Back to Journals » Clinical Interventions in Aging » Volume 16
Quantitative Assessment of Serum Amino Acids and Association with Early-Onset Coronary Artery Disease
Authors Xuan C , Li H, Tian QW, Guo JJ, He GW, Lun LM, Wang Q
Received 24 December 2020
Accepted for publication 25 February 2021
Published 15 March 2021 Volume 2021:16 Pages 465—474
DOI https://doi.org/10.2147/CIA.S298743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Chao Xuan,1 Hui Li,1 Qing-Wu Tian,1 Jun-Jie Guo,2 Guo-Wei He,3,4 Li-Min Lun,1 Qing Wang1
1Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Department of Cardiology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 3Center for Basic Medical Research & Department of Cardiovascular Surgery, TEDA International Cardiovascular Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, People’s Republic of China; 4Department of Surgery, Oregon Health and Science University, Portland, OR, USA
Correspondence: Chao Xuan
Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, No. 1677, Wutai Mountain Road, Qingdao (West Coast), 266500, People’s Republic of China
Tel +86-532-82919388
Email [email protected]
Qing Wang
Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, No. 59, Haier Road, Qingdao, 266100, People’s Republic of China
Tel +86-532-82913085
Email [email protected]
Background: Amino acids play essential roles in protein construction and metabolism. Our study aims to provide a profile of amino acid changes in the serum of patients with early-onset coronary artery disease (EOCAD) and identify potential disease biomarkers.
Methods: Ultra-performance liquid chromatography-multiple reaction monitoring-multistage/mass spectrometry (UPLC-MRM-MS/MS) was used to determine the amino acid profile of patients with EOCAD in sample pools. In the validation stage, the serum levels of candidate amino acids of interest are determined for each sample.
Results: A total of 128 EOCAD patients and 64 healthy controls were included in the study. Eight serum amino acids associated with disease state were identified. Compared with the control group, serum levels of seven amino acids (L-Arginine, L-Methionine, L-Tyrosine, L-Serine, L-Aspartic acid, L-Phenylalanine, and L-Glutamic acid) increased and one (4-Hydroxyproline) decreased in the patient group. Results from the validation stage demonstrate that serum levels of 4-Hydroxyproline were significantly lower in myocardial infarction (MI) patients (9.889 ± 3.635 μg/mL) than those in the controls (16.433 ± 4.562 μmol/L, p < 0.001). Elevated serum 4-Hydroxyproline levels were shown to be an independent protective factor for MI (OR = 0.863, 95% CI: 0.822– 0.901). The significant negative correlation was seen between serum 4-Hydroxyproline levels and cardiac troponin I (r = − 0.667) in MI patients.
Conclusion: We have provided a serum amino acid profile for EOCAD patients and screened eight disease state-related amino acids, and we have also shown that 4-Hydroxyproline is a promising target for further biomarker studies in early-onset MI.
Keywords: amino acid, coronary artery disease, myocardial infarction, 4-Hydroxyproline
Introduction
Cardiovascular disease (CVD) is a leading global cause of death. It has a high incidence rate in China and epidemiological studies indicate that the burden of CVD has rapidly and substantially increased, with approximately 2.4 million deaths in 2016.1 Although it generally affects the elderly, the number of young people suffering from the disease is increasing because of lifestyle changes. Early-onset coronary artery disease (EOCAD) is a CVD type, defined by the patient’s age (<50 years old) when the initial diagnosis of coronary heart disease (CAD) is made. The majority of patients are male.2,3 EOCAD patients have a poor long-term prognosis, meaning that identifying risk factors is critical for disease diagnosis and control.
Amino acids are the building blocks of proteins and play central roles as metabolic intermediates. Their chemical properties and combinations not only determine the structure and function of proteins but also regulate vital, disease-associated, metabolic pathways.4 Metabolomics is the comprehensive study of metabolites in bio-fluids, tissues, or cellular extracts and is a powerful tool for discovering biomarkers.5 As important metabolites, amino acids are also a relevant research target for metabolomics. Over the past two decades, metabolomic studies have demonstrated significant changes in blood amino acid levels in a variety of diseases, including diabetes, cancers, chronic kidney disease, and Alzheimer’s disease.6–9
The heart beats about 4 billion times in a person’s lifetime and it must continuously adapt to changing metabolic demands to meet substantial ongoing energy needs. It is therefore to be expected that metabolites are disturbed in CVD. Metabolomics studies have confirmed that metabolic changes occur in CVD and have identified some biomarkers.10,11 However, few studies have focused on serum amino acid changes in CAD patients, especially those with EOCAD. In this study, we quantified levels of 25 amino acids in the serum of patients with EOCAD using targeted metabolomic techniques to identify disease-related changes and screen for potential disease biomarkers. These biomarkers may help understand the pathophysiological process of early-onset coronary artery disease and may have special significance for the disease risk assessment and prediction.
