Back to Journals » OncoTargets and Therapy » Volume 13
Radiofrequency Ablation with Continued EGFR Tyrosine Kinase Inhibitor Therapy Prolongs Disease Control in EGFR-Mutant Advanced Lung Cancers with Acquired Resistance to EGFR Tyrosine Kinase Inhibitors: Two Case Reports
Authors Shi X , Zhou J, Qian C, Gao L, Wang B, Feng X
Received 8 April 2020
Accepted for publication 5 June 2020
Published 13 July 2020 Volume 2020:13 Pages 6789—6793
DOI https://doi.org/10.2147/OTT.S257431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Xuefei Shi, Jia Zhou, Caihua Qian, Liliang Gao, Bin Wang, Xueren Feng
Department of Respiratory Medicine, Huzhou Centre Hospital, Affiliated Centre Hospital Huzhou University, Huzhou, People’s Republic of China
Correspondence: Xueren Feng
Department of Respiratory Medicine, Huzhou Centre Hospital, Affiliated Centre Hospital Huzhou University, Huzhou, People’s Republic of China
Tel/ Fax +86 572 2555775
Email [email protected]
Objective: Lung cancer remains the leading cause of malignant tumor-related death globally. There is mounting evidence that a large proportion of patients harboring epidermal growth factor receptor (EGFR) mutation and treated with EGFR TKI experience oligoprogressive disease. The optimal treatment strategy for these patients is undetermined. Thus, in this article, we report two cases of EGFR-mutant NSCLC patients with locally resistant lesions achieving disease control via combination therapy.
Patients and Methods: We present two cases of lung adenocarcinoma patients that developed oligoprogressive disease during TKI treatment. For further treatment, the patient then received radiofrequency ablation.
Results: Through follow-up observation, we found that the addition of radiofrequency ablation might provide the clinical benefit of these two NSCLC patients.
Conclusion: Our two cases provide a promising treatment for oligoprogressive disease during the first-line EGFR-TKI therapy.
Keywords: radiofrequency ablation, EGFR-mutant non–small-cell lung cancer, oligoprogressive disease, first-line EGFR-TKI therapy
Introduction
On the basis of data collected by the National Cancer Institute (NCI) in 2018, lung cancer remains the second most diagnosed cancer and the foremost cause of cancer-related death in both women and men.1 Lung cancer continues to be a major public health problem worldwide due to the 5-year survival rate being as low as 15%.2 In recent years, the classification of lung cancer is more and more accurate based on the result of modern molecular biology techniques. Subsequently, the treatment of lung cancer becomes personalized with the development of molecular-targeted agents. Some examples of the more well-established include epidermal growth factor receptor (EGFR), K-Ras (KRAS) and anaplastic lymphoma kinase (ALK).3,4 It was reported that mtEGFR NSCLC respond dramatically to EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib.5 However, most patients undergo acquired resistance and disease progression usually within 9–14 months.6 A plenty of studies is ongoing to explore the mechanism of this acquired resistance. Nowadays, several mechanisms of resistance have been identified including a secondary EGFR-T790M mutation, alternative signaling pathways activation and epithelial-mesenchymal transition and transformation into small cell lung carcinoma (SCLC).7–9 The third-generation EGFR-TKI, such as osimertinib (AZD9291) and rociletinib (CO1686), irreversibly and selectively inhibits EGFR-TKI-sensitizing mutation and T790M resistance mutation while sparing wildtype EGFR.10,11 Osimertinib is an FDA-approved drug for first-line treatment of common sensitive EGFR mutations or for second line treatment of acquired resistance to first-generation EGFR-TKI via T790M mutation.11 Standard chemotherapy is substituted for an EGFR TKI at progression on account of acquired resistance to EGFR-TKI without T790M mutation. Although a few patients develop resistant EGFR-TKI therapy, the clinical evidence showed the sites growth indolently and asymptomatically. For these patients, a change in treatment might not be necessary and continued EGFR inhibition conjunction with local therapies seems to provide continued clinical benefit.12 Several studies suggest that local therapies including metastasectomy, radiation, and radiofrequency ablation plus continued with the original EGFR-TKI showed good efficacy in the patients with partial treatment progression. Indeed, Yu et al13 investigated the efficacy of local therapy with continued EGFR-TKI therapy specifically in patients with acquired resistance to EGFR TKIs therapy. The result indicated that continued treatment with an EGFR TKI following local therapy is associated with long PFS and OS. Moreover, Xu et al14 discovered that local ablative therapy plus continuation of EGFR-TKI could provide additional benefits for EGFR-mutant NSCLC patients with oligoprogression during first-line EGFR-TKI treatment and female, one metastatic lesion, EGFR exon 19 mutation and response to EGFR TKIs therapy were the independent prognostic factors. In clinical work, radiofrequency ablation or microwave ablation as local therapies for EGFR-TKI therapy with extracranial oligometastasis is widely used. According to a recent report conducted by Ni et al,15 microwave ablation consolidation therapy after first-line EGFR-TKI treatment had significantly improved PFS and OS than TKI monotherapy. However, there were few retrospective or prospective studies about the efficacy of radiofrequency ablation for the oligoprogressive lesions with continuous EGFR-TKI treatment. Thus, in this article, we report two cases of EGFR-mutant NSCLC patients with locally resistant lesions achieving disease control via combination therapy (continuous EGFR-TKI and radiofrequency ablation therapy).
