Back to Journals » Journal of Pain Research » Volume 15
QX-OH/Levobupivacaine: A Structurally Novel, Potent Local Anesthetic Produces Fast-Onset and Long-Lasting Regional Anesthesia in Rats
Authors Yang Y, Wang C, Liu J, Liao D, Zhang W , Zhou C
Received 9 October 2021
Accepted for publication 14 January 2022
Published 4 February 2022 Volume 2022:15 Pages 331—340
DOI https://doi.org/10.2147/JPR.S343500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Yang Yang,1,2 Chiyi Wang,1,2 Jin Liu,1,2 Daqing Liao,2 Wensheng Zhang,1,2 Cheng Zhou2
1Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Laboratory of Anesthesia and Critical Care Medicine, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu, 610041, Sichuan, People’s Republic of China
Correspondence: Cheng Zhou
Laboratory of Anaesthesia & Critical Care Medicine, Translational Neuroscience Center, The Research Units of West China (2018RU012), Chinese Academy of Medical Sciences, West China Hospital of Sichuan University, Chengdu, 610041, People’s Republic of China
, Tel +81-28-85164145
, Fax +81-28-85164039
, Email [email protected]; Wensheng Zhang
Department of Anaesthesiology and Laboratory of Anaesthesia & Critical Care Medicine, Translational Neuroscience Center, West China Hospital of Sichuan University, Chengdu, 610041, Sichuan, People’s Republic of China
, Tel/Fax +81-28-85164144
, Email [email protected]
Purpose: Local anesthetics (LAs) are an important alternative for postoperative analgesia; however, the short duration of LAs limits their use. Thus, we previously developed LL-1, a mixture of QX-OH and levobupivacaine (LB) that produces regional anesthesia for more than 10 h in rats. The aim of this study is to investigate the long-acting mechanism of LL-1 in vivo and in vitro.
Methods: Regional anesthetic effects and local toxicity of the LL-1, QX-OH and LB treatment groups were investigated in a sciatic nerve block rat model. Whole-cell patch-clamping recordings were used to measure the inhibition Nav currents (INa) in ND7/23 cells.
Results: The onset of LL-1 (35mM QX-OH+10mM LB) and 10 mM LB was 10 min, which was much faster than 35 mM QX-OH (27 [18, 60] min, t[12] = − 4.535, p = 0.001). The duration of LL-1 (35mM QX-OH+10 mM LB) was significantly longer than 35 mM QX-OH or 10 mM LB alone (F[3, 35] = 191.336, p < 0.0001). No differences in local tissue toxicity were found between LL-1 and LB. In patch-clamping recordings, 5 mM QX-OH produced ∼ 20% inhibition of INa currents. LB at 40 μM inhibited INa by 65.51%± 3.63%, while QX-OH 2 mM+LB 40 μM inhibited INa by 77.37%± 3.36% (t[14] = 2.358, p = 0.025), and QX-OH 5 mM+LB 40 μM inhibited INa by 83.88%± 1.57% (t[13] = 4.191, p = 0.0003). Furthermore, INa inhibition by QX-OH+LB was more persistent than that of LB alone during washout.
Conclusion: LL-1 can produce an additive and stable inhibition of Nagv currents, which can contribute to the long-lasting regional anesthetic action.
Keywords: long-lasting local anesthetics, QX-OH, levobupivacaine, voltage-gated sodium channel, Nav
Introduction
Local anesthetics (LAs) are an important alternative for postoperative analgesia, due to the limitations due to adverse effects associated with opioid use such as respiratory depression, nausea, vomiting, and constipation that can reduce patient satisfaction and safety.1 However, the duration of currently used LAs is insufficient to meet the demand for postoperative pain management.2–4 As a result, it is necessary to develop novel LAs with long-acting properties and tolerable toxicity.
