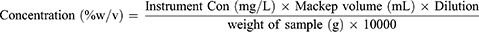Back to Journals » Risk Management and Healthcare Policy » Volume 17
Quantification of p-Phenylenediamine in Hair Dyes and Health Risk Implications in the UAE: Describing Discordances Between Regulations and Real-Life Practices
Authors Jairoun AA , Al-Hemyari SS , Shahwan M , Jairoun O, Zyoud SH
Received 15 September 2023
Accepted for publication 26 February 2024
Published 21 March 2024 Volume 2024:17 Pages 663—675
DOI https://doi.org/10.2147/RMHP.S440482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Ammar Abdulrahman Jairoun,1,2 Sabaa Saleh Al-Hemyari,2,3 Moyad Shahwan,4,5 Obaida Jairoun,6 Sa’ed H Zyoud7,8
1Health and Safety Department, Dubai Municipality, Dubai, United Arab Emirates; 2Discipline of Clinical Pharmacy, School of Pharmaceutical Sciences, Universiti Sains Malaysia (USM), Pulau Pinang, 11800, Malaysia; 3Pharmacy Department, Emirates Health Services, Dubai, United Arab Emirates; 4Department of Clinical Sciences, College of Pharmacy and Health Sciences, Ajman University, Ajman, 346, United Arab Emirates; 5Centre of Medical and Bio-allied Health Sciences Research, Ajman University, Ajman, 346, United Arab Emirates; 6College of Dentistry, Ajman University, Ajman, 44839, United Arab Emirates; 7Department of Clinical and Community Pharmacy, College of Medicine and Health Sciences, An-Najah National University, Nablus, 44839, Palestine; 8Clinical Research Centre, An-Najah National University Hospital, Nablus, 44839, Palestine
Correspondence: Ammar Abdulrahman Jairoun; Moyad Shahwan, Tel +971558099957, Email [email protected]; [email protected]
Background: p-Phenylenediamine (PPD) has been used over the past five decades as a primary precursor in the production of oxidative hair dyes. Numerous health dangers are associated with the short- and long-term use of PPD, raising concerns about its safety. For instance, mounting data suggests that PPD is linked to dermatitis and allergy cases.
Objective: To quantify the PPD content in hair dyes by measuring the PPD concentration after mixing the ingredients of commercial hair dyes.
Methods: A total of 290 permanent hair dyes were tested. RP-HPLC-DAD analysis was performed to determine and quantify the PPD content.
Results: The estimated mean of the PPD limit was 0.89 (95% CI [0.81– 0.96]). Of the 290 tested hair dyes, 7.2% (n = 21) exceeded the recommended PPD concentration after mixing. Significantly more hair dyes manufactured in India and China had a PPD content exceeding 2% after mixing compared to dyes from other regions (P = 0.001). Moreover, hair dyes manufactured in India and the UAE were more likely to have incomplete descriptions of the conditions of use and warnings on the label (P = 0.002).
Conclusion: The effectiveness of the current regulations relevant to these products should be reevaluated. Moreover, through the use of good manufacturing procedures (GMPs), research, and the reporting of adverse reactions, hair dyes should be subjected to better control and monitoring in terms of their safety and quality.
Keywords: PPD, gap analysis, allergy, contact dermatitis, permanent hair dyes, sensitization
Introduction
Hair dyes are currently available for various cosmetic uses, including hiding gray hair, changing the hair color, and enhancing the longevity of the natural hair color. The population increase and the popularity of contemporary hairstyles have created a strong demand for hair-coloring products.1,2 The hair dye industry has grown over time into a multibillion-dollar market, with permanent hair dyes accounting for over 70% of the market’s total revenue. The rise in popularity of hair dyes can be attributed to their favorable long-term benefits and simplicity of use.2 Human hair consists of two main structural components: the root and the shaft. The three layers that form the shaft are the cuticle, which is the outermost protective section composed of keratinized cells, the cortex, which is the central part and forms the bulk, and the medulla, which is the innermost layer.3,4 The diet, DNA, environment, and use of hair dyes can affect how human hair looks or feels.5 According to their composition and process of manufacture, hair dyes are categorized into the following groups: (1) vegetable hair dyes, the most popular of which is henna; (2) mineral hair dyes (silver nitrate or lead salts), which need to be applied gradually to achieve the desired result; and (3) synthetic hair dyes.6 Based on how permanent they are, synthetic hair dyes can be subdivided into four categories:
(1) Temporary hair dyes: These are acidic, textile, water-soluble dyes of large molecular weight that cannot penetrate the hair shaft. These dyes deposit on the cuticle surface until the point at which they are rinsed off.6,7
