Back to Journals » Drug Design, Development and Therapy » Volume 8
Quetiapine for acute bipolar depression: a systematic review and meta-analysis
Authors Suttajit S, Srisurapanont M , Maneeton N, Maneeton B
Received 8 March 2014
Accepted for publication 31 March 2014
Published 25 June 2014 Volume 2014:8 Pages 827—838
DOI https://doi.org/10.2147/DDDT.S63779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Sirijit Suttajit, Manit Srisurapanont, Narong Maneeton, Benchalak Maneeton
Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Background: Precise estimated risks and benefits of quetiapine for acute bipolar depression are needed for clinical practice.
Objective: To systematically review the efficacy and the tolerability of quetiapine, either as monotherapy or combination therapy, for acute bipolar depression.
Methods: We included all randomized, controlled trials (RCTs) comparing quetiapine with other treatments, including placebo, in patients with acute bipolar depression (bipolar I or II disorder, major depressive episode). Published and unpublished RCTs were identified using the Cochrane Central Register of Controlled Trials, MEDLINE®, Web of Knowledge™, CINAHL®, PsycINFO®, the EU Clinical Trials Register database, and ClinicalTrials.gov. The primary outcome was the change scores of depression rating scales.
Results: Eleven RCTs (n=3,488) were included. Two of them were conducted in children and adolescents. The change in depression scores was significantly greater in the quetiapine group compared with the placebo group (mean difference, [MD] =-4.66, 95% confidence interval [CI] -5.59 to -3.73). The significant difference was observed from week 1. Compared with placebo, quetiapine had higher incidence rates of extrapyramidal side effects, sedation, somnolence, dizziness, fatigue, constipation, dry mouth, increased appetite, and weight gain but lower risks of treatment-emergent mania and headache. Quetiapine treatment was associated with significant improvement of clinical global impression, quality of life, sleep quality, anxiety, and functioning.
Conclusion: Quetiapine monotherapy is effective for acute bipolar depression and the prevention of mania/hypomania switching. Its common adverse effects are extrapyramidal side effects, sedation, somnolence, dizziness, fatigue, constipation, dry mouth, increased appetite, and weight gain. The lower risk of headache in quetiapine-treated patients with acute bipolar depression should be further investigated. The evidence for the use of quetiapine combined with mood stabilizers in children and adolescents with acute bipolar depression is too small to support the clinical practice.
Keywords: efficacy, side effects, response, remission, antipsychotic, dropout
Background
Bipolar disorders are psychiatric illnesses defined by the presence of periodic episodes of mania/hypomania and depression. The lifetime prevalence of bipolar I, bipolar II, and subthreshold bipolar disorders are approximately 2%–4% of the general population.1 Their treatment costs are high as they normally require hospitalization.2 Moreover, bipolar disorders tend to be chronic and have complicated comorbidity, which leads to substantial disability.3 Up to 25%–55% of bipolar patients make a medically serious suicide attempt, and 10%–20% commit suicide.4 A depressive episode of bipolar disorder is associated with increased morbidity and mortality. Therefore, early recognition and effective treatment for its acute depressive episode not only reduces the treatment cost but also saves lives.
Quetiapine, a dibenzothiazepine derivative, is an atypical antipsychotic initially introduced for treating schizophrenia. A systematic review of quetiapine for schizophrenia reported that quetiapine is as effective as first-generation antipsychotics in the treatment of positive symptoms and general psychopathology, and causes fewer adverse effects, in terms of abnormal electrocardiogram, extrapyramidal effects, abnormal prolactin levels, and weight gain.5 At present, quetiapine is, not only approved for treatment but also, recommended as first-line treatment for acute bipolar depression by some guidelines.6,7
Quetiapine acts as an antagonist at 5-hydroxytryptamine (5-HT)1A, 5-HT2A, dopamine (D)1, D2, and histamine (H)1 receptors, as well as at adrenergic (α)1 and α2 receptors. Norquetiapine (N-desalkyl quetiapine) is an active metabolite of quetiapine with high affinity for norepinephrine transporters and partial agonism at serotonin 5-HT1A receptors.8 The mechanism by which quetiapine ameliorates depression may include 5-HT2A antagonism, 5-HT1A receptor partial agonism, α2b receptor antagonism, and D2 receptor antagonism.9 While the common side effects of quetiapine are somnolence, postural hypotension, dizziness, and dry mouth, some serious side effects include elevated blood glucose levels, diabetic coma, and ketoacidosis.5
Although precise estimated risks and benefits of quetiapine for acute bipolar depression are needed for clinical practice, an updated systematic review addressing this has not been performed. In this systematic review, we aimed to assess the efficacy and the tolerability of quetiapine, either as monotherapy or combination therapy, for acute depressive episode in patients with bipolar I or II disorder.