To improve the postoperative analgesia, we developed QX-OH, a hydroxyethyl derivative of QX-314, by substituting a hydroxyl group (-OH) for one of the hydrogen groups (-H) in QX-314.5,6 In rats, it has previously been reported that QX-OH produces longer-lasting analgesia with less local tissue damage than QX-314 in the sciatic nerve block model.7 QX-OH, conversely, has a half-hour onset period, which limits its clinical use.7 In the rat sciatic nerve block model, combining QX-OH with levobupivacaine (LB) (named LL-1) may elicit sensory block lasting 9 hours after knee surgery and with regional anesthesia lasting 17 hours, with an onset of less than 10 minutes.8–10 The mechanism by which LL-1 provides a long-lasting localized anesthetic effect, as well as the pharmacological interaction between QX-OH and LB, remains unexplained.
The voltage-gated sodium channel (Nav) is a critical ion channel required for initiation and propagation of the action potential.11 Almost all of the local anesthetics used in clinical practice are Nav channel blockers.12–16 However, it is unknown whether QX-OH can inhibit Nav channels alone or in combination with LB.
The purpose of this study was to examine the interaction of QX-OH and LB on INa inhibition and to compare the regional anesthetic effects and local toxicity of LL-1 with QX-OH and LB at relative concentrations in a standard sciatic nerve block model in rats.
Materials and Methods
Animals
Adult male and female Sprague-Dawley rats weighing between 250 and 300 g, aged between 2 and 3 months, were purchased from Chengdu Dossy Biological Technology Co., Ltd. (Chengdu, China). The animals were housed in a standard laboratory animal husbandry with a 12-hour light/dark cycle and free access to food and water. All experimental procedures in this study meet the guidelines of the Care and Use of Laboratory Animals by the US National Institutes of Health (NIH Publications No. 80-23, revised 1996). The Committee of Animal Care of the West China Hospital (Sichuan University, Chengdu, China) (Ethical Approval Number, 2015014A) approved all experimental protocols.
ND7/23 Cells Expressing Rat Sodium Channels
ND7/23 is a hybrid cell line of rat DRG and mouse N18Tg2 neuroblastoma, which is widely used for pharmacological measurement of the INa.17 ND7/23 cells (Binhui Biology Co., Shanghai, China) were cultured in DMEM (GIBCO-Invitrogen, Karlsruhe, Germany) containing 10% FBS and 1% penicillin/streptomycin (GIBCO-Invitrogen) in an 37°C and 5% carbon dioxide environment.18 Electrophysiological recordings were performed 48 hours post-plating.
Electrophysiological Recordings
Whole-cell patch-clamp recordings were performed at room temperature (~24–25°C) using an Axopatch 200B amplifier (Axon Instruments Inc, Foster City, CA, USA). Recording glass electrodes (B150-86-7.5, Sutter Instrument, Novato, CA, USA) were pulled to a resistance of 4–5 MΩ by a Micropipette Puller (P-97, Sutter Instrument). The internal solution contained (mM): CsF 120, NaCl 10, HEPES 10, EGTA 10, TEA-Cl 10, CaCl2 1, MgCl2 1, Mg-ATP 3, and GTP 0.3, at pH 7.3 adjusted with CsOH, and was filtered using a 0.2-µm pore membrane filter before pouring into the glass electrodes. The external solution contained (mM): NaCl 140, HEPES 10, KCl 3, MgCl2 1, CaCl2 2, TEA-Cl 20, D-glucose 5, at pH 7.38 adjusted with NaOH. The study solutions were applied using an in-house constructed gravity-driven application system. The tip of the perfusion cannula was placed near the target cell within 200 μm. Membrane current signals were filtered at 10 Hz and sampled at 20 kHz. INa was recorded by depolarization to −10 mV from holding potential of −70 mV in voltage-clamp mode.
Chemicals
QX-OH was synthesized in our laboratory based on the protocols of a registered patent (Invention Patent, China, ZL201410688865.1)8 (Figure 1). QX-314, levobupivacaine (LB), and lidocaine were purchased from Sigma-Aldrich Canada Ltd (Oakville, ON, Canada). QX-OH was diluted to working concentrations by ultrapure water (Milli-Q® integral water purification system, Merck Millipore, Germany) in animal experiments and by the external solution in patch-clamping recordings immediately before experiments.
