B, left panel). Despite the clear impact of RT alone on treated tumor growth curve, no abscopal effect has been produced, as the untreated tumor growth curve for this group was identical to controls (Figure 2B, right panel). In stark contrast, a significant abscopal effect was obtained in mice treated with NBTXR3+RT (p<0.001). In addition, a significant lengthening of the lifespan of animals treated with NBTXR3+RT was obtained (median survival 19.5 vs 14 for NBTXR3+RT and RT alone, respectively, p<0.01), while survival for the other groups was similar (Figure 2C and D).
NBTXR3+RT Increases Immune Cell Infiltrates in Both Tumors
To further investigate the mechanism driving the NBTXR3+RT abscopal effect, we repeated the experiment by sacrificing the animals 3 days after the last fraction of irradiation and measured CD4+, CD8+ and CD68+ cell densities in both tumors (Figure 3A). For CD4+, only irradiated tumors showed a marked difference compared to control groups. Nonetheless, no significant difference between RT and NBTXR3+RT has been observed. In contrast, significant increases in CD8+ and macrophages (CD68+) infiltrates (p<0.05) were observed in both treated and distant untreated tumors in NBTXR3+RT mice, compared to RT alone.
NBTXR3+RT Abscopal Effect Is Dependent on CD8+ Cells
To determine the role of CD8+ cells in the abscopal effect produced by NBTXR3+RT, we repeated an abscopal assay, including a group of mice previously depleted of their CD8+ cells before treatment with NBTXR3+RT. For the groups not depleted in CD8+ cells, the results were similar to those previously obtained, with a marked abscopal effect for the group of mice treated with NBTXR3+RT (Figure 3B). Interestingly, depletion of CD8+ cells completely abolished the abscopal effect. This shows that CD8+ cells drive the abscopal effect induced by RT-activated NBTXR3.
Discussion
In this article, we explored the impact of NBTXR3 activated by RT on the anti-tumor immune response. We demonstrated that NBTXR3 activated by RT more efficiently induced cancer cell death than RT alone, mainly by a significant increase of apoptosis. We also showed that NBTXR3+RT significantly increased necrosis, while RT alone had almost no impact. Interestingly, these results suggest that NBTXR3+RT amplified the main cell death mechanism triggered by RT but was also able to modulate another cell death pathway in these cells. This ability of NBTXR3 to modulate biological pathways set in motion by RT is consistent with our previous work on the enhancement of the cGAS-STING pathway in colorectal cancer cells.20 On the other hand, it is interesting to note that cell death levels induced by the highest doses of radiotherapy alone can be achieved (or even exceeded) with much lower radiotherapy doses, in the presence of NBTXR3 (eg, NBTXR3+4Gy vs 6Gy). These data demonstrate NBTXR3 radioenhancement ability, which opens interesting possibilities for RT dose reduction in clinical trials. Indeed, it has been shown by Bonvalot et al21 that NBTXR3 had a good safety profile. However, the expected adverse events of RT persist. If a reduced dose of RT with NBTXR3 can be as efficient as a standard dose of RT without NBTXR3, this made it possible to reduce the adverse events due to RT, thus significantly improving the quality of life of the patients.
NBTXR3 is administered by intratumoral injection and it was important to evaluate the distribution and persistence of the nanoparticles in tumors after administration. The μ-CT imaging showed both good distribution and persistence of nanoparticles in the tumor after at least 1 week. These results are in good agreement with previously published data,17 indicating that nanoparticles will be present at least throughout the duration of RT treatment of in vivo studies.