Objective
We aimed to systematically review the efficacy and the tolerability of quetiapine, either as monotherapy or combination therapy, for acute bipolar depression.
Methods
Types of studies and participants
We considered all relevant randomized, controlled trials (RCTs).
Types of participants
Participants included people with bipolar I or II disorder who currently had a major depressive episode, irrespective of the diagnostic criteria used, age, ethnicity, and sex.
Types of interventions
Quetiapine, as monotherapy or combination therapy, was investigated in comparison with placebo or other treatments. There was no restriction in the dose, dosage form, and frequency of treatment.
Types of outcome measures
Primary outcome measures
The primary outcome was the change in scores of depression rating scales (Montgomery-Asberg Depression Rating Scale [MADRS] and Children’s Depression Rating Scale, Revised [(CDRS-R]).10,11
Secondary outcome measures
Secondary outcomes measures were:
- Leaving the studies early: for any reason, for adverse events, or for inefficacy of treatment.
- Response: as defined by the individual studies.
- Relapse: as defined by the individual studies.
- Clinical global impression
- Anxiety: average change in the Hamilton Anxiety Rating Scale (HAM-A) score.14
- Quality of life: average change in the Quality of Life and Enjoyment and Satisfaction Questionnaire – Short Form (Q-LES-Q SF) score.15
- Sleep: average change in the Pittsburgh Sleep Quality Index (PSQI) score.16
- Disability: change in the Sheehan Disability Scale (SDS) score.17
- Adverse effects: number of participants with at least one serious adverse effect (ie, death, permanent damage, birth defects, or requires hospitalization) and at least one adverse effect; number of participants with treatment-emergent mania; number of participants with suicidal ideation; and clinically important specific adverse effects (death, sedation, somnolence, dry mouth, extrapyramidal side effects, dizziness, fatigue, constipation, headache, nausea, dyspepsia, increased appetite, decreased appetite, and weight gain).
4.1 Average change in Clinical Global Impressions-Severity (CGI-S) or Clinical Global Impression for Bipolar, severity of illness (CGI-BP-S) scale.12,13 The CGI-BP-S is a modified version of the CGI-S Scale for use in bipolar disorder. Both scales are 7-point scale which requires the clinician to rate the severity of the patient’s illness at the time of assessment.13 Therefore, in this study the CGI -S and the CGI-BP-S were combined in the same analysis and are mentioned as CGI-S/CGI-BP-S.
4.2 Average endpoint of the Clinical Global Impression-Improvement (CGI-I) scale.12
Search strategy for identification of studies
Electronic searches
The Cochrane Central Register of Controlled Trials, MEDLINE®, Web of Knowledge™, Cumulative Index to Nursing and Allied Health Literature (CINAHL®), PsycINFO®, the EU Clinical Trials Register database, and ClinicalTrials.gov were searched with the search strategy (September 2013): (quetiapine or seroquel) AND (bipolar depression or BD).
Searching other resources
The website of a pharmaceutical company that manufactures quetiapine (AstraZenecaTrials.com) was searched for relevant published and unpublished data with the same search strategy.
No language restriction was applied within the search tools.