2) Synthetic dyes: Most semi-permanent dyes are synthetic.8 These dyes are derived from coal tar components with low molecular weight. Thus, these products can freely diffuse in and out of the cortex and last longer than the temporary dyes.6–8
3) Permanent dyes: These dyes achieve deep penetration into the hair due to their low molecular weight. The use of permanent dyes results in permanent coloration.7 Despite being the type of hair dye that causes the most damage, permanent hair dyes represent 70% of hair dye sales.6,8–10 Prior to dyeing the hair, hair-bleaching chemicals that oxidize melanin in the hair cortex lighten the hue of the natural hair.11,12 Permanent hair dyes are created mechanically by oxidizing non-colored active components to create the desired shade. Thus, the phrase “oxidative hair dye” was coined.3,11 There are two key components within an oxidative hair dye formulation: the precursor and the coupler. Quinone diimine intermediates are intermediate compounds generated when combined with hydrogen peroxide (developer). As a result, the coupler and these compounds interact to create the appropriate hair dye molecule. Notably, the complete dyeing process requires both an alkaline medium and an oxidizing agent (often hydrogen peroxide) to ensure that the staining agents reach the cuticle. The oxidative dye products are, therefore, initially provided in two different bottles. The developer is in one vial, whereas the precursor and couplers are in another vial.1,11
p-Phenylenediamine (PPD) is a common chemical used as a permanent hair dye. PPD has the chemical formula C6H4(NH2)2, indicating that it is an organic substance. PPD is a white solid, although this aniline derivative can become darker owing to air oxidation.13 PPD is largely utilized as a constituent of engineering polymers and composites and is also used as an ingredient in hair dyes and as a substitute for henna.13,14
PPD is a monocyclic arylamine compound with the appearance of a white to light purple powder that, when exposed to air, oxidizes, first turning red, then brown, and finally, black.14 PPD is a chemical that can be found in henna, hair dyes, photographic-developing agents, temporary tattoos, dark-colored cosmetics, photocopying inks, printing inks, black rubber, gasoline, oils, and rubber compounds as an antioxidant.13,14 Other aniline analogs and derivatives (eg, 2.5-diamino hydroxyethylbenzene and 2.5-diaminotoluene) are sometimes used as PPD alternatives. PPD has been used regularly over the past five decades as a primary precursor in the production of oxidative hair dyes. PPD is increasingly preferred by hair dye producers because it offers a wide variety of hues.15 Nevertheless, numerous health dangers are associated with the short- and long-term use of PPD, raising concerns about its safety. For instance, mounting data suggests that PPD is linked to dermatitis and allergy cases.16–21 Moreover, PPD is regarded as a strong skin sensitizer. Hence, a patch test is advised before using any hair dye containing PPD.
In individuals with dermatitis, the reported prevalence of positive patch test reactions to PPD is 4.4% in Asia, 4.1% in Europe, and 6.0% in North America.22 The Cosmetics Regulation (EC) No. 1223/2009 states that the maximum concentration of PPD and its salts in oxidative hair dyes after mixing under oxidative circumstances is 2%, calculated as a free base.
The mixing ratio, warnings that hair colorants can cause severe allergic reactions, and a disclosure that the product is not intended for use on people younger than 16 years should also be included on the labels of hair dyes. Anyone who experiences a face rash, a sensitive, inflamed, or damaged scalp, or who has ever experienced a negative reaction after coloring their hair should also avoid using hair dyes. Consumers should also be informed that phenylenediamines are present and that the product should not be used to tint eyebrows or eyelashes.23
The cosmetics technical regulations of the United Arab Emirates (UAE) apply the same dosage limits and labeling requirements to hair dyes as those in EU regulations.24
Concerns about the safety of PPD have been raised due to the well-established evidence of risk associated with this chemical in cosmetic products. To avoid the health risks related to PPD use, recent studies have focused on developing novel PPD substitutes.25,26 Carefully defining a safe PPD limit in hair dyes that can deliver the desired coloring with the least amount of PPD exposure to the body is necessary. Thus, the current study aimed to quantify the PPD content in hair dyes by measuring PPD after mixing the ingredients of commercial products. The results of this study may help regulators determine appropriate PPD limits and regulations for cosmetics in general and hair dyes in particular.