Case Description
Case 1
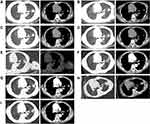
A 76-year-old female patient with a no-smoking history was hospitalized because of chest pain in November 2014. Chest CT examination showed a 2.2*2.0 cm mass in the left upper lobe of the lung (Figure 1A). A biopsy specimen taken by CT-guided fine-needle aspiration (FNA) showed a lung adenocarcinoma. In addition, the mutational analysis was performed and the result was the classic Epidermal Growth Factor Receptor gene (EGFR) Exon18 L858R mutation, which indicated EGFR-TKI sensitivity. Due to a diagnosis of bronchial asthma together with advanced age of the patient, the patient underwent EGFR-TKIs. From November 20, 2014, Icotinib Hydrochloride Tablets were administered at 125 mg dose orally three times a day. Follow-up CT showed mitigated local lesion (Figures 1B and C) and a partial response (PR) was achieved. However, disease progression of lung lesion occurred 21 months after the initiation of Icotinib Hydrochloride Tablets (Figure 1D). The patient was secondly hospitalized for radiofrequency ablation (Olympus 200-T20, 20 W and 9 KJ for 10 minutess) in September 2016 (Figure 1E). After radiofrequency ablation, needle-tract hemorrhage was observed but no hemoptysis happened. Oral administration of Icotinib Hydrochloride Tablets was continued after radiofrequency ablation. The chest CT scan was reexamined in the outpatient department and the result revealed a shrunken tumor miraculously in the left upper lobe of the lung after six months (Figure 1F). The patient achieved stable disease after radiofrequency ablation and continuous first generation of EGFR TKI until May 2018 (Figure 1G). For further treatment, she was presented to hospital in August 2018. The patient then received radiofrequency ablation again in the left upper lobe of the lung (Figure 1H) and continued oral Icotinib Hydrochloride Tablets. The parameters of radiofrequency ablation were 40 W up to 150 W for 12.5 minutes and 105 W for 94 seconds (Boston Scientific RF 3000TM Radiofrequency Generator, Boston Scientific Corporation). There were no pneumothorax, hemoptysis, or other complications. Until April 2019, the efficacy was evaluated as complete ablation (Figure 1I).
Case 2
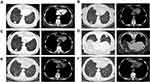
A 72-year-old woman, with no history of smoking, was diagnosed with lung adenocarcinoma (T1N0Mx) in March 2017 (Figure 2A). The mutational analysis was performed and the result showed that the EGFR was positive while ALK and ROS1 were negative. In consideration of severe chronic obstructive pulmonary disease, EGFR-TKI (gefitinib) was recommended as the treatment of choice. Regular follow-up in the outpatient department after two months showed a decrease in pulmonary lesion (Figure 2B). Yet, the patient was admitted to the hospital in October 2017 because chest CT scan revealed a progression of lung lesion (Figure 2C). The local radiofrequency ablation (Olympus 200-T20, 20 Wm and 9 KJ for 10 minutes) for treatment of oligoprogressive lesions in combination with continuation of the original TKIs was accepted (Figure 2D). Regular follow-up of chest CT indicated that the lesion was stable until May 2019 (Figures 2E and F).
These two patients had provided written informed consent for case publication. The case details publication was approved by the Medical Ethics Committee of Huzhou Centre Hospital.
Discussion
Despite the recent advances in anti-cancer drugs, the prognosis of lung cancer, especially in advanced stage of lung cancer, remain poor. The systemic chemotherapy or target drugs are commonly recommended as standard management when the patients were diagnosed with advanced lung cancer. However, most patients will eventually experience disease progression. Generally, they will change antineoplastic drugs according to the result of molecule detection. Yet, the optimal treatment strategy for NSCLC patients with slow progression or with a limited number of metastatic sites remains controversial. Emerging evidence shows that active systemic therapy combined with local treatment might extend the survival benefit in NSCLC patients with oligoprogressive disease.14,16,17 Shang et al17,18 compared the efficacy of local treatment alone or chemotherapy plus local ablative treatment and chemotherapy alone or chemotherapy plus local ablative treatment in NSCLC patients with oligometastases after surgery.Their results implied that local treatment combined with chemotherapy might be an optimistic treatment strategy for some specific oligometastatic NSCLC patients. Similarly, in a study of Yu et al13 which reviewed outcomes of 18 patients who acquired resistance to EGFR TKI and received elective local therapy, local therapy with continued treatment with an EGFR TKI demonstrated it was well tolerated with a survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. In the latter studies, doctors pay increasing attention to the clinical benefit of first-line continual EGFR-TKI plus local ablative therapy in limited metastatic NSCLC patients.14,19 These data suggested that local therapy might potentially prevent switching systemic or osimertinib therapy prematurely. However, most studies emphasize the effect of local therapy and seem to fail to take into account the fact that local therapy is diversified, including surgery, radiotherapy, microwave ablation and radiofrequency ablation. Therefore, it is still uncertainwhether one local therapy could extend the survival benefit in patients with slow progression.