Methods of the reviews
Selection of studies
Review authors SS and MS independently inspected the citations identified from the searches. We identified all relevant reports and obtained the full papers for reassessment. The retrieved articles were assessed independently by SS and NM for inclusion, according to the aforementioned inclusion criteria.
Data extraction
SS and BM independently extracted data from the included studies. Any disagreement was discussed with MS and decisions documented, and if necessary, we contacted the study authors for clarification.
Quality assessment
SS and MS independently assessed risk of bias, using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions.18 This set of criteria is based on the evidence of associations between effect overestimates and high risk of bias found in trial articles, such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
Measures of treatment effects
Data was entered into the Review Manager (RevMan) software (Version 5.2; The Nordic Cochrane Centre, Copenhagen, Denmark). For a dichotomous outcome, we calculated the risk ratio (RR) and its 95% confidence interval (CI) based on the random-effects model as this takes into account any difference between studies, even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive than odds ratios and that odds ratios tend to be misinterpreted as RR by clinicians.19,20 This misinterpretation then leads to an overestimate of the treatment effect.
For a continuous outcome, we estimated a mean difference (MD) between groups. Mean differences were based on the random-effects model as this takes into account any difference between studies even if there is no statistically significant heterogeneity.
Dealing with missing data
For missing participants due to drop out, intention to treat (ITT) was used, when available. When standard errors instead of standard deviations were presented, the former were converted to the standard deviations. If standard deviations were not reported and could not be calculated from the available data, the weighted mean of standard deviations from other studies was used.
Subgroup analyses
The subgroup analysis of children and adolescents was performed.
Assessment of heterogeneity
An I2 of 50% or more, accompanied by a statistically significant chi-square statistic, was interpreted as a significant heterogeneity of data, and reasons for heterogeneity were explored. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance.21 If the inconsistency was high and clear reasons were found, the data were presented separately.
Results
Results of the search
The overall search strategy yielded 1,525 reports of which 25 were considered as relevant and closely inspected. Of the 25 full-text papers, 14 were excluded because they did not completely match the inclusion criteria. Eleven studies, with 3,488 participants, fulfilled the inclusion criteria. All were short-term trials, with a duration range of 1–12 weeks (Figure 1).
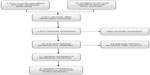  | Figure 1 Study flow diagram. |
Nine22–30 and two studies31,32 included adult and child/adolescent participants, respectively. Seven studies23–27,29,30 used fixed dosing of 300 mg or 600 mg of quetiapine. Overall, quetiapine was given in a dose range of 150–600 mg. Quetiapine monotherapy was compared with placebo,24,25,27,29–32 sertraline,22 paroxetine,25 lithium,30 quetiapine plus lithium,23 and Interpersonal and Social Rhythm Therapy (IPSRT)28 (Table 1).
Risk of bias in included studies
All included studies were described as randomized. While nine trials were “double blind”, one each was “open label” and “rater blind”. The “last observation carried forward” method was used to compensate for attritions in all studies. No study had the problem of selective reporting; nevertheless, most studies were sponsored by AstraZeneca (London, UK), a manufacturer of quetiapine (Figure 2).
  | Figure 2 Risk of bias summary. |
Quetiapine versus placebo
Depressive symptoms
There was a significant difference, favoring quetiapine, on the change in scores of the MADRS and the CDRS-R depression rating scales at the end of the studies (MD −4.66, 95% CI −5.59 to −3.73).24,25,27,29–32 The mean differences with quetiapine 300 mg/day and quetiapine 600 mg/day were superior to those with placebo (MD –4.72, 95% CI −6.02 to −3.42 and MD −4.92, 95% CI −6.32 to −3.51, respectively) (Figure 3).
  | Figure 3 Quetiapine versus placebo: average change in total depressive scores. |
Leaving the study early
Overall, dropout rates were not significantly different between groups (RR 0.99, 95% CI 0.88 to 1.13).24,25,27,29–32 While dropouts due to inefficacy were significantly lower in the quetiapine group (RR 0.31, 95% CI 0.19 to 0.53), dropouts due to adverse events were significantly higher in the quetiapine group (RR 1.88, 95% CI 1.20 to 2.96). The data of dropouts due to side effects were heterogeneous (I2=66%, χ2=29.66, df=10, P=0.001). The heterogeneity was likely due to the difference in dropouts between adults and children/adolescents since the dropouts due to side effects were higher in the quetiapine group in adults but not in children/adolescents.