Methods and Materials
Sample Collection (Sampling Method)
Local business directories were examined to identify pharmacies, para-pharmacies, and cosmetics and personal care product stores that offer personal care products, including hair dyes and cosmetics, in the UAE. The 2100 individual outlets found were then added to the sampling framework, which was an Excel spreadsheet that contained the relevant information about the stores, including their names, addresses, Email addresses, and phone numbers. Each store was given a unique business ID number. These numbers were subjected to a basic random-sample selection procedure to produce the study sample. The researchers subsequently visited the locations of the selected stores to purchase the products as samples. Products were chosen based on the main selection criterion of being a permanent hair dye containing the oxidative ingredient PPD. For each product that met this criterion, one item was randomly chosen at each sample location. The country of manufacture was not considered during the sampling. A reference code was assigned to each sample to ensure that each product could be tracked and that no product would be sampled multiple times. For each sample, the product name, brand name, barcode, batch number, item category, subcategory, size/volume, dosage form, recommended dose, country of origin/manufacturer, and the store from which it had been sampled were recorded. For any products that were essentially identical (ie, had identical names, formulations, and manufacturers, yet were for sale at multiple outlets), only the first product was tested and all other samples were returned. Products with the same name but from different manufacturers or offered in different formats were treated as distinct products and thereby tested individually. All products were immediately sent to the laboratory for same-day testing (Figure 1).
 |
Figure 1 Sampling method. |
Instrumentation and Data Acquisition Conditions
Materials and Regents
Materials
Amber volumetric flasks (Gulf Scientific Glass, Al Hidd, Bahrain), amber autosampler vials, 5 mL syringes, and 0.22 µm PTFE syringe filters (Biomed Scientific Ltd., Guangdong, China) were used.
Reagents
Phosphate buffer (0.025 M/L), 0.1% heptanesulfonic acid sodium salt at pH 6.0 (3.06 g of potassium dihydrogen phosphate monobasic + 0.38 g of disodium hydrogen phosphate), 1 g of 1-heptanesulfonic acid sodium salt, 0.5% sodium ascorbate in phosphate buffer, 1 M sodium hydroxide, 2 M O-phosphoric acid were obtained from Sigma-Aldrich (Switzerland). Acetonitrile was of HPLC grade (Merck, Kenilworth, NJ, USA) and the water was deionized using a Milli-Q water purification system. (Merck)
Instrumentation
An Agilent (Hanover, USA) high-performance liquid chromatography (HPLC) system with a 1260 Infinity II diode array detector (DAD) and Open Lab-Chemstation software was used. The column was a Synergi RP 80A (250 × 4.6 mm, 4.0 µm; Phenomenex, Torrance, USA) with a 10 µL sample loop.
The pH meter used (Sartorius, Gottingen, Germany) had an accuracy of 0.01 pH units.
The analytical balance (Sartorius) used had a max. 200 g range.
Micropipettes (100–1000 µL; Transpette, Wertheim, Germany) were used.
A Qualilife sonicator (China) was used.
HPLC Acquisition Conditions
RP-HPLC-DAD analysis was performed using the Agilent 1260 Infinity II. For quantification, a Synergi-RP 80A column (250 × 4.6 mm, 4.0 µm) was used with mobile phase A (0.025 M/L phosphate buffer + 0.1% heptane sulfonic acid sodium salt at 6.0 pH) and mobile phase B (acetonitrile). Chromatographic separation was achieved with gradient elution (Table 1). A volume of 10 μL of the sample was injected into the chromatographic system at a flow rate of 1.0 mL/min. The column temperature was 25 °C, and the wavelength was 280 nm. The peaks of the determined components were identified by comparing their retention times with those of standards, and the run time was 15 min (Table 1).
 |
Table 1 Gradient Program |
Preparation of Test Portions
Approximately 0.5 g of each hair dye as a homogenous sample was added into a 25 mL amber stoppered flask and 10 mL of 0.5% sodium ascorbate solution was added in four parts. Then, 10 mL of acetonitrile was added in six parts, and the mixture was sonicated to complete disintegration, cooled to room temperature, and diluted to the appropriate volume with acetonitrile. The two phases were separated by centrifugation (6000 rpm), and then the supernatant was filtered through a 0.22 µm PTFE syringe filter and put into an amber HPLC autosampler vial.
Calibration Standards and Linearity Procedure
The reference material was p-phenylenediamine (PPD; CAS# 106–50-3) (Figure 2).
 |
Figure 2 Chemical structure of p-phenylenediamine (PPD). |
Calibration standards of PPD were prepared at concentrations of 20.0, 50.0, 100.0, 250.0, 500.0, and 1000.0 mg/L by using four parts of 0.5% sodium ascorbate solution and six parts of acetonitrile as a diluent in amber glassware.
Validation Methodology
The method was fully validated according to the ICH (International Conference on Harmonization) guidelines by determining the linearity, precision, accuracy, limit of detection (LOD), and limit of quantification (LOQ). The selectivity of the method was proven by the chromatographic peak resolution obtained for PPD, o-phenylenediamine (OPD), and m-phenylenediamine (MPD).
