Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Quantifying the Economic Impact of Delayed Multiple-Inhaler Triple Therapy Initiation in Patients with COPD: A Retrospective Cohort Study of Linked Electronic Medical Record and Hospital Administrative Data in England
Authors Sansbury LB, Wood RP , Anley GA, Nam Y, Ismaila AS
Received 31 March 2021
Accepted for publication 23 August 2021
Published 8 October 2021 Volume 2021:16 Pages 2795—2808
DOI https://doi.org/10.2147/COPD.S312853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Leah B Sansbury,1 Robert P Wood,2 Glenn A Anley,3 Yein Nam,2 Afisi S Ismaila4,5
1Epidemiology, Value Evidence and Outcomes, GlaxoSmithKline, Research Triangle Park, NC, USA; 2Observational Research, Adelphi Real World, Bollington, UK; 3UK Health Outcomes, GlaxoSmithKline, Uxbridge, UK; 4Value Evidence and Outcomes, GlaxoSmithKline, Collegeville, PA, USA; 5Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila
Value Evidence and Outcomes, GlaxoSmithKline, 1250 South Collegeville Road, Collegeville, PA, 19426-0989, USA
Tel +1 919 315 8229
Email [email protected]
Purpose: To assess if early multiple-inhaler triple therapy (MITT) initiation in patients with chronic obstructive pulmonary disease (COPD) reduces subsequent healthcare resource utilization (HCRU), direct medical costs, and acute exacerbations of COPD (AECOPDs).
Patients and Methods: This retrospective, longitudinal cohort study used electronic health records and linked hospital administrative data in England. COPD patients with an AECOPD between July 2012 and May 2016 (index), and who subsequently started MITT within 180 days were eligible. Patients with an AECOPD 6 months prior to index were excluded. HCRU, direct healthcare costs, and AECOPDs were assessed in the following 24-month period for early (≤ 30 days) and delayed (31– 180 days) MITT initiators.
Results: A total of 934 patients were included in the analysis and categorized as early (n=367, 39%) or delayed (n=567, 61%) MITT initiators. Mean patient age was 68.5 years and 53.2% were male. A significantly higher proportion of delayed MITT initiators required ≥ 1 outpatient appointment (all-cause) compared with early MITT initiators (87% vs 79%; p=0.0016). A significantly higher proportion of delayed MITT initiators required ≥ 1 COPD‑related inpatient stay versus early MITT initiators (47% vs 40%; p=0.0262). Over the 24-month follow-up, mean all-cause and COPD-related total healthcare costs were significantly higher in delayed MITT initiators compared with early MITT initiators (all‑cause: £ 11,348 vs £ 8126; p=0.0011; COPD-related: £ 7307 vs £ 4535; p=0.0009).
Conclusion: Delayed initiation of multiple-inhaler triple therapy was associated with higher all-cause and COPD-related costs, suggesting that earlier initiation of triple therapy in COPD patients may help reduce the economic burden on the healthcare system.
Keywords: chronic obstructive pulmonary disease, England, exacerbation, healthcare utilization, multiple-inhaler triple therapy, triple therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. In the United Kingdom (UK), COPD is the second most prevalent lung disease, with approximately 1.8‒2% of the population in England and Scotland (>1 million people) estimated to have been diagnosed with the disease.1,2 Exacerbations are a key clinical feature of COPD, defined as an acute increase in the severity of symptoms, such as dyspnea, coughing, and wheezing, that result in additional treatment.3 While acute exacerbations of COPD (AECOPDs) have a substantial impact on patients’ quality of life, they also have a considerable economic burden.2 According to National Health Services (NHS) Digital statistics, over 130,000 patients with a COPD diagnosis code were admitted into hospital in England in 2019–2020.4 Each year in the UK, COPD costs the NHS approximately £1.9 billion, which constitutes 29% of the total cost of respiratory illness, second only to asthma (£3 billion).2
With multiple classes of drugs now available for the pharmacological treatment of COPD in the UK, there are numerous maintenance therapy options available to clinicians to prevent future AECOPDs. These include inhaled corticosteroids (ICS), long-acting beta agonists (LABAs) and long-acting muscarinic antagonists (LAMAs). Both the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and UK National Institute for Health and Care Excellence (NICE) guidelines recommend that triple therapy with ICS/LAMA/LABA should be initiated in patients who continue to experience symptoms and remain at risk of AECOPD despite receiving dual therapy with LAMA/LABA or ICS/LABA.3,5 Historically, patients received triple therapy via multiple inhalers (multiple-inhaler triple therapy; MITT). In 2017, the first once-daily, single-inhaler triple therapy (SITT; fluticasone furoate/umeclidinium/vilanterol) was approved by the European Medicines Agency (EMA) as a long-term maintenance treatment for patients with COPD.6 Two other SITTs are approved by the EMA; beclomethasone/formoterol/glycopyrronium bromide was approved in 2017,7 and formoterol/glycopyrronium bromide/budesonide in 2020,8 both of which require twice-daily dosing.