Response and remission
The overall response rate, defined as ≥50% reduction on the depression rating scale scores, was higher in the quetiapine group at the end of the studies (RR 1.31, 95% CI 1.23 to 1.40; number needed to treat [NNT] 6, 95% CI 5 to 8)24,25,27,29–32 (Figure 4). In one study24 reporting the response rates at week 1, quetiapine was also significantly superior to placebo (RR 1.92, 95% CI 1.32 to 2.79; NNT 11, 95% CI 7 to 23).
  | Figure 4 Quetiapine versus placebo: response rate at end point. |
The overall remission rate, defined variously by the authors, was higher in the quetiapine group (RR 1.36, 95% CI 1.24 to 1.49; NNT 6, 95% CI 5 to 7).24,25,27,29–32 The significant superiority in this respect was found in the subgroups treated with quetiapine 300 mg/day and 600 mg/day (Figure 5).
  | Figure 5 Quetiapine versus placebo: remission rate at end point. |
Clinical global impression
There was a significant difference, favoring the quetiapine group, in the change in scores of both the CGI-S/CGI-BP-S24,25,27,29–32 (MD −0.45, 95% CI −0.56 to −0.34) and the CGI-I (MD −0.62, 95% CI −0.76 to −0.49). The significant differences were found in both subgroups treated with quetiapine 300 mg/day and 600 mg/day.
Anxiety
There was a significant difference, favoring quetiapine, in the change of the HAM-A (MD −2.44, 95% CI −3.34 to −1.55).24,25,29,30,32 The significant differences were found in both quetiapine 300 mg/day and 600 mg/day. The data were heterogeneous (I2=52%, χ2=16.53, df=8, P=0.04). The heterogeneity might have been due to directions of effect and one outlier of the analysis,32 in children/adolescents. Excluding this study, there was no heterogeneity, and there was a significant difference, favoring quetiapine (MD −2.89, 95% CI −3.55 to −2.22).
Quality of life
Three studies24,25,29 used the Q-LES-Q SF for the assessment of quality of life and found the superiority of quetiapine in terms of the change scores (MD 2.95, 95% CI 1.70 to 4.20). However, the subgroup analysis showed that the significant difference was found only in the subgroup treated with quetiapine 300 mg/day and not that treated with quetiapine 600 mg/day.
Sleep
There was only one study24 assessing the quality of sleep, using the PSQI. Participants receiving quetiapine were significantly improved in terms of quality of sleep (MD −2.31, 95% CI −2.95 to −1.66). The significant differences were found on both quetiapine doses (300 mg/day and 600 mg/day).
Disability
Three studies25,29,30 used the SDS for the assessment of the disability. There was a significant difference, favoring quetiapine, in the change of SDS scores (MD −1.42, 95% CI −2.32 to −0.53). The significant differences were found on both quetiapine doses (300 mg/day and 600 mg/day).
Adverse events
The participants having at least one adverse event was significantly higher in the quetiapine group (RR 1.18, 95% CI 1.12 to 1.25; number needed to harm [NNH] 13, 95% CI 9 to 26).24,27,30,31 The subgroup analysis showed that the significant difference was found only in the subgroup treated with quetiapine 300 mg/day (RR 1.21, 95% CI 1.13 to 1.30) but not in that with quetiapine 600 mg/day (RR 1.21, 95% CI 0.97 to 1.51). Moreover, there was no significant difference between quetiapine and placebo in the likelihood of having at least one serious adverse event (RR 0.85, 95% CI 0.49 to 1.48).24,25,27,29–32
Treatment-emergence mania was less likely in the quetiapine groups compared with the placebo groups (RR 0.58, 95% CI 0.37 to 0.92).24,25,29–32 The significant difference was found only in the subgroup treated with quetiapine 600 mg/day (RR 0.57, 95% CI 0.43 to 0.76) but not in that with quetiapine 300 mg/day (RR 0.64, 95% CI 0.22 to 1.87). The overall data were heterogeneous (I2=67%, χ2=24.34, df=8, P=0.002), although there was no heterogeneity in the quetiapine 600 mg/day group. The heterogeneity might have been due to one outlier30 and quetiapine dosage (Figure 6).