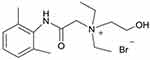 |
Figure 1 Chemical structure of QX-OH. This figure illustrated the chemical structure of QX-OH. |
Sciatic Nerve Block Model
Rats were randomly assigned to four groups (n = 8/each group) and received LL-1 (a combination of 35 mM QX-OH and 10 mM levobupivacaine), 35 mM QX-OH, and 10 mM levobupivacaine (equivalent to ~0.3%) at a volume of 200 μL, respectively. Under 2% isoflurane general anesthesia, rats were shaved, disinfected, and injected with study solutions vertically into the bone layer below the midpoint between the largest trochanter and the ischial tuberosity of the left leg.19 Neurobehavioral evaluation was performed 10, 18, 24, and 30 min, and 1, 2, 4, 6, 8, 10, 12, 18, and 24 hours after injection.
The sensory block was assessed using a hotplate (Model RB-200 Hotplate Analgesia Meter; Chengdu Technology & Market Co., Ltd., China). Setting the temperature at ~55°C, the hotplate was used to inflict thermal noxious stimulation on rats and the cut-off time of thermal stimulation was set at 12s to avoid burns.20 The time from the onset of thermal stimulation to the withdrawal response of the rat was defined as the paw withdrawal latency (PWL). The baseline PWL of rats was measured on 3 consecutive days before the experiments and calculated as the mean value of the 3 days. Then, PWL was calculated as the percentage maximal possible effect (%MPE) according to the following equation: (%MPE) = (test PWL − baseline)/(cutoff time − baseline) *100%.21 The %MPE of above 50% was defined as an effective sensory block, and the complete sensory block was defined as %MPE reaching 100%. The onset time of the sensory block was defined as the time from injection to the first time when %MPE was more than 50% (10 min was the minimal time required for recovery from isoflurane anesthesia). The offset time was defined as the first time when %MPE again decreased to lower than 50%. The duration from onset to offset of sensory block was defined as the duration of sensory block.
The motor function of the injected leg was assessed by the postural extensor thrust test.7 An electronic scale was used to measure the muscle strength of the injected hind limb in an upright posture and the force that the paw generated against the platform of the scale was the strength of the limb. Before the experiments, we measured the muscle strength for 3 consecutive days and calculated the mean value as the baseline. After injection of study solutions, the effective motor block was defined as the strength of the limb decreased by more than 50% compared to baseline. The onset of motor block was defined as the time from the injection to the time of the first detectable effective block (10 min was the minimal time required for recovery from isoflurane anesthesia). The offset was defined as the time when the strength of the limb again increased to more than 50%. The duration from the onset to offset of motor block was defined as the duration of motor block.
Histological Analysis
Samples of sciatic nerves and adjacent muscle that were exposed to study solutions were selected on the 7th and 14th days after recovery of the sciatic nerve block. Eight rats in each group were randomly euthanatized on the 7th or 14th day, respectively. Pathological section, hematoxylin-and-eosin (HE) staining, and fast blue staining were performed according to standard steps.22 Two skilled technicians blinded to the treatment of rats evaluated the degree of the inflammation, fibrosis, degeneration within epineurium, the inflammation, hemorrhage, fibrosis outside epineurium, and the inflammation of muscle using the Light Microscopy Imager A2 (Carl Zeiss, Germany). Stained tissues were scored according to the following scale: 0, indicated normal; 1 indicated ~0–25% of the area involved; 2 indicated ~25–50% of the area involved; 3 indicated ~50–75% of the area involved; and 4 indicated ~75–100% of the area involved.7,23
Statistical Analysis
Data were plotted and analyzed using SPSS software version 24.0 (IBM Corp., Armonk, NY, USA), Origin (OriginLab Corporation, MA, USA), and GraphPad Prism (GraphPad Software, San Diego, California, USA). The normal distribution of data was confirmed using the Shapiro–Wilk test. Continuous data were reported as mean ± SEM or median (Interquartile range) as appropriate. Categorical data were analyzed using the Chi-square test or Fisher’s exact test. A P-value of <0.05 was recognized as statistically significant. Differences were analyzed using the Student’s t-test and Mann–Whitney U-test with post-hoc Bonferroni analysis.