Patients and Methods
Subjects
A total of 128 CAD patients were recruited from the Affiliated Hospital of Qingdao University between September 2019 and July 2020. All were younger than 50, met the diagnostic criteria for CAD, and diagnosis was confirmed by coronary angiography. CAD was defined by the presence of; at least 50% stenosis in one or more of the major coronary arteries, clinical symptoms of angina, changes in cardiac troponin, and typical disease electrocardiogram patterns. Based on the severity of the clinical presentations, each case was put into one of two groups: CAD with (n = 64) and without myocardial infarction (MI) (n = 64). Sixty-four age and gender-matched control subjects who did not show any signs or symptoms of cardiovascular events were recruited from a pool of Health Management Centre volunteers who had a similar geographic background. The study protocol conformed to the 1975 Helsinki declaration guidelines and was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University. Informed consent (written or verbal) was obtained from all participants and recorded on an electronic datasheet. Participants generally sign informed consent. But it is not possible to obtain the person’s signature (illiteracy, physical disability or other limiting condition) and verbal consent is an alternative. Verbal informed consent was acceptable and approved by the ethics committee of The Affiliated Hospital of Qingdao University.
Sample Preparation and Biochemical Measurements
All participants fasted for at least 8 hours and blood samples were collected on the morning of the second day after admission. Whole blood was collected using BD® anticoagulant-free vacuum blood collection tubes, allowed to stand at 4 °C for half an hour, then centrifuged at 3500 rpm for 10 minutes and the serum collected. Two hundred microliters of serum from each sample was taken and mixed with eight samples from the same group to make one sample pool. All samples were frozen at −80 °C until required for analysis.
Serum activity/concentrations of fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), serum creatinine (SCr), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and lipoprotein(a)(Lp(a)) were determined using an automatic biochemistry analyzer (Hitachi HCP-7600, Hitachi, Japan).
Metabolite Extraction and Standard Solution
Samples were defrosted in an ice water bath then vortexed for 30 s and centrifuged at 10,000 rpm for 10 min at 4 °C. Forty microliters of each sample was transferred to a tube and 160 μL of pre-cooled extraction solution (Acetonitrile-methanol, 1:1, 500 nmol/L internal standard solution, −20 °C) was added. Samples were swirled for 30 s, sonicated for 10 min at 0 °C, then incubated at −20 °C for 1 h before being centrifuged at 12,000 rpm for 15 min at 4 °C. Eighty microliters of clear supernatant was transferred to an auto-sampler vial for ultra-performance liquid chromatography-multiple reaction monitoring-multistage/mass spectrometry (UHPLC-MRM-MS/MS) analysis.
A stock solution (10 mmol/L) was obtained by dissolving or diluting each standard substance. A portion of each stock solution was transferred to a 10 mL flask to make a mixed working standard solution. A series of calibration solutions were obtained by diluting the standard solution, in turn, each of which contained 400 nmol/L of the internal standard mixture.
UHPLC-MRM-MS Analysis
UHPLC separation was performed using an Agilent 1290 Infinity II series UHPLC System (Agilent Technologies), equipped with a Waters ACQUITY UPLC BEH Amide column (100 × 2.1 mm, 1.7 μm). The mobile phase A was a 1% aqueous solution of formic acid, and the mobile phase B was a 1% acetonitrile solution of formic acid. The elution gradient used is shown in Table S1. The column temperature was 35 °C, the sample-plate temperature was 4 °C, and the sample volume injected was 1 μL. An Agilent 6460 triple quadrupole mass spectrometry (Agilent Technologies, USA) with an electrospray interface and multiple reaction monitoring (MRM) modes was used for the quantitative analysis. The parameters of the typical ion source were as follows, N2 Flow: 5 L/min, N2 Temperature: 300 °C, Sheath Gas Flow: 11 L/min, Sheath Gas Temperature: 250 °C, Capillary Voltage: +4000/-3500 V, Nozzle Voltage: +500/-500 V, and Nebulizer: 45 psi. Several of the most sensitive transitions were used in the MRM scan mode to optimize the collision energy for each Q1/Q3 pair (Table S2). Of the optimized MRM transitions per analyte, the Q1/Q3 pairs which showed the highest sensitivity and selectivity were selected as a “quantifier” for quantitative monitoring. The additional transitions acted as a “qualifier” to verify target analyte identities. MRM data were collected and processed with Agilent® Mass Hunter Work Station Software Version B.06.00 (Agilent Technologies, Santa Clara, CA, USA).