  | Figure 6 Quetiapine versus placebo: treatment-emergent mania. |
Compared with placebo, quetiapine caused more adverse effects of extrapyramidal side effects (RR 2.77, 95% CI 2.12 to 3.62; NNH 8, 95% CI 7 to 10),24,25,27,29–31 sedation (RR 3.32, 95% CI 2.71 to 4.06, NNH 8, 95% CI 7 to 9),24,25,27,29,30,32 somnolence (RR 3.74, 95% CI 2.86 to 4.90; NNH 7, 95% CI 6 to 8),24,25,27,29,30 dizziness (RR 2.18, 95% CI 1.73 to 2.74; NNH 14, 95% CI 11 to 20),24,25,27,29,30,32 fatigue (RR 1.57, 95% CI 1.16 to 2.13; NNH 35, 95% CI 21 to 132),24,25,27,29 constipation (RR 2.05, 95% CI 1.50 to 2.81; NNH 25, 95% CI 18 to 41),24,25,27,29,30 dry mouth (RR 3.65, 95% CI 3.04 to 4.40; NNH 5, 95% CI 4 to 6),24,25,27,29,30,32 increased appetite (RR 2.81, 95% CI 1.58 to 5.01; NNH 26, 95% CI 18 to 48),24,25,27,32 and weight gain (RR 2.33, 95% CI 1.34 to 4.03; NNH 29, 95% CI 19 to 57).24,27,29,31 Nevertheless, the quetiapine group reported a lower incidence rate of headache than did the placebo group (RR 0.68, 95% CI 0.53 to 0.86).24,25,27,29,30,32 The incidence rates of nausea and diarrhea were not significantly different between the quetiapine and placebo groups (RR 0.77, 95% CI 0.56 to 1.0724,25,27,29,30 and RR 0.64, 95% CI 0.40 to 1.01,24,30 respectively).
Subgroup analyses in children and adolescents
There was no significant difference between quetiapine and placebo on the change in total score of the Children’s Depression Rating Scale™-Revised (CDRS-R) in child and adolescent participants (MD −1.82, 95% CI −5.98 to 2.34).31,32 The response rate, the remission rate, overall dropout rate (due to any reason, inefficacy, or side effects) and rates of anxiety, adverse events (extrapyramidal side effects, sedation, dizziness, dry mouth, headache, increased appetite, and weight gain), and serious adverse events were not significantly different between groups. However, quetiapine was superior to placebo with respect to change in CGI-BP-S scores (MD −0.26, 95% CI −0.51 to −0.02).31,32
Quetiapine versus other treatments
Quetiapine versus selective serotonin reuptake inhibitors (SSRIs)
Monotherapy
The Efficacy of Monotherapy Seroquel in BipOLar DEpressioN (EMBOLDEN) II study investigated the efficacy and tolerability of quetiapine monotherapy compared with paroxetine monotherapy and placebo.25 It was found that the decrease in MADRS scores in the quetiapine group (both 300 mg/day and 600 mg/day) was significantly greater than that in the paroxetine group. However, the dose of paroxetine used in the study was relatively low, at 20 mg/day. The efficacy of paroxetine might be higher at a higher dose. The proportions of participants having at least one adverse event were similar among the three groups, at 65.8%, 70.1%, and 69.4% for quetiapine 300 mg/day, quetiapine 600 mg/day, and paroxetine, respectively. Nevertheless, the proportion of participants having at least one serious adverse event was higher in the paroxetine group compared with the quetiapine 300 mg/day and 600 mg/day groups, at 7.4%, 0.4%, and 3.7%, respectively.25
Combination therapy
A randomized pilot study compared the efficacy of quetiapine and sertraline for acute bipolar depression as adjunctive treatment to previous mood stabilizers.22 At week 8, there was no significant difference in the change of MADRS score between the quetiapine and the sertraline groups (MD −19.4, 95% CI −24.2 to −14.5 and MD −18.2, 95% CI −24.8 to −11.6, respectively). The proportion of participants having at least one adverse event was slightly but not significantly higher in the quetiapine group compared with the sertraline group (85.7% and 69.2%, respectively) (P=0.303). None of the participants in either group reported any serious adverse event.22
Quetiapine versus lithium