Previous real-world studies suggest that patients with COPD do not always receive appropriate therapy.9–11 A study of more than 20,000 patients with COPD treated in UK primary care found that many patients were not treated in line with GOLD recommendations; 28% of patients received no pharmacological treatment, despite experiencing AECOPDs.9 Another study of patients treated in UK primary care included 4000 newly diagnosed COPD patients without a history of exacerbation; nearly half of the patients received an ICS-containing therapy, despite GOLD guidelines recommending that ICS treatment should be reserved for exacerbating patients.10 A similar study examined prescribing patterns in a cohort of nearly 25,000 patients with COPD in a UK primary care setting. The analysis showed that COPD management was often not in line with NICE or GOLD guidelines; a substantial percentage of patients with risk of AECOPD or who were symptomatic did not receive any treatment, and ICS were frequently prescribed without airflow limitation severity, AECOPD history, and asthma diagnosis being taken into consideration.11 These findings highlight a need to address the timing of treatment initiation within the COPD care pathway. This will ensure that patients receive the most appropriate therapy at the right time, optimizing patient outcomes and reducing burden on healthcare systems.
A small number of real-world studies have been carried out in Spain and the United States to investigate if the timing of triple therapy initiation affects the risk of future AECOPDs, and their associated costs.12,13 This study aimed to assess if earlier initiation of MITT with ICS/LAMA/LABA in exacerbating patients reduces subsequent healthcare resource utilization (HCRU), direct medical costs, and AECOPDs in England.
Patients and Methods
Study Design
This was a retrospective, longitudinal cohort study performed using data from the Clinical Practice Research Datalink GOLD (CPRD-GOLD) primary care database. CPRD-GOLD is a longitudinal, anonymized research database derived from over 940 primary care practices covering >19 million patients,14 which contains data on all recorded patient interactions within the primary care setting for participating practices. This includes patient demographics, symptoms and diagnoses, prescriptions issued in primary care, referrals to specialists and secondary care, immunizations, tests performed, and lifestyle information.15
The CPRD-GOLD database is linked to secondary care records from the Hospital Episode Statistics (HES) database. HES is a data warehouse of information relating to all inpatient admissions, outpatient attendances, and Accident & Emergency (A&E) admissions at NHS hospitals in England,16 which contains data on all patient interactions relating to the hospital environment.17 It captures data on basic patient demographics, diagnoses, surgeries/procedures, and administrative information (including dates and methods of admission/discharge and waiting times). The use of linked CPRD/HES data was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol number 19_046; approved 12-Mar-2019).
The study design is shown in Figure 1. Patients who had a moderate-to-severe AECOPD (index date) between July 2012 and May 2016 with subsequent MITT initiation within 180 days were included in the study. The earliest AECOPD within the indexing period was considered the index date. MITT initiation was defined as the first day on which there was any overlap of an ICS, LABA, and LAMA (in two or three devices).