In this article, we show two case reports describing the outcome of oligoprogressive NSCLC patients. These two patients were both female and initially received EGFR-TKI treatment. During the treatment, oligoprogressive state occurred and radiofrequency ablation was performed. The combination of EGFR-TKI plus local radiofrequency ablation therapy provide additional benefits for these two people, which were consistent with previous studies. Nonetheless, there are some limitations that should be acknowledged. First, this was a case report, which reflected the phenomenon instead of the result of retrospective or prospective studies. Secondly, these two patients did not have a re-biopsy, resulting in the possible acquired resistance mechanisms of these two people being unclear. Thirdly, safety data on radiofrequency ablation side-effects should be further confirmed, despite the apparent good tolerability of radiofrequency ablation in these two cases. Given the limitations expressed above, we emphasize that future retrospective studies or prospective trials need to be conducted in order to validate our findings.
Acknowledgment
We apologize to all researchers whose relevant contributions were not cited due to space limitations.
Disclosure
The authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi:10.3322/caac.21442
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332
3. Pirker R, Filipits M. Personalized treatment of advanced non-small-cell lung cancer in routine clinical practice. Cancer Metastasis Rev. 2016;35:141–150. doi:10.1007/s10555-016-9612-6
4. Rocco G, Morabito A, Leone A, Muto P, Fiore F, Budillon A. Management of non-small cell lung cancer in the era of personalized medicine. Int J Biochem Cell Biol. 2016;78:173–179. doi:10.1016/j.biocel.2016.07.011
5. Yan D, Parker RE, Wang X, et al. MERTK promotes resistance to irreversible EGFR tyrosine kinase inhibitors in non-small cell lung cancers expressing wild-type EGFR family members. Clin Cancer Res. 2018;24:6523–6535. doi:10.1158/1078-0432.CCR-18-0040
6. Matikas A, Mistriotis D, Georgoulias V, Kotsakis A. Current and future approaches in the management of non-small-cell lung cancer patients with resistance to EGFR TKIs. Clin Lung Cancer. 2015;16:252–261. doi:10.1016/j.cllc.2014.12.013
7. Dorantes-Heredia R, Ruiz-Morales JM, Cano-Garcia F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5:401–412. doi:10.21037/tlcr.2016.07.10
8. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi:10.1126/scitranslmed.3002003
9. Soucheray M, Capelletti M, Pulido I, et al. Intratumoral heterogeneity in EGFR-mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res. 2015;75:4372–4383. doi:10.1158/0008-5472.CAN-15-0377
10. Igawa S, Ono T, Kasajima M, et al. Impact of EGFR genotype on the efficacy of osimertinib in EGFR tyrosine kinase inhibitor-resistant patients with non-small cell lung cancer: a prospective observational study. Cancer Manag Res. 2019;11:4883–4892. doi:10.2147/CMAR.S207170
11. Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;12:63. doi:10.1186/s13045-019-0759-9
12. Chen Q, Quan Q, Ding L, et al. Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget. 2015;6:24904–24911. doi:10.18632/oncotarget.4570
13. Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi:10.1097/JTO.0b013e31827e1f83
14. Xu Q, Liu H, Meng S, et al. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J Cancer. 2019;10:522–529. doi:10.7150/jca.26494
15. Ni Y, Ye X, Yang X, et al. Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J Cancer Res Clin Oncol. 2020;146:197–203. doi:10.1007/s00432-019-03043-6
16. Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, Phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi:10.1200/JCO.19.00201
17. Shang S, Su Y, Zhu Z, et al. Local ablative therapy with or without chemotherapy for non-small-cell lung cancer patients with postoperative oligometastases. Cancer Manag Res. 2018;10:6421–6429. doi:10.2147/CMAR.S185592
18. Shang S, Wang L, Su Y, et al. Local therapy combined with chemotherapy versus chemotherapy for postoperative oligometastatic non-small-cell lung cancer. Future Oncol. 2019;15:1593–1603. doi:10.2217/fon-2018-0923
19. Juan O, Popat S. Ablative therapy for oligometastatic non-small cell lung cancer. Clin Lung Cancer. 2017;18:595–606. doi:10.1016/j.cllc.2017.03.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.