Results
LL-1 Blocked Na+ Currents in ND7/23 Cells
QX-OH Alone Produced a Small Inhibition of the INa
To evaluate the main components of LL-1, we performed inhibition comparisons between QX-OH and its prototype QX-314 on INa, lidocaine was used as the positive control, and the extracellular solution was set as the blank control (Figure 2A). After an extracellular perfusion for 10 min, lidocaine at a concentration of 5 mM produced a significant inhibition of INa by 88.08% ± 3.79%, QX-OH at 5 mM inhibited INa by 19.28% ± 3.63%, 5 mM QX-314 inhibited INa by 9.48% ± 2.05%, and in the control group with an only extracellular solution produced inhibition of INa by 7.05% ± 7.32%. The inhibition of INa was statistically significant for 5 mM QX-OH, 5 mM QX-314, 5 mM lidocaine, and the blank control group (F (3, 28)=192.36, p < 0.0001, one-way ANOVA with Bonferroni post hoc test). The inhibition of INa by 5 mM QX-OH was lower than that obtained with lidocaine 5 mM (t (10)=17.638, p< 0.001; two independent sample t-test). No significant differences were detected in the inhibition of INa at 5 mM QX-OH and 5mM QX-314 (t (10)=−1.962, p=0.07; two independent sample t-test), or for 5 mM QX-314, or the control group (t (11)=0.239, p=0.814; two independent sample t-test) (Figure 2B). The Figure 2C showed the currents down trend in four groups after perfusion with drugs.
Combined QX-OH and LB Produced an Additive and Persistent Inhibition of INa
To evaluate the interaction of QX-OH and LB on inhibition of the INa, the IC50 (median inhibitory concentration) of LB was also determined by fitting the averaged dose-response curve with the Hill equation, which yielded an IC50 of 66.85 ± 1.77 μM with a Hill coefficient of −1.529 for the INa (n = 5–8) (Figure 3A and B). However, we determined that QX-OH could only provide a small inhibition of the INa at 5 mM, while at 2 mM the INa, changed modestly, thus we could not evaluate the IC50 of the QX-OH directly.
Next, we evaluated different combinations of LB+QX-OH to determine any combinatory effects. LB at a concentration of 40 μM (slightly lower than IC50) was used for combination exposures. The initial concentration of QX-OH used in the combination was set at 140 μM based on the ratio of QX-OH and LB in LL-1 (3.5:1) in vivo. In addition, two other higher concentrations of QX-OH (2 mM and 5 mM) were also tested. In total, four groups were evaluated: 40 μM LB alone; 40 μM LB+140 μM QX-OH; 40 μM LB+2 mM QX-OH; and 40 μM LB+ 5 mM QX-OH.
To compare the effects of different mixing ratios of bupivacaine and QX-OH, we performed inhibition comparisons among LB 40 μM alone, LB 40 μM+QX-OH 140 μM, LB 40 μM+QX-OH 2 mM and LB 40 μM+QX-OH 5 mM (Figure 3D). The inhibition of the INa was significantly different among the four groups (F (3, 52)=8.248, p = 0.0001, one-way ANOVA with the Bonferroni post hoc test) (Figure 3C). The inhibition of the INa by LB 40 μM+QX-OH 140 μM (64.38%±3.50%) was similar to the effect produced by LB 40 μM alone (65.51%±3.63%, t[12]=−0.223, p = 0.825; two independent sample t-test) (Figure 3C). LB 40 μM+QX-OH 2 mM produced a larger inhibition of INa (77.37%±3.36%) than LB 40 μM alone (t[14]=2.358, p = 0.025; two independent sample t-test) (Figure 3C). Exposure to LB 40 μM+QX-OH 5 mM produced a larger inhibition of the INa (83.88%±1.57%) than exposure to LB 40 μM alone (t[13]=4.191, p = 0.0003; two independent sample t-test) (Figure 3C). The inhibition of the INa was similar between the LB 40 μM+QX-OH 5 mM and LB 40 μM+QX-OH 2 mM treatment groups (t[13]=1.703, p = 0.100; two independent sample t-test) (Figure 3C). Because QX-OH exposure at 5 mM inhibited the INa by ~20%, QX-OH and LB might produce an additive inhibition on the INa.