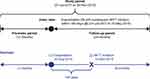 |
Figure 1 Study design. Abbreviation: MITT, multiple-inhaler triple therapy. |
Study Population
The study cohort included patients aged ≥35 years at index, with a diagnosis of COPD identified by International Classification of Diseases (ICD)-10 or Read code (Supplementary Table 1) in the 12 months prior to index, and who were continuously registered with a general practitioner (GP) throughout the period of observation (ie, 12 months of data available prior to the index date, and 24 months of data available following MITT initiation). Eligible patients also had a 6-month AECOPD-free period prior to the index date and no prescription history for MITT in the 12-month period prior to indexing (ie, MITT naïve). Patients were excluded from the study if they had any record of specific conditions within the study period (for example, lung transplant, cystic fibrosis, bronchiectasis).
The timing of MITT prescription following index was used to define two study groups: an early cohort (prescription ≤30 days from the index date) and a delayed cohort (31‒180 days from the index date). Patients were observed for study outcomes from the date of MITT initiation for 24 months (follow-up period).
Demographic and Clinical Variables
The following demographic and clinical variables were assessed in the 12 months prior to index (ie, at baseline), in order to describe the study cohort: age, sex, Strategic Health Authority of GP practice (region), body mass index, Charlson Comorbidity Index (CCI), specific comorbidities (such as depression, anxiety, gastroesophageal reflux disease, acute myocardial infarction, congestive heart failure, stroke, herpes zoster, and hypertension), current asthma diagnosis, Medical Research Council (MRC) dyspnea (Grades 1‒5), COPD GOLD grade (A, B, C, or D), maintenance therapy prescribed, and smoking status (current smoker, former smoker, non-smoker, unknown). Where more than one record existed, the record closest to the index date was used.
Outcomes
Healthcare Resource Utilization and Costs
All-cause and COPD-related HCRU including medication use, GP appointments, outpatient appointments, A&E admissions, and hospitalizations were assessed in the 12 and 24 months following MITT initiation. Prescriptions for COPD medications (short-acting bronchodilators [SABD], ICS, LABA, LAMA, ICS/LABA, and LAMA/LABA) were considered COPD-related. GP consultations with a Read code for COPD or a prescription for a COPD treatment recorded on the same day were classified as COPD-related. Outpatient appointments within the respiratory medicine (Read code 340), respiratory physiology (Read code 341), or programmed pulmonary rehabilitation (Read code 342) departments, and hospitalizations, with a primary or secondary diagnosis code for COPD were classified as COPD-related.
Direct medical costs were quantified via the application of appropriate English-specific sources of unit cost data for each resource use. This included the 2018 Personal Social Services Research Unit (PSSRU) “costs of health and social care” document18 to calculate consultation costs, the NHS prescription services drug tariff19 to calculate medication costs and the 2017/2018 HRG4+ Local Payment Grouper to calculate secondary care costs.20
Exacerbations
AECOPD events were assessed in the 12 and 24 months following MITT initiation date.
AECOPDs were defined using a previously validated algorithm.21 Moderate AECOPDs were defined by the presence of an event for one of the four following reasons: 1) those requiring prescriptions for both antibiotics and oral corticosteroids (irrespective of cause) on the same date for 5‒14 days each; 2) presence of ≥2 worsening respiratory symptoms (eg, cough, breathlessness, sputum, and/or purulence), and a prescription for antibiotics or oral corticosteroids (or both) on the same date; 3) lower respiratory tract infection medical code; and/or 4) AECOPD-specific medical code. Severe AECOPDs were those requiring hospital admission, identified via HES using ICD-10 codes. Exacerbation events occurring within 14 days of a prior event were assumed to be part of the same exacerbation episode, and the episode end date adjusted accordingly. A 14-day period free of exacerbation events was applied to distinguish between distinct exacerbation episodes and ensure that a relapse was not categorized as a separate episode. Exacerbation episodes encompassing both moderate and severe exacerbation events were graded as severe, ie, the worst severity was assumed.