Calibration solutions were subjected to UPLC-MRM-MS/MS analysis using the methods described above and the results summarized in Table S3. Concentration (nmol/L) is on the X-axis, and the Y-axis is the ratio of peak areas in the calibration curves. The least-squares method was used for regression fitting. 1/x weighting was applied in the curve fitting since it provided the highest accuracy and correlation coefficient. If the signal-to-noise ratio (S/N) of a calibration concentration was close to or less than 20, or the recovery rate exceeds the range of 80–120%, the calibration point of that concentration was excluded.
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The calibration solution was diluted 2-fold in turn and analyzed via UHPLC-MRM-MS/MS. The lower limits of detection (LLOD) and quantitation (LLOQ) were determined using the S/N. The LLOD was defined as the compound concentration at which the S/N was 3, and the LLOQ was defined as the compound concentration at which the S/N was 10 (US FDA guideline for bioanalytical method validation). The method’s precision was evaluated by the relative standard deviation (RSD) of repeated injections of QC samples (Table S4) and accuracy was assessed by the recovery rate of QC samples. The spiked recovery rate is the percentage difference between the mean measured concentration and spiked concentration.
Experiment Protocol
The study was divided into two stages. In the first stage, 200 μL serum was taken from each sample and mixed into a sample pool with 8 other samples from the group. There were 8 sample pools in the CAD with MI group, 8 in the CAD without MI group, and 8 in the control group. UHPLC-MRM-MS/MS was used to analyze the levels of 25 serum amino acids and screen for potential differences between groups. In the second stage, UHPLC-MRM-MS/MS was used to verify the screened differential amino acids of interest in each sample and study their relationship with CAD.
Statistical Analysis
The results are presented as the mean ± standard deviation (SD). Comparisons of categorical variables were using the chi-square or Fisher exact tests. For independent samples, a t-test or one-way ANOVA was used to assess any group differences, and a post hoc least significant difference (LSD) test was used to evaluate any relevant interactions. Logistic regression was used to test the interactive effects of variables on the observed association and Pearson correlation coefficients were used to uncover interrelationships. Significance was set at p < 0.05 and all statistical analyses were performed with the Statistical Package for Social Sciences SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism Software 8 (GraphPad Software Inc., San Diego, CA, USA).
Results
A total of 128 CAD patients (mean age 44.27 ± 5.26 years) and 64 healthy controls (mean age 44.82 ± 5.12 years) were involved in the study. The clinical features of all participants are summarized in Table 1. Twenty-five amino acids were quantified by UHPLC-MRM-MS/MS and the details of the targeted amino acids are shown in Table 2. For all analytes, the LLODs ranged from 2.44 to 97.66 nmol/L, the LLOQs from 4.888 to 1562.50 nmol/L, and the correlation coefficients of the regression fitting were all above 0.9967. Recovery rates for all analytes were determined to be 91.3%–106.2%, with all RSDs below 3.5%.
 |
Table 1 Demographic and Clinical Characteristics of EOCAD Patients and Controls |
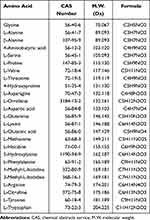 |
Table 2 Targeted Amino Acid Information |
In the first stage, every eight serum samples were combined into a single sample pool. The study included CAD with and without MI groups, and control group, with each group containing 8 sample pools. Of the 25 amino acids examined, differences in serum levels between groups of eight were seen (One-way ANOVA, p < 0.05).
A significant difference in the serum level of L-Phenylalanine was found between the three groups. L-Phenylalanine levels in the CAD with MI group (97.928 ± 12.982μmol/L, n = 8) were significantly increased compared to the control (70.945 ± 5.411μmol/L, n = 8, p < 0.001) and CAD without MI groups (82.473 ± 11.789 μmol/L, n = 8, p = 0.008). A significant difference was also detected between the control and CAD without MI groups (p = 0.041).