After washout, the INa in the 40 μM LB group was significantly higher than that of 40 μM LB+2 mM QX-OH (t[13]=3.017, p = 0.006; two independent sample t-test) (Figure 3E), while the INa was markedly inhibited in LB 40 μM+QX-OH 5 mM treatment group compared with exposure to LB 40 μM alone (t[15]= −7.174, p < 0.0001; two independent sample t-test) (Figure 3E). All the cells were alive without rundown during washout throughout and the recording could continue for a long time during the washout. Therefore, the co-application of QX-OH and LB produced a steady block of the INa.
LL-1 Produced a Fast-Onset and Long-Lasting Sciatic Nerve Block in vivo
The onset time of sensory and motor block by LL-1 and 10 mM LB were immediate after recovery from general anesthesia by isoflurane, which was recorded as 10 min for all the rats (Figure 4A). While the onset time of nerve block induced by exposure to 35 mM QX-OH (27 [18, 60] min, t[12]= −4.535, p = 0.001; two independent sample t-test), Figure 4D] was significantly delayed compared with exposure to LL-1.
The offset of sensory and motor block showed no significant difference among four groups (Figure 4B–E). There was a significant difference among the four groups in the duration of the sensory block (F (3, 35)=191.336, p < 0.0001, one-way ANOVA with Bonferroni post hoc test, Figure 4C). Exposure to LL-1 produced a sensory block with a total duration of 11 (11.8, 12.8) h, which was longer than that following exposure to QX-OH [7.7 (6.5, 9) h, t (12)= 11.419, p < 0.0001; two independent sample t-test], QX-314 [11.6 (9.7, 11.6) h, t (7)= 3.453, p = 0.009; two independent sample t-test] or LB [2.8 (2.8, 2.8), t (12)= 11.419, p < 0.0001; two independent sample t-test] (Figure 3C). For the motor block, the total duration of the four groups was significantly different (F[3, 35]=63.052, p < 0.0001, one-way ANOVA with Bonferroni post hoc test, Figure 4F). LL-1 produced a motor block with a total duration of 11 (11.8, 12.8) h, which was longer than that observed following exposure to QX-OH [6.7 (5.5, 9.7) h, t (12)= 6.393, p = 0.00015; two independent sample t-test], QX-314 [11.6 (9.7, 11.6) h, t (7)= 3.584, p = 0.002; two independent sample t-test] or to LB [2.8 (2.8, 2.8) hour, t (12)=29.989, p < 0.0001; two independent sample t-test] (Figure 4F).