Statistical Analysis
HCRU and associated direct medical costs (all-cause and COPD-related), and the number of subsequent AECOPDs, following MITT initiation were reported separately for 12 and 24 months following index, and split by early versus delayed initiators. For nominal variables, results were presented as frequency and percentage. For numeric variables, results were presented as mean and standard deviation (SD). Statistical significance between MITT initiation subgroups was assessed using a t-test for numeric outcomes and Fisher’s exact or a Chi-squared test for nominal outcomes. All tests were two-sided in nature and a significance level of p<0.05 was used, and no adjustments for multiple testing were applied.
Results
A total of 117,540 patients with COPD were extracted from the CPRD-GOLD database who were eligible for linkage to HES data. Of the 68,955 (59%) patients with at least one moderate-to-severe AECOPD, 11,025 (16%) initiated MITT within 180 days of the index AECOPD and had a COPD diagnosis in the 12 months prior to, or on the date of, index. Of these patients, 934 (0.8% of the initial extract) met the study criteria and were included in this analysis (Figure 2). These patients were further subcategorized as early (n=367; 39%) or delayed (n=567; 61%) MITT initiators.
Baseline demographics and clinical characteristics are presented in Table 1. The mean age at index was 68.5 years and 53.2% of the study cohort were male. The mean (SD) CCI score was 0.8 (1.2). The most frequently reported co-morbidities were hypertension (14.5%) and acute myocardial infarction (3.1%). Most patients had a GOLD grade of A or B (A: 36.9%; B: 34.8%) and the most frequently prescribed maintenance therapies during baseline were SABDs (84.2%), ICS/LABA (51.6%), and LAMA (41.9%). There were no significant differences in smoking status between the two groups (p=0.0881).
 |
Table 1 Baseline Demographics and Clinical Characteristics of Study Cohort |
For the majority of characteristics, there were no significant differences between the early and delayed MITT initiated subgroups. However, significantly more patients in the delayed subgroup reported hypertension compared with the early subgroup (16.8% vs 10.9%; p=0.0133), and there were significantly more patients with GOLD grade D in the delayed initiators versus the early initiators (18.4% vs 9.8%; p=0.0344 [p-value applies to the comparison of all GOLD grades]).
HCRU
Regarding HCRU in the first 12 months following MITT initiation, the number of patients requiring at least one outpatient appointment (all-cause) was higher in the delayed MITT initiators compared with early MITT initiators (76% vs 69%; p=0.0189). A higher proportion of delayed MITT initiators also required at least one inpatient stay (all-cause) compared with early MITT initiators (40% vs 34%; p=0.0381). Delayed initiators had a lower mean number of COPD-related GP consultations compared with early MITT initiators (1.4 vs 1.6; p=0.0295) (Table 2).
 |
Table 2 All-Cause and COPD-Related HCRU in the 12 and 24 Months Following MITT Initiation for Early (≤30 Days) versus Delayed (31–180 Days) MITT Initiators |
All-cause HCRU in the 24 months following MITT initiation was numerically higher for delayed MITT initiators compared with early MITT initiators across all types of HCRU recorded, but in most cases statistical significance was not reached (Table 2). A significantly higher proportion of delayed MITT initiators required at least one outpatient appointment compared with early MITT initiators (87% vs 78%; p=0.0016). Similar trends were observed for COPD-related HCRU, with the exception of mean number of GP consultations (delayed: 2.5; early: 2.7; p=0.1532). The mean number of COPD-related inpatient stays was significantly higher for delayed MITT initiators versus early MITT initiators (2.0 vs 1.4; p=0.0045), with a higher proportion of delayed MITT initiators requiring at least one inpatient stay (47% vs 40%; p=0.0262).
Costs
The mean cost per patient in the first 12 months following MITT initiation was £3031 for the entire study cohort. Mean all-cause and COPD-related costs were significantly higher for delayed MITT initiators compared with early MITT initiators (all-cause: £5541 vs £4190; p=0.0220; COPD-related: £3491 vs £2321; p=0.0183) (Figure 3A). All individual components of all-cause costs were numerically higher for delayed MITT initiators compared with early MITT initiators. Costs associated with all-cause related inpatient stays were significantly different between patient subgroups (delayed: £3043; early: £1935; p=0.038), with inpatient stays accounting for approximately half of total costs on average (delayed: 55%; early: 46%). Costs associated with COPD-related inpatient stays also differed significantly between patient subgroups (delayed: £2637; early: £1470; p=0.0174) and accounted for >60% of COPD-related total costs (delayed: 76%; early: 63%).