The EMBOLDEN I study compared the efficacy and tolerability of quetiapine monotherapy with those of lithium monotherapy and placebo.30 At week 8, the quetiapine 600 mg/day group, but not quetiapine 300 mg/day group, was found to have significantly greater changes in the MADRS scores than the lithium group (MD −2.49) (P=0.013). The proportions of participants reporting serious adverse events were similar among the quetiapine groups and the lithium group (3.8% and 2.6% for quetiapine 300 and 600 mg/day, 2.2% for lithium). However, the proportions of participants with clinically relevant weight gain (>7% from baseline) were higher in the quetiapine groups compared with the lithium group (4.6% and 8.3% for quetiapine 300 and 600 mg/day, 2.4% for lithium).30
Quetiapine versus quetiapine plus lithium
A single-blinded RCT compared the efficacy and safety between quetiapine 300 mg/day monotherapy and quetiapine in combination with lithium for acute bipolar depression.23 There was no significant difference in the change of MADRS total scores at week 8 between the quetiapine and the combination treatment groups (MD −21.6 and −21.9, respectively) (P=0.334). In addition, the response rates in both groups were similar (83.8% for quetiapine, 83.6% for quetiapine combined with lithium). However, the proportion of participants having weight gain was higher in the combination treatment group compared with the monotherapy group (23.5% and 12.8%, respectively).23
Quetiapine versus psychotherapy
A small randomized pilot study compared the feasibility and acceptability of quetiapine and the IPSRT for acute bipolar II depression.28 The IPSRT consists of interpersonal relationship therapy, psychoeducation, and behavioral interventions. There was no significant group-by-time interaction on the MADRS total scores. The response rates were not significantly different between groups (27% in the quetiapine group, 29% in the IPSRT group).28 Due to the low doses of quetiapine (50–300 mg/day) and the small sample size (N=25), limited conclusions could be drawn from this study.
Quetiapine extended release (XR) versus quetiapine immediate release (IR)
By using the self-reported modified Bond−Lader visual analog scale score,33 Riesenberg et al26 compared the sedative effect of quetiapine XR and IR. Between 1 and 3 hours after administration, 50 mg quetiapine XR had a significantly lower sedative effect than did quetiapine IR (P=0.009). The sedative intensities were not significantly different between the groups at 4 to 14 hours. The proportion of participants with at least one adverse event was significantly higher in the quetiapine IR group compared with the quetiapine XR group (71.0% versus 57.1%).26
Discussion
Our review supports the efficacy of quetiapine monotherapy for acute bipolar depression. Quetiapine can improve depression within 8 weeks of treatment, as demonstrated by a greater reduction of depression severity as well as higher response and remission rates and lower dropouts due to inefficacy, compared with placebo. Quetiapine is also associated with improved clinical global impression, quality of life, quality of sleep, anxiety, and functioning.
A higher dropout rate due to adverse events suggests that quetiapine is less tolerable than placebo. Its common side effects include sedation, somnolence, dizziness, fatigue, constipation, dry mouth, increased appetite, and weight gain. Despite its rapid dissociation from D2 receptors and high affinity for 5-HT2A receptors, our meta-analysis still found an increased risk of extrapyramidal side effects in bipolar depressed patients treated with quetiapine.34 Nevertheless, the comparable rates of serious adverse events between quetiapine and placebo suggest that quetiapine is safe for most patients with acute bipolar depression.