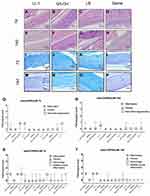
Local Tissue Toxicity of LL-1 Was Similar to LB Alone
The tissue morphology indicated indistinguishable inflammation among rats who received LL-1 (Figure 5A, E, I and M), QX-OH (Figure 5B, F, J and N), LB (Figure 5C, G, K and O), and saline (Figure 5D, H, L and P). The inflammation scores of the sciatic nerve and skeletal muscle were 1 (0, 1) in the LL-1 group, which were not significantly different compared to the LB group (0 [0, 1], t[8] = −0.966, p = 0.35; two independent sample t-test), but was slightly higher than that observed in the saline group (0[0,0], t[8] = −3.416, p = 0.011; two independent sample t-test) on the 7th day; and in the LL-1 group 0.5 (0, 1), compared within the LB group (0 [0, 0.75], t[8] = −1, p = 0.334; two independent sample t-test) on the 14th day after recovery of nerve block. The inflammation scores of the sciatic nerve and skeletal muscle were higher than that in the saline group on 14th day (0[0,0], t[8] = −2.646, p = 0.033; two independent sample t-test) (Figure 5R and T). The pathological scores of nerve fiber degeneration were higher in the LL-1 group than that in the LB group on the 7th day after nerve block (t[8[= −4.583, p = 0.003; two independent sample t-test, Figure 5Q), which is higher than that in the saline group (0[0,0], t[8]= −4.583, p = 0.003; two independent sample t-test) while no differences were found on the 14th day between the two groups (t[8] = −0.607, p = 0.554; two independent sample t-test), and there was no significant difference compared with the saline group on the 14th day (0[0,0], t[8]= −1.528, p = 0.170; two independent sample t-test). For other pathological scores including fibrosis and hemorrhage, no difference was found (p > 0.05 by Kruskal–Wallis test) (Figure 5Q–T).
Discussion
Our present study indicates that LL-1 can produce a long-acting and fast-onset regional anesthetic effects with tissue toxicity similar to that of LB alone. The combination of QX-OH and LB induces a steady and additive inhibition of sodium currents, which may contribute to the fast-onset and long-acting nerve block of LL-1.
In our previous studies, we investigated the toxicity, pharmacokinetics, and pharmacodynamics of LL-1 in knee surgery and the sciatic nerve block rat model.7,8,10 LL-1 can provide sensory block with duration ~9 hour after knee surgery8 and regional anesthesia with duration ~17 hour in the sciatic nerve block rat models,6 while the toxicity of QX-OH in local tissue was similar with a relevant concentration of LB.7,24 However, it is still unknown why a combination of QX-OH and LB can provide longer analgesia than either QX-OH or LB alone. To explore the possible mechanisms for the long-lasting nerve block of LL-1, we investigated the effects of LL-1 on voltage-gated sodium channels, which are the principal molecular target of local anesthetics.
The maximum reduction in INa showed an additive interaction for QX-OH and LB. The application of LB at a concentration of ~IC50 induced a concentration-dependent inhibition of sodium currents by 65.51% ± 3.63%. The application of QX-OH alone at a concentration of 5 mM reduced the INa by 19.28% ± 11.47%. When QX-OH was added to LB at the above-mentioned concentrations, the INa was reduced by 83.88% ± 1.57%. This result is below that reported in our previous study, we found that the neuron uptake of QX-OH was greater in LL-1 than QX-OH alone and the uptake of bupivacaine was similar the treatment group exposed to LL-1 with bupivacaine, which indicated an additive effect between QX-OH and LB.25 Thus, QX-OH and LB may produce an additive interaction on the inhibition of sodium currents.
In addition, the residual sodium currents quickly recovered after washout of 40 μM LB, while these currents remained unchanged after washout of 40 μM LB+5 mM QX-OH. These results indicated that the inhibition of the INa induced by the combination of QX-OH and LB was more sustained than that observed by LB alone, which could at least partly account for the long-lasting regional anesthetic effects observed following exposure to LL-1.
LL-1 shows a similar low degree of nerve inflammation compared to LB exposure at the corresponding concentration. The maximum of the inflammation scores following LL-1 exposure was one point, which revealed that QX-OH did not enhance the local tissue toxicity of LB, while LL-1 presented low local toxicity and a good safety profile. Co-injection of LAs with additives is a relatively better method when compared with continuous infusion of LAs or a controlled-release local anesthetic formulation, which both present a high risk of local tissue incompatibility, mechanical injury, and infections.23 The combination of QX-314 and bupivacaine has an acceptable safety profile.7 Moreover, QX-OH presents considerably lower local toxicity than QX-314 at the same concentration.7 Therefore, the combination of QX-OH and LB shows a higher possibility and value in clinical translation. In addition, in our experiment, we did not observe any behavioral manifestations of central nervous system or cardiac toxicities for LL-1.