Similar results were observed in the 24 months following MITT initiation (Figure 3B). Mean all-cause total direct healthcare costs were significantly higher for delayed MITT initiators compared with early MITT initiators (£11,348 vs £8126; p=0.0011). All individual components of cost were numerically higher for the delayed MITT initiators. Costs differed significantly between the patient subgroups for all-cause A&E visits (delayed: £704; early: £529; p=0.0498) and inpatient stays (delayed: £6402; early: £3768; p=0.0025). Inpatient stays accounted for approximately half of the total costs on average (delayed: 56%; early: 46%).
Mean COPD-related direct healthcare costs were also significantly higher for delayed MITT initiators compared with early initiators at 24 months (£7307 vs £4535; p=0.0009). The costs associated with COPD-related inpatient stays was significantly higher for the delayed MITT initiators (£5702 vs £2977; p=0.0009) and accounted for over two-thirds of the COPD-related total costs in delayed MITT initiators (78%).
Exacerbations
In the first 12 months following MITT initiation, the mean number of moderate-to-severe AECOPDs was higher for the early MITT initiators compared with the delayed MITT initiators, but statistical significance was not reached (1.5 vs 1.4) (Table 3). A similar result was observed for the moderate only AECOPDs (1.4 vs 1.2). There was no difference observed between the mean number of severe AECOPDs in early versus delayed MITT initiators.
 |
Table 3 Frequency of Exacerbations in the 12 and 24 Months Following MITT Initiation for Early (≤30 Days) versus Delayed (31–180 Days) MITT Initiators |
In the 24 months following MITT initiation, the mean number of moderate-to-severe AECOPDs was higher for the delayed MITT initiators compared with the early MITT initiators (2.9 vs 2.6), but statistical significance was not reached. A similar trend was observed for moderate only (2.6 vs 2.4) and severe AECOPDs (0.3 vs 0.2) (Table 3).
Discussion
This is the first study conducted in England to determine if early versus delayed initiation of MITT is associated with reduced HCRU and direct medical costs in patients who experienced a recent AECOPD. The frequency of future AECOPDs in relation to the timing of MITT initiation was also examined. The study showed that MITT initiation within 30 days of an AECOPD led to lower all-cause and COPD-related direct costs compared with delayed MITT initiation (31–180 days). The mean difference in all-cause and COPD-related total direct costs between early and delayed MITT initiators in the 24 months following MITT initiation was substantial (£3222 vs £2772 per patient, respectively), with inpatient length of stay being the main driver of these differences. Limited differences were observed in other elements of HCRU evaluated, and in the frequency of AECOPDs, after initiating MITT. However, it should be noted that there were significantly more delayed initiators categorized as GOLD grade D compared with early initiators, which could account for some of the differences observed.