With L-Methionine and L-Glutamic acid, the L-Methionine levels in CAD without MI (38.263 ± 6.830 μmol/L, n =8, p = 0.020) and CAD with MI groups (41.641 ± 3.151 μmol/L, n = 8, p = 0.001) were significantly higher than the control group (32.028 ± 4.076 μmol/L, n = 8). The L-Glutamic acid levels in the CAD without MI (176.280 ± 32.170 μmol/L, n = 8, p = 0.042) and CAD with MI groups (211.995 ± 61.575 μmol/L, n = 8, p = 0.001) were significantly higher than the control group (131.046 ± 20.339 μmol/L, n = 8).
Compared to the control (14.622 ± 1.237 μmol/L, n = 8, p < 0.001) and CAD without MI groups (13.785 ± 3.031 μmol/L, n = 8, p < 0.001), the 4-Hydroxyproline levels were lower in the CAD with MI group (8.503 ± 1.467 μmol/L, n = 8, Figure 1). The opposite was seen with L-Aspartic acid. The L-Aspartic acid levels in the CAD with MI group (63.870 ± 15.143 μmol/L, n = 8) were significantly higher than the control (43.140 ± 5.700 μmol/L, n = 8, p = 0.001) and CAD without MI groups (51.493 ± 9.076 μmol/L, n = 8, p = 0.031).
Significant differences between the control and CAD with MI groups were seen in L-Tyrosine, L-Arginine, and L-Serine. The L-Tyrosine levels in the CAD with MI group (122.558 ± 12.331 μmol/L, n = 8) were higher than the control (100.578 ± 12.785 μmol/L, n = 8, p = 0.011), but no significant difference was found with the levels of the CAD without MI group (116.025 ± 20.472 μmol/L, n = 8, p = 0.413). The L-Arginine levels in the CAD with MI group (147.619 ± 15.357 μmol/L, n =8) were higher than the control (120.111 ± 13.681 μmol/L, n = 8, p = 0.007), but not the CAD without MI groups (136.484 ± 23.943 μmol/L, n = 8, p = 0.235). The L-Serine levels in the CAD with MI group (171.436 ± 22.250 μmol/L, n = 8) was higher than control (142.516 ± 9.832 μmol/L, n = 8, p = 0.005), but not the CAD without MI groups (136.484 ± 23.943 μmol/L, n = 8, p = 0.235). C) L-Serine levels in the CAD with MI group (171.436 ± 22.250 μmol/L, n = 8) was higher than control (142.516 ± 9.832 μmol/L, n = 8, p = 0.005), but not the CAD without MI groups (154.917 ± 20.684 μmol/L, n = 8, p = 0.088).
4-Hydroxyproline was the only amino acid which had reduced levels in the CAD with MI group and was thus selected for further validation. In the validation stage, 4-Hydroxyproline levels were measured in all 192 patient and control samples. 4-Hydroxyproline levels in MI patients (9.889 ± 3.635 μg/mL, n = 64) were significantly lower than those in the control samples (16.433 ± 4.562 μmol/L, n = 64, p < 0.001, Figure 2). After adjusting for age, body mass index, hypertension, diabetes, smoking status, drinking status, FBG, TG, TC, SCr, HDL-C, LDL-C, and Lp(a), the elevated serum 4-Hydroxyproline levels were thought to be an independent protective factor for MI (OR = 0.863, 95% CI: 0.822–0.901, p < 0.001, Table 3). The main results are shown in Table 3. A significant negative correlation was seen between serum 4-Hydroxyproline levels and cardiac troponin I (r = −0.667, p < 0.001, Figure 3) in MI patients.
 |
Table 3 Associations Between Serum 4-Hydroxyproline Levels and Presence of MI |
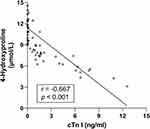 |
Figure 3 Negative correlation between serum 4-Hydroxyproline and cardiac troponin I (cTn I) levels in MI patients (r = −0.667, 95% CI: −0.7847 to −0.5046, p < 0.001). |
Discussion
In the study, we quantified the levels of 25 amino acids in the serum of EOCAD patients and identified eight that were related to the disease state. 4-Hydroxyproline was validated because it was the only amino acid with low serum levels in the disease group. Our results showed that 4-Hydroxyproline levels were significantly decreased in patients with early-onset MI, and that elevated serum levels were a protective factor for EOCAD.