Atypical antipsychotic medications may have a role in the treatment of headache, especially migraine.35 Several neurotransmitters, such as dopamine and serotonin, may be involved in the pathophysiology of migraine.35 Quetiapine may prevent headache via its 5-HT2A and D2 antagonistic effects. The present findings that the quetiapine group had lower incidence of headache than the placebo group are in line with recent findings that quetiapine was beneficial for participants with treatment-resistant migraine.36
Our meta-analysis also suggests that quetiapine, especially 600 mg/day, has a protective effect against treatment-emergent mania. This efficacy is imperative in the treatment of bipolar depression since SSRIs and other antidepressants are associated with the high switching rates. Moreover, quetiapine may have higher efficacy but less incidence of serious adverse events than paroxetine in the treatment of bipolar depression.25 The aforementioned findings support the avoidance of antidepressant monotherapy for bipolar depression, as recommended by some guidelines.7
Five RCTs compared the efficacy and tolerability of quetiapine 300 mg and 600 mg/day. Taken together, these two doses were equi-effective. However, the dose of 600 mg/day may have a superior effect on sleep quality. The EMBOLDEN I study also found that quetiapine 600 mg/day, but not quetiapine 300 mg/day, was superior to lithium.30 However, the 300 mg/day dose may be superior to the 600 mg/day dose with respect to quality of life and weight gain. Participants who received quetiapine 300 mg/day, but not quetiapine 600 mg/day, had better quality of life than those treated with placebo. Moreover, quetiapine 600 mg/day caused more weight gain than did quetiapine 300 mg/day, although other adverse events were similar between the two groups.
The evidence for quetiapine combination therapy for acute bipolar depression is too small to guide practice. Quetiapine in combination with lithium may not be different from quetiapine monotherapy in terms of efficacy.23 Also, there was no significant difference between quetiapine and sertraline as adjunctive treatment to the ongoing treatment of mood stabilizers.22
Some limitations should be taken into account in interpreting the present findings. First, most of the studies were highly controlled (eg, had highly restricted inclusion and exclusion criteria for a participant), which may limit the application of the results to the real world. Second, because only a few studies compared quetiapine with lithium or SSRIs, making decisions on treatment choices may still be difficult. Little is known about the accurate risks and benefits of quetiapine for child/adolescent bipolar depression as only two studies with small sample size have been carried out in this population. Third, most of the studies were sponsored by a pharmaceutical company manufacturing quetiapine. The evidence so far suggests that pharmaceutical companies are more likely to report positive results of their medications than other funding sources.37
In conclusion, this systematic review underscores the efficacy of quetiapine monotherapy for acute bipolar depression. Quetiapine may have a protective effect on treatment-emergent mania and headache. Because there is little evidence to support the uses of quetiapine in combination with mood stabilizers and quetiapine for children and adolescents with acute bipolar depression, more studies in these areas are needed.
Disclosure
SS received honoraria and/or consultacy fees from AstraZeneca, Janssen-Cilag, Lundbeck, and Thai-Otsuka. MS received honoraria, consultancy fees, research grants, and/or travel reimbursement from AstraZeneca, Janssen-Cilag, Johnson&Johnson, Lundbeck, Thai-Otsuka, Sanofi-Aventis, and Servier. NM received travel reimbursement from Thai-Otsuka and Lundbeck. BM received honoraria and/or travel reimbursement from GlaxoSmithKline and Pfizer.