The mean cost per patient in the first 12 months following MITT initiation was £3031 for the entire study cohort. This result is comparable to an earlier retrospective cohort study of CPRD data from UK patients that estimated the annual cost of COPD management to be £2108 per patient, excluding medication costs.22 Our findings also reflect those reported in other recent studies. A study performed in the United States reported total costs in the first 12 months of follow-up were higher in COPD patients who delayed triple therapy compared with patients who started triple therapy early.12 A Spanish study reported that early MITT initiation following an AECOPD that required hospital intervention resulted in lower HCRU, AECOPD rates, and COPD-related direct costs in the first 12 months of follow-up compared with delayed MITT initiation.13 Additionally, an observational study in Canada reported an increase in exacerbations, emergency room visits, and concomitant medication use in COPD patients who were not escalated to triple therapy following an exacerbation, compared with those who were.23 Other studies have also reported reduced mortality (all-cause and respiratory-related) and hospital admissions for patients treated with triple therapy versus ICS/LABA, and suggest that earlier initiation of any treatment, not just MITT, may have beneficial effects.24,25 It should, however, be noted that ICS-containing treatments are associated with an increased risk of pneumonia among COPD patients with severe disease and/or a history of exacerbation.26–28 Although an increased risk of pneumonia has not been observed among COPD patients with moderate airflow limitation,29 physicians should remain aware of this possible side effect in all COPD patients receiving triple therapy. Due to the risks associated with ICS use, current COPD treatment guidelines recommend taking blood eosinophil counts into consideration when deciding to initiate ICS treatment in combination with a LABA and/or LAMA.3 As the current study was conducted prior to the inclusion of this recommendation in treatment guidelines, it is likely that blood eosinophil counts were not considered when initiating MITT in our study.
In line with the 2020 GOLD recommendations3 and NICE guidelines,5 the use of triple therapy is becoming increasingly important in UK clinical practice. A retrospective cohort study of COPD patients in the UK found that 23% of patients received initial treatment with MITT, and almost half of patients who initially received a LAMA or LABA went on to receive triple therapy within the next 2 years.30 As such, it is key that triple therapy is initiated at the correct time in the treatment pathway. The authors of the aforementioned Spanish study noted the importance of understanding the drivers in escalation to triple therapy. As in the current study, the study population included patients with COPD experiencing an AECOPD, and as such, the AECOPD was the driver of MITT initiation.13 As we have shown, this led to beneficial effects in terms of reduced HCRU and costs when treatment was given sooner, rather than delayed, in patients experiencing an AECOPD.13 There is a need for clinician education to improve awareness of the timing of prompt therapeutic intervention to prevent future AECOPDs.12 Similar findings from real-world studies in different populations may therefore be reassuring to clinicians. A reduction in AECOPDs leading to hospitalization, particularly in vulnerable subgroups of COPD patients, is particularly relevant in the current coronavirus disease 2019 pandemic.
The current study considered triple therapy administered via two or three devices, which may have had different frequencies of administration. Studies including TRILOGY (NCT01917331), IMPACT (NCT02164513), TRIBUTE (NCT02579850), and ETHOS (NCT02465567) have compared the efficacy of ICS/LAMA/LABA SITT and dual-bronchodilator therapies, showing that SITT was associated with a reduced number of AECOPDs.31–34 Economic analysis of the IMPACT study showed SITT to be more cost-effective compared with dual therapies.35 Based on these findings, the introduction of triple therapy at an earlier point in the disease pathway could help to reduce the frequency of AECOPDs and their associated HCRU and costs. In addition, the early initiation of triple therapy may also be beneficial in patients who are at risk of AECOPD. SITT was only recently introduced in the UK and, therefore, has the potential of improving adherence as the uptake of SITT increases over time. Increased adherence to treatment may in turn improve patient outcomes, with a consequent reduction in the economic burden of COPD on the healthcare system, as quantified by this study. This should be the focus of future studies.
Our findings should be considered within the context of the study limitations. This includes the potential for misclassification bias. Treatments prescribed in the secondary care setting are not captured within linked CPRD/HES data. This means that whilst most COPD prescriptions initiated in secondary care would be continued by a GP, a patient prescribed triple therapy during an AECOPD requiring hospital intervention may not have received a subsequent prescription from the GP in the 30 days following the AECOPD, and thus may have been incorrectly classified as a delayed MITT initiator; this may have reduced the treatment effect of early MITT initiation. It should also be noted that SITT is now widely available for patients in England; however, SITT use was not considered in this analysis. A control group (patients who received non-MITT/SITT inhaled maintenance therapy) would also be useful to include in future studies. Another limitation is that the AECOPD washout period likely excluded patients who exacerbate frequently (ie, those with a more severe condition), thus impacting on generalizability of the study findings. The requirement for patients to have at least 24 months of follow-up may have introduced bias, whereby patients who died within 24 months following MITT initiation were not included in this study. As this was a retrospective database study, there is potential for misclassification or under-ascertainment of COPD and/or AECOPD, which could have led to an underestimation of total costs. Another limitation is that COPD-related costs, such as hospital interactions, were defined as healthcare services with an associated diagnosis of COPD; this may have underestimated the true costs incurred due to COPD. In addition, data on the occurrence of pneumonia were not collected, which may have impacted inpatient length of stay and costs. However, each of these limitations would have affected both early and delayed MITT initiators (ie, non-differential bias). Finally, the analyses reported in this paper are descriptive only. Further research would be of value to adjust for any unknown potential confounding factors. Although the study was conducted using data exclusively from patients in England, the authors consider the sample size to be representative of a wider UK population.