CAD is a complex disease caused by genetic factors and environmental interactions. The relationship between CAD and endogenous/exogenous lipid metabolism, nutrition, inflammation, and immune response has been widely studied. With the rapid development of metabolomics, the relationship between circulating metabolites and CVD has also been widely concerned. Notably, several large prospective studies have identified a range of circulating metabolites associated with cardiovascular events, including amino acids, glycolysis-related metabolites, ketone bodies, inflammatory factors, fatty acids, lipids, and others. Amino acids mainly include phenylalanine/tyrosine,12 glutamate,13,14 branching amino acids (BCAAs),15,16 tryptophan-related metabolites,17 and modified arginine.18–20 Besides, some studies have focused on the relationship between amino acid profiles and CAD. In one of these studies, researchers from Duke University quantified plasma levels of 15 amino acids in CAD patients and indicated that in patients referred for cardiac catheterization, levels of branched-chain amino acids and catabolites are predictors of cardiovascular events.21 However, the quantitative results of these 15 amino acids have not been published in the study. A subsequent study from this group quantified the plasma levels of Proline, Leucine/Isoleucine, Valine, Methionine, Glutamic acid, and Citrulline, and showed that Leucine/Isoleucine, Glutamic acid, and Methionine levels are significantly elevated in CAD patients.10 In 2005, Obeid also attempted to find differential amino acid levels in the plasma of CAD patients by HPLC. Limited by test methodology and sample size, they found that of the 23 amino acids tested, only cysteine was significantly elevated in the plasma of CAD patients tested.22 Circulating BAACs (isoleucine, leucine, and valine) have been previously identified as significant predictors of CAD. A large prospective study of American women demonstrated that circulating BCAAs levels were associated with the long-term risk of CAD, comparable in magnitude to LDL cholesterol.23 Similar results have also been observed in the Chinese population.24,25 In previous studies, we examined the effects of homocysteine (Hcy), asymmetric dimethylarginine (ADMA), L-citrulline, and L-arginine on arterial endothelial function and their associations with the risk of CAD.26–30
To the best of our knowledge, a complete description of serum amino acid levels of EOCAD is lacking. Compared to late-onset CAD, EOCAD may have different pathogenic mechanisms and a wide range of biomarkers have already been identified for EOCAD, including genes, proteins, and other bio-macromolecules. Besides, gender differences are present in many diseases and are especially prevalent in CVD. Males tend to suffer from CAD earlier than females. As a result, the majority of subjects with EOCAD in our study are male. In our previous studies, the MTHFR C677T polymorphism was shown to be associated with a risk of EOCAD,31 serum ADMA levels were associated with the presence and severity of EOCAD,27 and that there is a close relationship between lipid metabolism and early-onset MI.32 Because of the different dietary structures, serum amino acid levels may differ in CAD patients of different ages.33,34 At the screening stage of this study, one sample pool was composed of eight serum samples. We quantified 25 amino acids in 24 serum sample pools of three groups (CAD without MI group, MI group, and control group) and differences in a total of eight different amino acids were identified. Significant differences in L-Phenylalanine, L-Methionine, and L-Glutamic acid levels were seen between the CAD without MI and control groups. Significant differences in L-Phenylalanine, L-Methionine, L-Tyrosine, 4-Hydroxyproline, L-Glutamic acid, L-Aspartic acid, L-Arginine, and L-Serine levels were seen between the CAD with MI and control groups. Compared to the CAD without MI group, in the CAD with MI group, L-Phenylalanine and L-Glutamic acid levels were significantly increased, and 4-Hydroxyproline levels were decreased. Notably, 4-Hydroxyproline was the only amino acid whose levels decreased in the serum of the disease group serum, suggesting that it may play a specific role in CAD pathogenesis.
Because there is a paucity of systematic studies on the correlation between non-BCAAs and CVD, it is difficult to discuss causes and possible mechanisms of changes in serum amino acid levels in CAD patients. L-Phenylalanine, a precursor of L-Tyrosine, is an essential amino acid and both are also precursors for catecholamines.35 Murr et al showed that elevated serum L-phenylalanine and L-Tyrosine levels in CAD patients may be associated with immune activation and inflammatory response.36
We recently focused on the relationship between one-carbon metabolism and CVD. We found that various metabolites in the metabolic pathway are closely related to the risk of CAD.37 Hcy is formed in the one-carbon metabolism pathway during the metabolism of methionine to cysteine, and can also be used to regenerate methionine by vitamin B12.38 Other studies have shown that serum Hcy levels are significant in CAD patients and that it is an independent risk factor for the disease.39–41
Although glutamic acid is a non-essential amino acid, almost all living beings use it in protein biosynthesis. Animal studies have shown that exogenous glutamic acid protects hypoxic cardiomyocytes and enhances anaerobic ATP synthesis in mitochondria to reduce myocardial contracture.42,43 Elevated serum levels of L-Glutamic acid may be a compensatory response to myocardial ischemia and can promote the formation of anaerobic ATP, enhancing the body’s resistance to severe hypoxia. Recent studies have shown that specific genetic variations can disrupt glutamic acid metabolism and lead to CVD.3,44
L-Glutamic acid is a precursor of L-Arginine45 which is the substrate of nitric oxide synthase and the biological precursor of nitric oxide. It is vital for the maintenance of vascular endothelial function, which is closely related to coronary heart disease.46 The results of this study indicate that L-glutamate and L-Arginine serum levels are significantly elevated in CAD patients, especially those with MI.