References
Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. | |
Woods SW. The economic burden of bipolar disease. J Clin Psychiatry. 2000;61 Supp 13:S38–S41. | |
Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68(2–3):167–181. | |
Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford: Oxford University Press; 1990. | |
Suttajit S, Srisurapanont M, Xia J, Suttajit S, Maneeton B, Maneeton N. Quetiapine versus typical antipsychotic medications for schizophrenia. Cochrane Database Syst Rev. 2013;5:CD007815. | |
Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force On Treatment Guidelines For Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11(2):81–109. | |
Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. | |
Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology. 2008;33(10):2303–2312. | |
Yatham LN, Goldstein JM, Vieta E, et al. Atypical antipsychotics in bipolar depression: potential mechanisms of action. J Clin Psychiatry. 2005;66 Suppl 5:40–48. | |
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. | |
Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J Child Adolesc Psychopharmacol. 2010;20(6):513–516. | |
Guy W, ed ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. | |
Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. | |
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. | |
Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. | |
Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. May 1989;28(2):193–213. | |
Sheehan DV. The Anxiety Disease. New York: Scribner’s; 1983. | |
Higgins JPT Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated Mar 2011]. Oxford: The Cochrane Collaboration; 2011. | |
Boissel JP, Cucherat M, Li W, et al. [The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use]. Thérapie. 1999;54(4):405–411. French. | |
Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575–1600. | |
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | |
AstraZeneca [Webpage on Internet]. Effectiveness of Quetiapine XR versus Sertraline in acute depression as add-on therapy to previous mood stabilizer treatment: a pilot study. 2012. Available from: http://www.astrazenecaclinicaltrials.com/Submission/View?id=1406. Accessed September 17, 2013. | |
AstraZeneca [Webpage on Internet]. A Randomised, Multi-Centre Study to Compare the Efficacy and Safety of Extended Release Quetiapine Fumarate (Seroquel XR) Tablets as Mono-Therapy or in Combination with Lithium in the Treatment of Patients with Acute Bipolar Depression. 2012. Available from: http://astrazenecagrouptrials.pharmacm.com/Submission/View?id=1405. Accessed September 17, 2013. | |
Calabrese JR, Keck PE, Jr., Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. July 2005;162(7):1351–1360. | |
McElroy SL, Weisler RH, Chang W, et al. EMBOLDEN II (Trial D1447C00134) Investigators. A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry. 2010;71(2):163–174. | |
Riesenberg RA, Baldytcheva I, Datto C. Self-reported sedation profile of quetiapine extended-release and quetiapine immediate-release during 6-day initial dose escalation in bipolar depression: a multicenter, randomized, double-blind, phase IV study. Clin Ther. 2012;34(11):2202–2211. | |
Suppes T, Datto C, Minkwitz M, Nordenhem A, Walker C, Darko D. Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression. J Affect Disord. 2010;121(1–2):106–115. | |
Swartz HA, Frank E, Cheng Y. A randomized pilot study of psychotherapy and quetiapine for the acute treatment of bipolar II depression. Bipolar Disord. 2012;14(2):211–216. | |
Thase ME, Macfadden W, Weisler RH, et al. BOLDER II Study Group. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol. 2006;26(6):600–609. | |
Young AH, McElroy SL, Bauer M, et al. EMBOLDEN I (Trial 001) Investigators. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I). J Clin Psychiatry. 2010;71(2):150–162. | |
AstraZeneca [Webpage on Internet]. An 8-week, Multicenter, Double-blind, Randomized, Parallel-group, Placebo-controlled Study of the Efficacy and Safety of Quetiapine Fumarate (SEROQUEL) Extended-Release in Children and Adolescent Subjects with Bipolar Depression. 2011. Available from: http://www.astrazenecaclinicaltrials.com/Submission/View?id=1464. Accessed September 17, 2013. | |
DelBello MP, Chang K, Welge JA, et al. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord. 2009;11(5):483–493. | |
Bond A, Lader M. The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology. 1974;47(3):211–218. | |
Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47(1):27–38. | |
Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14(1):27–35. | |
Krymchantowski AV, Jevoux C, Moreira PF. An open pilot study assessing the benefits of quetiapine for the prevention of migraine refractory to the combination of atenolol, nortriptyline, and flunarizine. Pain Med. 2010;11(1):48–52. | |
Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326(7400):1167–1170. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