Conclusions
In conclusion, this study found that although frequencies of AECOPDs after treatment initiation were comparable, the all-cause and COPD-related costs were higher in those delaying MITT treatment initiation compared with those starting MITT early, in both the 12 months and the 24 months following MITT initiation. Management of COPD through earlier initiation of MITT may therefore reduce the economic burden on the healthcare system.
Abbreviations
A&E, Accident & Emergency; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; EMA, European Medicines Agency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HCRU, healthcare resource utilization; HES, Hospital Episode Statistics; ICD, International Classification of Diseases; ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LoS, length of stay; MITT, multiple-inhaler triple therapy; NHS, National Health Services; NICE, National Institute for Health and Care; PSSRU, Personal Social Services Research Unit; SABD, short-acting bronchodilator; SD, standard deviation; SITT, single-inhaler triple therapy; UK, United Kingdom.
Data Sharing Statement
The data analyzed in this publication are derived from the Clinical Practice Research Datalink (www.cprd.com) and Hospital Episode Statistics database (https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics). Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to each database can be requested via the respective websites.
Ethics Approval and Informed Consent
Approval of this study was provided by the GlaxoSmithKline Protocol Review Committee and by the Independent Scientific Advisory Committee (ISAC), which reviewed the protocol and approved access to Clinical Practice Research Datalink data (ISAC study no. 19_046). Generic ethical approval for ISAC-approved observational research using the CPRD was granted by a Health Research Authority Research Ethics Committee (East Midlands-Derby, REC reference number 05/MRE04/87). Patient consent was not required as anonymized patient-level data were used in this analysis.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Fiona Goodwin and Rebecca Cunningham of Aura, a division of Spirit Medical Communications Group Limited and was funded by GlaxoSmithKline. These data have previously been presented in abstract/poster form at the European Respiratory Society 30th Annual Congress (Afisi Ismaila, Robert Wood, Glenn Anley, Yein Nam, Daniel Bluff, Leah Sansbury. Economic impact of delayed multiple-inhaler triple therapy initiation in COPD patients. European Respiratory Society – 30th Annual Congress. 2020;56(64):2426). This study is based, in part, on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Copyright © 2020, re-used with the permission of The Health & Social Care Information Centre.17 All rights reserved.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GlaxoSmithKline (study HO-17-17594/206974). The sponsor was involved in study conception and design, data interpretation, and the decision to submit the article for publication. The sponsor was also given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
The authors declare the following conflicts of interest during the last three years in relation to this manuscript: LBS was an employee of and/or held stocks/shares in GlaxoSmithKline at the time the study was conducted. LBS is currently affiliated with Medical Affairs, Ultragenyx Pharmaceutical Inc., Novato, CA, USA. GAA is an employee of and/or holds stocks/shares in GlaxoSmithKline and is currently affiliated with Speciality & Primary Care, GlaxoSmithKline, Brentford, UK. RPW is a current employee of Adelphi Real World, who received funding from GlaxoSmithKline to conduct this research. YN was an employee of Adelphi Real World at the time the study was conducted and is currently affiliated with Integrated Real World Evidence & Solutions, IQVIA, London, UK. ASI is an employee of and/or holds stocks/shares in GlaxoSmithKline and an unpaid faculty member at McMaster University, Hamilton, ON, Canada. The authors report no other conflicts of interest in this work.
