Back to Journals » OncoTargets and Therapy » Volume 9
Radiation dose reduction for patients with extranodal NK/T-cell lymphoma with complete response after initial induction chemotherapy
Authors Wang L, Bi X, Xia Z, Huang H, Jiang W, Zhang Y
Received 6 July 2016
Accepted for publication 3 September 2016
Published 26 September 2016 Volume 2016:9 Pages 5875—5881
DOI https://doi.org/10.2147/OTT.S116591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Liang Wang,1,2,* Xi-wen Bi,1,3,* Zhong-jun Xia,1,2 Hui-qiang Huang,1,3 Wen-qi Jiang,1,3 Yu-jing Zhang1,4
1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 2Department of Hematologic Oncology, 3Department of Medical Oncology, 4Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Previous studies have found that radiotherapy (RT) dose less than 50 Gy resulted in inferior outcomes for early stage extranodal NK/T-cell lymphoma (ENKTL). Nowadays, induction chemotherapy (CT) followed by RT consolidation is often used. For patients who get complete response (CR) after CT, whether RT dose can be safely reduced or not remains unknown. This retrospective study compared the survival outcomes between patients who received higher dose (>50 Gy) and lower dose (≤50 Gy) RT after CR was attained by CT. One hundred and forty four patients of early stage ENKTL got CR after induction CT and received RT consolidation. Thirty-one patients received lower dose RT (median 46 Gy, range, 36–50 Gy), and 113 patients received higher dose RT (median 56 Gy, range, 52–66 Gy). In univariate survival analysis, age >60, local tumor invasion, and non-asparaginase-based CT were associated with inferior progression-free survival (PFS) and overall survival (OS). However, there were no differences in PFS and OS between patients treated with higher and lower dose RT, which was confirmed in the multivariate survival analysis. Furthermore, reduced dose RT did not affect local control rate. Most common RT-related side effects were grade 1/2 mucositis and dermatitis, and the incidence rate of grade 3 mucositis or dermatitis was lower in patients treated with reduced dose RT (9.7% vs 15.0% for mucositis, and 6.5% vs 17.7% for dermatitis). In conclusion, this study found that RT dose could be safely reduced without compromising survival outcomes and further improved RT-related side effects. Prospective randomized controlled trials are warranted to validate our findings.
Keywords: extranodal NK/T-cell lymphoma, radiotherapy, prognosis, asparaginase, radiation-related toxicity
Introduction
Extranodal NK/T-cell lymphoma (ENKTL), closely associated with Epstein–Barr virus (EBV) infection, is relatively common in East Asia, Southeast Asia, and Central and South America.1 Most patients of ENKTL have early stage disease at diagnosis, and thus radiotherapy (RT) has been adopted as the primary treatment during the past decades.2 RT alone or combination of RT and consolidative chemotherapy (CT) can achieve a complete response (CR) rate of 87% and 5-year overall survival (OS) rate of 71%.3 However, local relapse or systemic failure is very common in patients who are treated with RT alone.4 Therefore, increasing evidence has proven the benefit of CT in reducing the risk of disease relapses. Previous studies have demonstrated poor efficacy with anthracycline-based CT (such as CHOP or EPOCH regimens) due to overexpression of multidrug resistance genes in ENKTL cells.5 In recent years, asparaginase-based regimens have been demonstrated to be highly active in the treatment of ENKTL, and can attain a CR rate of 70% in early stage ENKTL.6–10 Increasingly cancer centers are using a combination of asparaginase-based CT and RT for the treatment of early stage ENKTL, although the optimal combination strategy has not been defined yet.
Previous studies using RT as primary treatment for early stage ENKTL concluded that higher dose (>50 Gy) and extended involved-field RT (IFRT) can get superior outcomes than lower dose (<50 Gy) and small IFRT.11 However, with increasing application of asparaginase-based CT as primary treatment for early stage ENKTL patients, many patients can get CR before RT.9,10 Thus, in order to reduce the toxicity of higher dose RT and improve the quality of life, whether the RT dose can be safely reduced or not without compromising survival outcomes needs to be investigated in patients with CR after induction CT.
Materials and methods
Patients
From January 2003 to December 2015, a total of 221 patients of early stage ENKTL with complete follow-up information in Sun Yat-sen University Cancer Center received CT as primary treatment, among whom 144 patients got CR before RT. Only patients with disease in upper aerodigestive tract were included in this study. Sun Yat-sen University Cancer Center Research Ethics Board has approved us to use the data in this retrospective study, and all patients included in this study gave their written informed consent for publishing the medical information at their first visit to our center.