Only a few studies have focused on the correlation between aspartic acid, serine, and CVD. Elevated serum L-Aspartic acid levels may be related to its involvement in antiplatelet aggregation during acute MI,47 and L-Serine may play an antioxidant role.48
As early as 2009, Vallejo et al observed that 4-Hydroxyproline plasma levels were significantly decreased in patients with acute coronary syndrome using plasma fingerprinting with GC-MS technology.49 This indicated that 4-Hydroxyproline is an attractive compound for further studies. In this study, 4-Hydroxyproline was once again identified as being associated with MI. 4-Hydroxyproline levels were significantly decreased in CAD with MI patients, however, no significant decrease was seen in CAD without MI patients. Since 4-Hydroxyproline is related to collagen stability, this suggests that decreased serum 4-Hydroxyproline levels are associated with ventricular remodeling.50 Our results show that there is a significant negative correlation between 4-Hydroxyproline and cardiac troponin I levels, suggesting a potential relationship between 4-Hydroxyproline and myocardial injury. We expect 4-Hydroxyproline will become a novel biomarker for predicting fibrosis and ventricular remodeling after MI.
The development and progression of atherosclerosis eventually lead to the occurrence of CAD. Protein and amino acid metabolism affect the process of atherosclerosis through immune regulation, glucose and lipid metabolism, and other aspects. However, the relationship between abnormal protein and amino acid metabolism and CAD has been underestimated until now. Our study provides a profile of the serum amino acid changes in EOCAD patients. Although this is a preliminary study, these observations may open up a new field in the study of myocardial ischemia injury.
Our study has some limitations. Firstly, due to funding reasons, we have not measured all the identified amino acids individually except 4-Hydroxyproline, and the relationship between other amino acids and EOCAD still needs to be verified by large sample size studies. Secondly, because amino acid determination requires fresh samples and female patients with EOCAD are rare. Although there may be differences in serum amino acid levels between genders, subgroup analysis could not be performed. Finally, coronary angiography was not performed in healthy controls as they were age- and sex-matched individuals with no signs or symptoms of CAD.
In conclusion, we initially quantified serum levels of 25 amino acid levels in EOCAD patients and identified eight amino acids associated with disease status. Compared to the control group, serum levels of L-Arginine, L-Methionine, L-Tyrosine, L-Serine, L-Aspartic acid, L-Phenylalanine, and L-Glutamic acid were higher in the EOCAD group, and 4-Hydroxyproline levels were lower. 4-Hydroxyproline appears to be a promising amino acid for further biomarker studies of predicting fibrosis and ventricular remodeling after MI.