One of the desired effects after RT is the generation of a strong anti-tumor immune response able to generate an abscopal effect. Unfortunately, this effect is rarely observed in patients.22 Based on previous preclinical and clinical results,17,19-21 we hypothesized that the radio-enhancement effect of NBTXR3 may be strong enough to generate this effect. To test this hypothesis, we used immuno-competent mice carrying a subcutaneous tumor on both flanks. Only right tumors received treatment, while left tumors remained untreated. Thanks to this approach, we demonstrated that tumors treated with NBTXR3+RT produced a significant abscopal response. The generation of this abscopal effect is particularly noteworthy since treated tumor growth control was similar for RT alone and NBTXR3+RT groups. This suggests that tumor microenvironment modifications triggered by NBTXR3+RT treatment were stronger (and/or different) to prime an anti-tumor immune response able to produce an abscopal effect, compared to radiotherapy alone. This hypothesis is supported by the comparison of immune cell densities in the treated and distant untreated tumors for the different groups. Remarkably, we measured a significant increase in CD8+ infiltrates (known to play a central role in cancer cell destruction) both in treated and untreated tumors of NBTXR3+RT group, compared to RT alone. We also demonstrated that this abscopal effect was highly dependent on CD8+ cells, as their depletion results in the complete abolishment of the abscopal effect.
Conclusion
Taken together, our results clearly demonstrate the benefit of NBTXR3 on the antitumor immune response activation, particularly at the level of adaptive response mediated by CD8+ cells. NBTXR3 activated by RT efficiently transformed the tumor in an in situ vaccine, with systemic effect on a distant untreated lesion. As such, NBTXR3 could be an innovative solution to overcome the current limitations of RT. This study may have important implications for the combination of immunotherapeutic agents with radiotherapy.
Abbreviations
RT, radiotherapy; µCT, micro X-ray computed tomography.
Acknowledgments
The authors thank Dr. Katherine Jameson, Clinical Lead at Nanobiotix for proofreading this article and wise advice.
Disclosure
P. Zhang, A. Darmon, J. Marill, N. Mohamed Anesary, and S. Paris are employees of Nanobiotix. The authors report no other conflicts of interest in this work.
References
1. Harrington KJ, Billingham LJ, Brunner TB, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer. 2011;105(5):628–639. doi:10.1038/bjc.2011.240
2. Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5(173):173sr172. doi:10.1126/scitranslmed.3005148
3. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2(6):286–294. doi:10.1016/j.trecan.2016.05.002
4. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–379. doi:10.1038/nrclinonc.2016.211
5. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi:10.1084/jem.20052494
6. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi:10.1146/annurev-immunol-032712-100008
7. Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25(1):11–17. doi:10.1016/j.semradonc.2014.07.005
8. Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4(8):858–864. doi:10.1007/s11671-009-9334-6
9. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi:10.4049/jimmunol.174.12.7516
10. Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–523. doi:10.1667/RR3031.1
11. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi:10.1001/jama.2016.4059
12. Cummings B, Keane T, Pintilie M, et al. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85(1):7–16. doi:10.1016/j.radonc.2007.09.010
13. Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734.
14. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi:10.1016/j.ijrobp.2003.09.012
15. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi:10.1158/1078-0432.CCR-09-0265
16. Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi:10.1016/S1470-2045(09)70082-8
17. Maggiorella L, Barouch G, Devaux C, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8(9):1167–1181. doi:10.2217/fon.12.96
18. Pottier A, Borghi E, Levy L. New use of metals as nanosized radioenhancers. Anticancer Res. 2014;34(1):443–453.
19. Marill J, Anesary NM, Zhang P, et al. Hafnium oxide nanoparticles: toward an in vitro predictive biological effect? Radiat Oncol. 2014;9:150. doi:10.1186/1748-717X-9-150
20. Marill J, Mohamed Anesary N, Paris S. DNA damage enhancement by radiotherapy-activated hafnium oxide nanoparticles improves cGAS-STING pathway activation in human colorectal cancer cells. Radiother Oncol. 2019;141:262–266. doi:10.1016/j.radonc.2019.07.029
21. Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, Phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–1159. doi:10.1016/S1470-2045(19)30326-2
22. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1):25–37. doi:10.1016/j.currproblcancer.2015.10.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.