Some new sodium channel blockers have been recently described. Selective Nav1.7 inhibitors are a research direction in the field of analgesia, Ahuja et al first described GX-936, a arylsulfonamide antagonists, and its related inhibitors could bind to the activated state of voltage-sensor domain IV and opposed VSD4 deactivation.26 Bankar et al made an additional improvement on the arylsulfonamides, and developed a Nav 1.7 blocker with analgesic activity in inflammatory and neuropathic pain models having a 10-fold decrease in EC50.27 Cannabidiol26–28 is another type of analgesia; it non-selectively inhibits Nav channels from the inactivated state by preventing channel opening. Compared to these Nav channel blockers, LL-1 did not exert a direct effect on the IC50 and showed extremely prolonged block time. The different effects of LL-1 were mainly attributed to the special properties of its component QX-OH. QX-OH can only inhibit the Nav at concentrations over 5 mM, and the inhibition rate is only about 20%. If the concentration is reduced, the inhibition rate of QX-OH is similar to that of the control group alone. Only when QX-OH was combined with LB at a certain ratio, can LL-1 increase its potency and persistently inhibit Nav.
There are still limitations to our study. First, we speculated that there may be a synergetic or additive effect between QX-OH and LB both in nerve block and inhibition of Nav. However, analysis of synergetic effects using isobologram analysis could not be performed because QX-OH only minimally inhibits Nav even at the 5 mM concentration, thus we could not calculate the IC50 of QX-OH. Second, in the histological analysis, we use semi-quantitative assessment to evaluate the neuronal-specific damage, as HE and Fast blue staining are inadequate to precisely assess all the pathological features; histological analysis such as Masson’s Trichrome stain and immunohistochemical staining may be needed in the subsequent studies.
Conclusions
LL-1 (QX-OH+LB) can produce a fast-onset and long-lasting regional anesthetic effects with low local tissue toxicity in rat sciatic nerve block models. QX-OH and LB induce additive and constant inhibition of sodium currents. The fast-onset, remarkably long-lasting anesthetic effects, and low local tissue toxicity indicate the great potential for the clinical application of the QX-OH and LB combination.
Acknowledgments
The authors thank Li Li, Fei Chen, and Chunjuan Bao from the Institute of Clinical Pathology, West China Hospital of Sichuan University, for processing histological staining.
Funding
This work was supported by the National Natural Science Foundation of China (grants No. 81571353, J.L. and No. 81771486, C.Z.); and the Science & Technology Department of Sichuan Province (2019YJ0029, C.Z.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31(4):499–504. doi:10.1016/j.bpa.2017.07.002