Treatments
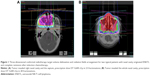
All 144 patients received CT as primary treatment (anthracycline-based regimens, n=93; asparaginase-based regimens, n=51) and got CR before initiation of RT. As previously reported,9 IFRT was delivered using 6-MeV linear accelerator using conventional planning RT, 3-dimensional conformal treatment planning, or intensity modulated RT. The RT prescription was 200 cGy per fraction, once a day, and 5 fractions every week (Figure 1).
Response and toxicity criteria
The treatment efficacy was evaluated after each two cycles of CT, before and after RT using MRI or PET-CT scan. Follow-up visits were done every 3 months within the first 2 years, every 6 months within the next 3 years, and annually thereafter for the whole life. The National Cancer Institute Common Terminology Criteria of Adverse Events v3.0 was used to grade all treatment toxicities.
Statistical analysis
OS was defined as the interval from the date of diagnosis of ENKTL to the date of death or the date of last follow-up visit for patients who were still alive. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression or death, or to the date of last follow-up visit, whichever came first. Survival analysis was done using the Kaplan–Meier method with log-rank test. Two-sided P<0.05 was considered statistically significant. SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analysis.
Results
Patients’ characteristics and treatments
All patients’ characteristics are shown in Table 1. The median age was 41 years old (range 11–75), and 18 patients (12.5%) were older than 60; 63.9% of patients had stage I disease, and serum lactate dehydrogenase (LDH) level was elevated in only 19.4% patients; 55.6% of patients had local tumor invasion. In this study, all patients received CT followed by RT consolidation, and 64.6% of patients had anthracycline-based CT regimens, and the remaining 35.6% of patients had asparaginase-based CT regimens. All patients got CR after at least two cycles of CT. Thirty-one patients (21.5%) received reduced dose RT (median 46 Gy, range, 36–50 Gy), and 113 patients (78.5%) received higher dose RT (median 56 Gy, range, 52–66 Gy). There were no significant differences between patients treated with higher dose RT or lower dose RT in regard to clinical characteristics (P>0.05).
Survival analysis and relapse patterns
Till January 2016, 27 patients died of ENKTL, and the median follow-up time was 42 months (range, 2–135 months) for surviving patients. Five-year OS rate was 76%. Relapsed disease occurred in 46 patients (including 35 patients treated with higher dose RT and eleven patients with lower dose RT). Among those 46 patients, most of them (29/46, 63.0%) had systemic relapses, including gastrointestinal tract invasion, skin invasion, liver invasion, lung invasion, bone marrow invasion, or hemophagocytic syndrome. Seventeen patients (37.0%) had locoregional relapse within the radiation field (ten patients with higher dose RT and seven patients with lower dose RT). Overall, 5-year PFS rate was 64%, and 5-year local control rate (LCR) was 85%. As is shown in Figure 2, whether patients received higher dose RT or lower dose RT did not affect OS and PFS (P=0.375 and 0.809, respectively). Patients with older age, local tumor invasion, and treated with anthracycline-based CT regimens had significantly inferior OS and PFS (P<0.05). A subgroup survival analysis was also done in patients treated with higher RT dose (56–66 Gy) and lower RT dose (36–46 Gy), and similar results can be reached, which showed that OS and PFS were not affected by RT dose (Figure 3). As is shown in Figure 4, no significant difference was found in 5-year LCR between patients treated with higher dose RT and lower dose RT. In a multivariate Cox regression survival analysis including age, Eastern Cooperative Oncology Group Performance Status score, LDH level, local tumor invasion, induction CT regimens, Ann-Arbor stage, and RT dose (Table 2), only age (>60 years old) and induction CT regimens (anthracycline-based regimens) were found to be independent poor prognostic factors for PFS and OS, and lower RT dose (≤50 Gy) did not affect the survival outcomes.
Treatment toxicities
The most common toxicities related to RT were mucositis, dermatitis, and dysphagia, but most of these adverse events were mild in severity. Grade 3 mucositis and dermatitis occurred in 15% and 17.7% of patients treated with high dose RT, both higher than that in patients treated with low dose RT (9.7% and 6.5%, respectively). The incidence rate of grade 3 dysphagia was 6.5% and 8.8% in patients treated with low dose and high dose RT, respectively. No RT-related deaths occurred in this study.
Discussion
ENKTL, a distinct subtype of non-Hodgkin lymphoma, is very sensitive to RT, and it is reported that RT with or without CT can result in very high CR rate.2,3,11,12 However, systemic relapse is very common in patients treated with RT alone, and it is now recommended that CT should be added in combination with RT to reduce relapse rate, especially for patients with high risk factors.5 Due to primary resistance to anthracyclines by ENKTL, previous CHOP regimen resulted in very low control rate.13 With the advent of asparaginase, most patients can get CR even before RT.8–10 Thus, it is rational to reduce the RT dose in patients who get CR before RT in order to decrease RT-related toxicities and improve the quality of life. In this retrospective study, we found that patients receiving lower dose RT (≤50 Gy) can achieve equal survival outcomes as patients treated with higher dose RT (>50 Gy), and also lower dose RT did not affect the LCR, suggesting it may be safe to reduce the RT dose in patients who got CR before RT.