Data Sharing Statement
The datasets used and/or analyzed during this current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the study was supported by grants from the National Natural Science Foundation of China (no. 81672073), China Postdoctoral Science Foundation (no. 2016M590620), and Shandong Medical and Healthy Outstanding Personalities Cultivation Project (no. 3522).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203–212. doi:10.1038/s41569-018-0119-4
2. Filippi E, Sentinelli F, Romeo S, et al. The adiponectin gene SNP+276G>T associates with early-onset coronary artery disease and with lower levels of adiponectin in younger coronary artery disease patients (age <or=50 years). J Mol Med. 2005;83(9):711–719. doi:10.1007/s00109-005-0667-z
3. Ichihara S, Yamamoto K, Asano H, et al. Identification of a glutamic acid repeat polymorphism of ALMS1 as a novel genetic risk marker for early-onset myocardial infarction by genome-wide linkage analysis. Circ Cardiovasc Genet. 2013;6(6):569–578. doi:10.1161/CIRCGENETICS.111.000027
4. Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25(11):558–566. doi:10.1016/j.tem.2014.07.002
5. Hollywood K, Brison DR, Goodacre R. Metabolomics: current technologies and future trends. Proteomics. 2006;6(17):4716–4723. doi:10.1002/pmic.200600106
6. Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in Prediabetes and Diabetes: a Systematic Review and Meta-analysis. Diabetes Care. 2016;39(5):833–846. doi:10.2337/dc15-2251
7. Kühn T, Floegel A, Sookthai D, et al. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016;14(1):13. doi:10.1186/s12916-016-0552-3
8. Hocher B, Adamski J. Metabolomics for clinical use and research in chronic kidney disease. Nat Rev Nephrol. 2017;13(5):269–284. doi:10.1038/nrneph.2017.30
9. Ibáñez C, Simó C, Martín-álvarez PJ, et al. Toward a predictive model of Alzheimer’s disease progression using capillary electrophoresis-mass spectrometry metabolomics. Anal Chem. 2012;84(20):8532–8540. doi:10.1021/ac301243k
10. Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207–214. doi:10.1161/CIRCGENETICS.109.852814
11. Dagogo-Jack S. Metabolomic prediction of diabetes and cardiovascular risk. Med Princ Pract. 2012;21(5):401–403. doi:10.1159/000339203
12. Wurtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774–785. doi:10.1161/CIRCULATIONAHA.114.013116
13. Zheng Y, Hu FB, Ruiz-Canela M, et al. Metabolites of Glutamate Metabolism Are Associated With Incident Cardiovascular Events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) Trial. J Am Heart Assoc. 2016;5(9):e003755. doi:10.1161/JAHA.116.003755
14. Ottosson F, Smith E, Melander O, Fernandez C. Altered asparagine and glutamate homeostasis precede coronary artery disease and type 2 diabetes. J Clin Endocrinol Metab. 2018;103(8):3060–3069. doi:10.1210/jc.2018-00546
15. Ruiz-Canela M, Toledo E, Clish CB, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED Trial. Clin Chem. 2016;62(4):582–592. doi:10.1373/clinchem.2015.251710
16. Magnusson M, Lewis GD, Ericson U, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi:10.1093/eurheartj/ehs424
17. Yu E, Ruiz-Canela M, Guasch-Ferre M, et al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevencion con Dieta Mediterranea (PREDIMED) Study. J Nutr. 2017;147(3):314–322.
18. Meinitzer A, Seelhorst U, Wellnitz B, et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study). Clin Chem. 2007;53(2):273–283. doi:10.1373/clinchem.2006.076711
19. Ottosson F, Ericson U, Almgren P, et al. Dimethylguanidino valerate: a lifestyle-related metabolite associated with future coronary artery disease and cardiovascular mortality. J Am Heart Assoc. 2019;8(19):e012846. doi:10.1161/JAHA.119.012846
20. O’Sullivan JF, Morningstar JE, Yang Q, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394–4402. doi:10.1172/JCI95995
21. Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163(5):844–850.e1. doi:10.1016/j.ahj.2012.02.005
22. Obeid OA. Plasma amino acid concentrations in patients with coronary heart disease: a comparison between U.K. Indian Asian and Caucasian men. Int J Vitam Nutr Res. 2005;75(4):267–273. doi:10.1024/0300-9831.75.4.267
23. Tobias DK, Lawler PR, Harada PH, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US Women. Circ Genom Precis Med. 2018;11(4):e002157. doi:10.1161/CIRCGEN.118.002157
24. Chen T, Ni Y, Ma X, et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep. 2016;6(1):20594. doi:10.1038/srep20594
25. Yang RY, Wang SM, Sun L, et al. Association of branched-chain amino acids with coronary artery disease: a matched-pair case-control study. Nutr Metab Cardiovasc Dis. 2015;25(10):937–942. doi:10.1016/j.numecd.2015.06.003
26. Zhang SY, Xuan C, Zhang XC, et al. Association Between MTHFR gene common variants, serum homocysteine, and risk of early-onset coronary artery disease: a case-control study. Biochem Genet. 2020;58(2):245–256. doi:10.1007/s10528-019-09937-x
27. Xuan C, Liu ZF, Wang Q, et al. Increased serum concentrations of asymmetric dimethylarginine (ADMA) in patients with early-onset coronary artery disease. Clin Chim Acta. 2017;464:195–199. doi:10.1016/j.cca.2016.11.028
28. Xuan C, Lun LM, Zhao JX, et al. L-citrulline for protection of endothelial function from ADMA-induced injury in porcine coronary artery. Sci Rep. 2015;5:10987.