2. Leone S, Di Cianni S, Casati A, Fanelli G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008;79(2):92–105.
3. Kuusniemi K, Poyhia R. Present-day challenges and future solutions in postoperative pain management: results from PainForum 2014. J Pain Res. 2016;9:25–36. doi:10.2147/JPR.S92502
4. Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol. 2011;164(1):48–58. doi:10.1111/j.1476-5381.2011.01391.x
5. Blumberg PM. Lighting a backfire to quench the blaze: a combined drug approach targeting the vanilloid receptor TRPV1. Mol Interv. 2007;7(6):310–312. doi:10.1124/mi.7.6.5
6. Zhao W, Yin Q, Liu J, Zhang W, Yang L. Addition of dexmedetomidine to QX-314 enhances the onset and duration of sciatic nerve block in rats. Can J Physiol Pharmacol. 2018;96(4):388–394. doi:10.1139/cjpp-2017-0331
7. Zhang Y, Yang J, Yin Q, Yang L, Liu J, Zhang W. QX-OH, a QX-314 derivative agent, produces long-acting local anesthesia in rats. Eur J Pharm Sci. 2017;105:212–218. doi:10.1016/j.ejps.2017.05.039
8. Zhao W, Yang J, Zhang Y, Liu J, Zhang W. QX-OH/Levobupivacaine: fixed-dose combination to provide a long-acting postoperative pain of knee surgery in rodents. Eur J Pharm Sci. 2018;111:418–424. doi:10.1016/j.ejps.2017.10.025
9. Wang Q, Yin Q, Yang J, et al. Evaluation of the cardiotoxicity and resuscitation of rats of a newly developed mixture of a QX-314 analog and levobupivacaine. J Pain Res. 2017;10:737–746. doi:10.2147/JPR.S126396
10. Yin Q, Zhang Y, Lv R, et al. A fixed-dose combination, QXOH/ levobupivacaine, produces long-acting local anesthesia in rats without additional toxicity. Front Pharmacol. 2019;10:243. doi:10.3389/fphar.2019.00243
11. Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11(2):178–186. doi:10.1038/nn2040
12. Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1(3):175–182. doi:10.2174/1568026013395335
13. Catterall WA, Swanson TM. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol Pharmacol. 2015;88(1):141–150. doi:10.1124/mol.114.097659
14. Gawali VS, Lukacs P, Cervenka R, et al. Mechanism of modification, by lidocaine, of fast and slow recovery from inactivation of voltage-gated Na (+) channels. Mol Pharmacol. 2015;88(5):866–879. doi:10.1124/mol.115.099580
15. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381–387. doi:10.1124/mol.113.090472
16. Leffler A, Fischer MJ, Rehner D, et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008;118(2):763–776. doi:10.1172/JCI32751
17. Wood JN, Bevan SJ, Coote PR, et al. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990;241(1302):187–194.
18. Stoetzer C, Martell C, de la Roche J, Leffler A. Inhibition of voltage-gated Na+ channels by bupivacaine is enhanced by the adjuvants buprenorphine, ketamine, and clonidine. Reg Anesth Pain Med. 2017;42(4):462–468. doi:10.1097/AAP.0000000000000596
19. Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82(4):1013–1025. doi:10.1097/00000542-199504000-00026
20. Shankarappa SA, Sagie I, Tsui JH, et al. Duration and local toxicity of sciatic nerve blockade with coinjected site 1 sodium-channel blockers and quaternary lidocaine derivatives. Reg Anesth Pain Med. 2012;37(5):483–489. doi:10.1097/AAP.0b013e31826125b3
21. Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199(2):87–90. doi:10.1016/0304-3940(95)12022-V
22. Feldman AT, Wolfe D. Tissue processing and hematoxylin and Eosin staining. Methods Mol Biol. 2014;1180:31–43.
23. Yin Q, Li J, Zheng Q, et al. The quaternary lidocaine derivative QX-314 in combination with bupivacaine for long-lasting nerve block: efficacy, toxicity, and the optimal formulation in rats. PLoS One. 2017;12(3):e0174421. doi:10.1371/journal.pone.0174421
24. Zhang Y, Yin Q, Gong D, et al. The preclinical pharmacological study of a novel long-acting local anesthetic, a fixed-dose combination of QX-OH/levobupivacaine, in rats. Front Pharmacol. 2019;10:895. doi:10.3389/fphar.2019.00895
25. Zhang Y, Gong D, Zheng Q, Liu J, Zhang W. LC-MS/MS method for preclinical pharmacokinetic study of QX-OH, a novel long-acting local anesthetic, in sciatic nerve blockade in rats. J Pharm Biomed Anal. 2017;146:161–167. doi:10.1016/j.jpba.2017.07.028
26. Ahuja S, Mukund S, Deng L, et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science (New York, NY). 2015;350(6267):aac5464
27. Bankar G, Goodchild SJ, Howard S, et al. Selective Na(V)1.7 Antagonists with Long Residence Time Show Improved Efficacy against Inflammatory and Neuropathic Pain. Cell reports. 2018;24(12):3133–3145.
28. Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 2018;293(43):16546–16558. doi:10.1074/jbc.RA118.004929
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.