References
1. McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6(1):1–10. doi:10.1038/srep31893
2. Trueman D, Woodcock F, Hancock E; British Lung Foundation. Estimating the economic burden of respiratory illness in the UK; 2017. Available from: http://allcatsrgrey.org.uk/wp/wpfb-file/pc-1601_-_economic_burden_report_final_8cdaba2a-589a-4a49-bd14-f45d66167795-pdf/.
3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
4. National Health Services Digital. Hospital admitted patient care activity 2019-20: diagnosis; 2020. Available from: https://files.digital.nhs.uk/37/8D9781/hosp-epis-stat-admi-diag-2019-20-tab%20supp.xlsx.
5. NICE. Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2018. Available from: https://www.nice.org.uk/guidance/ng115.
6. European Medicines Agency. Trelegy Ellipta - summary of product characteristics; 2017. Available from: https://www.ema.europa.eu/en/documents/product-information/trelegy-ellipta-epar-product-information_en.pdf.
7. European Medicines Agency. Trimbow - summary of product characteristics; 2017. Available from: https://www.ema.europa.eu/en/documents/product-information/trimbow-epar-product-information_en.pdf.
8. European Medicines Agency. Trixeo Aerosphere - summary of product characteristics; 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/trixeo-aerosphere-epar-product-information_en.pdf.
9. Gruffydd-Jones K, Brusselle G, Jones R, et al. Erratum: changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: a real-world study. NPJ Prim Care Respir Med. 2017;27:17004.
10. Chalmers JD, Poole C, Webster S, Tebboth A, Dickinson S, Gayle A. Assessing the healthcare resource use associated with inappropriate prescribing of inhaled corticosteroids for people with chronic obstructive pulmonary disease (COPD) in GOLD groups A or B: an observational study using the Clinical Practice Research Datalink (CPRD). Respir Res. 2018;19(1):63.
11. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi:10.2147/COPD.S62750
12. Bogart M, Glassberg MB, Reinsch T, Stanford RH. Impact of prompt versus delayed initiation of triple therapy post COPD exacerbation in a US-managed care setting. Respir Med. 2018;145:138–144. doi:10.1016/j.rmed.2018.10.013
13. Mainar AS, Huerta A, Artieda RN, Monsó E, Landis SH, Ismaila AS. Economic impact of delaying initiation with multiple-inhaler maintenance triple therapy in Spanish patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2121. doi:10.2147/COPD.S211854
14. Medicines & Healthcare Products Regulatory Agency. Release notes: CPRD GOLD November 2020; 2020. Available from: https://www.cprd.com/data-highlights.
15. CPRD-GOLD. Primary care data for public health research; 2021. Available from: https://cprd.com/primary-care.
16. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
17. NHS Digital. Hospital Episode Statistics (HES); 2019. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed May 27, 2020.
18. PSSRU. Personal Social Service Research Unit - unit costs of health and social care; 2018. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/#sections.
19. NHS prescription services. NHS prescription cost analysis; 2017. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2016.
20. NHS HRG4+. NHS HRG4+, local payment grouper; 2018. Available from: https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/payment-hrg4-2017-18-local-payment-grouper.
21. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
22. Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65.
23. Tavares R, Zhang W, Dang-Tan T, et al. The healthcare burden of non-compliance to pharmacotherapeutic escalation recommendations for COPD. Eur Respir J. 2016;48:PA300.
24. Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting β-agonist therapy in COPD. Chest. 2012;141(1):81–86.
25. Akazawa M, Biddle AK, Stearns SC. Economic assessment of early initiation of inhaled corticosteroids in chronic obstructive pulmonary disease using propensity score matching. Clin Ther. 2008;30:1003–1016.
26. Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647.
27. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27–34.
28. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223.
29. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: the SUMMIT trial. Respir Med. 2017;131:27–34.
30. Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS One. 2014;9(9):e105296.
31. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973.
32. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680.
33. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084.
34. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
35. Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