29. Xuan C, Chang FJ, Liu XC, et al. Endothelial nitric oxide synthase enhancer for protection of endothelial function from asymmetric dimethylarginine-induced injury in human internal thoracic artery. J Thorac Cardiovasc Surg. 2012;144(3):697–703. doi:10.1016/j.jtcvs.2012.01.020
30. Xuan C, Tian QW, Li H, Zhang BB, He GW, Lun LM. Levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and risk of coronary artery disease: a meta-analysis based on 4713 participants. Eur J Prev Cardiol. 2016;23(5):502–510. doi:10.1177/2047487315586094
31. Xuan C, Bai XY, Gao G, Yang Q, He GW. Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8140 cases and 10,522 controls. Arch Med Res. 2011;42(8):677–685. doi:10.1016/j.arcmed.2011.11.009
32. Xuan C, Li H, Li LL, et al. Screening and identification of pregnancy zone protein and leucine-rich Alpha-2-Glycoprotein as potential serum biomarkers for early-onset myocardial infarction using protein profile analysis. Proteomics Clin Appl. 2019;13(3):e1800079. doi:10.1002/prca.201800079
33. Sarwar G, Botting HG, Collins M. A comparison of fasting serum amino acid profiles of young and elderly subjects. J Am Coll Nutr. 1991;10(6):668–674. doi:10.1080/07315724.1991.10718185
34. Kouchiwa T, Wada K, Uchiyama M, et al. Age-related changes in serum amino acids concentrations in healthy individuals. Clin Chem Lab Med. 2012;50(5):861–870. doi:10.1515/cclm-2011-0846
35. Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137(6Suppl 1):1539S–1547S.
36. Murr C, Grammer TB, Meinitzer A, Kleber ME, März W, Fuchs D. Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: the Ludwigshafen risk and cardiovascular health study. J Amino Acids. 2014;2014:783730.
37. Xuan C, Tian QW, Zhang SY, et al. Serum adenosine deaminase activity and coronary artery disease: a retrospective case-control study based on 9929 participants. Ther Adv Chronic Dis. 2019;10:2040622319891539. doi:10.1177/2040622319891539
38. Ramakrishnan S, Sulochana KN, Lakshmi S, Selvi R, Angayarkanni N. Biochemistry of homocysteine in health and diseases. Indian J Biochem Biophys. 2006;43(5):275–283.
39. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6. doi:10.1186/1475-2891-14-6
40. de Bree A, Verschuren WM, Blom HJ, Nadeau M, Trijbels FJ, Kromhout D. Coronary heart disease mortality, plasma homocysteine, and B-vitamins: a prospective study. Atherosclerosis. 2003;166(2):369–377. doi:10.1016/S0021-9150(02)00373-8
41. Mangoni AA, Jackson SH. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med. 2002;112(7):556–565. doi:10.1016/S0002-9343(02)01021-5
42. Bittl JA, Shine KI. Protection of ischemic rabbit myocardium by glutamic acid. Am J Physiol. 1983;245(3):H406–12. doi:10.1152/ajpheart.1983.245.3.H406
43. Pisarenko OI, Solomatina ES, Ivanov VE, Studneva IM, Kapelko VI, Smirnov VN. On the mechanism of enhanced ATP formation in hypoxic myocardium caused by glutamic acid. Basic Res Cardiol. 1985;80(2):126–134. doi:10.1007/BF01910459
44. Qi L, Qi Q, Prudente S, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA. 2013;310(8):821–828. doi:10.1001/jama.2013.276305
45. Utagawa T. Production of arginine by fermentation. J Nutr. 2004;134(10 Suppl):2854S–2857S. doi:10.1093/jn/134.10.2854S
46. Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85(3):342–350. doi:10.1136/heart.85.3.342
47. Kalra K, Franzese CJ, Gesheff MG, et al. Pharmacology of antiplatelet agents. Curr Atheroscler Rep. 2013;15(12):371.
48. Maralani MN, Movahedian A, ShH J. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res Pharm Sci. 2012;7(4):209–215.
49. Vallejo M, García A, Tuñón J, et al. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal Bioanal Chem. 2009;394(6):1517–1524. doi:10.1007/s00216-009-2610-6
50. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78(1):929–958. doi:10.1146/annurev.biochem.77.032207.120833
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