In this study, either anthracycline-based or asparaginase-based CT regimens were used. Though we only enrolled patients who got CR after induction CT, it is interesting to find that both PFS and OS were in favor of asparaginase-based regimens, indicating that asparaginase is a very important drug in controlling minimal residue disease (MRD). As is known, EBV infection plays a critical role in tumorigenesis of ENKTL,14,15 and previous studies have demonstrated that post-treatment EBV-DNA load can predict the survival outcomes.15–18 We also found that post-treatment EBV-DNA positivity correlates with poor PFS and OS, and can predict high relapse rate, which indicates that post-treatment EBV-DNA may be used as MRD of ENKTL.19 We also found that in all patients with CR after induction CT, the percentage of post-treatment EBV-DNA positivity was significantly higher in patients who received anthracycline-based regimens (data submitted elsewhere, not shown here), which may explain our findings that induction regimens significantly correlated with survival outcomes even though all patients got CR before RT. Kim et al demonstrated that post-treatment positron emission tomography (PET) in combination with EBV-DNA positivity can precisely predict the relapse rate and survival outcomes in patients with ENKTL.20 Thus, we recommend that both PET scan and EBV-DNA test should be done before RT in order to better define the treatment response.
Several previous studies have indicated that RT with higher dose (>50 Gy) and extended radiation field can improve the LCR in ENKTL.11,21 However, most of these studies were done in the era of pre-asparaginase, when CT induced very low CR rate in patients with ENKTL.10,13 With general application of asparaginase in the treatment of ENKTL, most patients can achieve CR (even PET negativity and EBV-DNA negativity).9,10,19 Major toxicities related with RT for ENKTL patients were mucositis, dermatitis, or dysphagia, all of which significantly affect the quality of life.2,9,11 Our results suggest that reducing the incidence rate of these toxicities by lowing the RT dose is feasible and meaningful. However, due to the small sample size of patients who received lower dose RT, various induction CT regimens, and retrospective nature of this study, our findings need to be validated in well-designed prospective clinical trials. In fact, a Phase III clinical trial evaluating the efficacy and safety of decreasing RT dose to <50 Gy for patients who get CR after pegaspargase-based induction CT is going on (ChiCTR-TRC-14005218).
In conclusion, this study found that RT dose could be safely reduced without compromising survival outcomes and further decreased RT side effects for patients who got CR after induction CT. Prospective randomized controlled trials are warranted to validate this finding.
Acknowledgments
We appreciate the cooperation of all doctors of Sun Yat-sen University Cancer Center.
This work was granted by National Natural Science Foundation of China (contract/grant number: 81400159).
Disclosure
The authors report no conflicts of interest in this work.
References
Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. | ||
Li YX, Wang H, Jin J, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82(5):1809–1815. | ||
Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181–189. | ||
Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18(1):54–63. | ||
Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83(10):795–799. | ||
Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. | ||
Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–4416. | ||
Lin N, Song Y, Zheng W, et al. A prospective phase II study of L-asparaginase-CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol. 2013;6:44. | ||
Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119(2):348–355. | ||
Wang L, Wang WD, Xia ZJ, Zhang YJ, Xiang J, Lu Y. Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol. 2014;31(3):860. | ||
Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70(1):166–174. | ||
Li YX, Liu QF, Fang H, et al. Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin Cancer Res. 2009;15(8):2905–2912. | ||
Wang L, Xia ZJ, Huang HQ, Lu Y, Zhang YJ. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/IIE extranodal natural killer/T cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J Hematol. 2012;96(5):617–623. | ||
Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162–1170. | ||
Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein-Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18(5):305–319. | ||
Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin Cancer Res. 2002;8(1):29–34. | ||
Chan KC, Zhang J, Chan AT, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 2003;63(9):2028–2032. | ||
An X, Wang FH, Ding PR, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117(16):3750–3757. | ||
Wang L, Wang H, Wang JH, et al. Post-treatment plasma EBV-DNA positivity predicts early relapse and poor prognosis for patients with extranodal NK/T cell lymphoma in the era of asparaginase. Oncotarget. 2015;6(30):30317–30326. | ||
Kim SJ, Choi JY, Hyun SH, et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. 2015;2(2):e66–e74. | ||
Isobe K, Uno T, Tamaru J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106(3):609–615. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.