Calibration standards of PPD at concentrations of 20.0, 50.0, 100.0, 250.0, 500.0, and 1000.0 mg/L were prepared in a diluent (four parts of 0.5% sodium ascorbate solution and six parts of acetonitrile). The solutions were kept in amber glass vials at −20 °C for long-term storage. The linearity of the method was tested in the range of 20–1000 mg/L with a correlation coefficient value greater than 0.995.
The LOD was determined using analyte-free samples (hair dye) with a signal-to-noise ratio of at least 3:1. The LOQ was estimated based on a signal-to-noise ratio of at least 10. The LOD and LOQ of the method were tested in the range of 5.0–50.0 mg/kg. The LOD and LOQ of the method were 0.025% w/v and 0.10% w/v, respectively.
The repeatability test for retention time (RT) and peak area was carried out by injecting the standard mixtures of three analytes (PPD, OPD, and MPD) at concentrations of 100.0 mg/L six times a day. The relative standard deviation (RSD) of the intraday precision for RT and peak area was not more than 1.4% and 6.6%, respectively.
Accuracy and precision were estimated by spiking six individual solutions at concentrations ranging from the LOQ to medium and high calibration levels. PPD, OPD, and MPD were spiked at 20.0, 100, and 800 mg/kg in analyte-free hair dye and each of the six spiked samples was analyzed. The %RSD and %Recoveries are listed in Table 2.
 |
Table 2 Accuracy and Precision on Day 1 |
The reproducibility test was performed by injecting the standard mixtures of the three analytes at concentrations of 20.0, 100, and 800 mg/kg six times for 2 days in analyte-free hair dye, and analyzing each of the spiked samples. The %RSD and %Recoveries are listed in Table 3.
 |
Table 3 Accuracy and Precision on Day 2 (Reproducibility) |
For the quality control and quality assurance (QC/QA), 100 mg of PPD was transferred in a diluent into a 10 mL volumetric flask to achieve a concentration of 10,000 mg/L. Serial dilutions were produced up to a concentration of 100 mg/L. This stock solution was stored in an amber glass vial at −20 °C. The quality control of the standard was performed with the 100 mg/L concentration, prepared separately from the different/same lot, and the recovery was within the range of 90–110%. For the quality control of the samples, an analyte-free matrix was spiked at a concentration of 100 mg/kg, and the recovery was within the range of 80–120%. To test the duplicate sample preparation, unknown samples were taken in duplicate, and the variation was not more than 10%. To evaluate the spiked sample preparation, unknown samples were spiked at a concentration of 100 mg/kg, and recovery within the range of 80–120% was obtained. To test the standards, the same standard (100 mg/L) preparation was injected at the end of the sequence, and the recovery was within the range of 90–110%.
Limit of Quantification (LOQ)
The LOD was determined using analyte-free samples (hair dye) with a signal-to-noise ratio of at least 3:1. The LOQ was estimated based on a signal-to-noise ratio of at least 10. The LOD and LOQ of the method were tested in the range of 5.0–50.0 mg/kg. The LOD and LOQ of the method were 0.025% w/v and 0.10% w/v, respectively.
PPD Concentration Reporting
PPD quantifications were performed using the peak area. The peak area for each standard solution and sample was calculated. Calibration curves were constructed for the concentrations of the standard solutions used. The concentrations of PPD, OPD, and MPD from the unknown samples were calculated using the following formula:
Ethical Considerations
Approval for this study was obtained from the Institutional Review Board of An-Najah National University (reference number Int.R.March.2021/14).
Statistical Analysis
SPSS version 26 (Chicago, IL, USA) was used to perform the data analysis, with all qualitative variables presented as frequencies or percentages. The PPD content (%) was measured for each sampled hair dye product. Subsequently, the estimated mean of the PPD content was calculated for each product. A one-way ANOVA test was used to identify the significant differences in the PPD content according to the sample’s characteristics. Chi-square and Fisher’s exact tests were used to compare the prevalence of PPD exceeding 2% after mixing. Statistical significance was assumed for p-values below 0.05.
Results
Characteristics of Permanent Hair Dye Samples
A total of 290 permanent hair dyes were tested in this study. The manufacturing countries were as follows: 203 (70%), European Union; 42 (14.5%), United Arab Emirates; 23 (7.9%), Turkey; 12 (4.1%), India; and 10 (3.4%), China. Of the total, 19% (n = 19) did not have clear instructions regarding the conditions of use or warnings on the label (Table 4).
 |
Table 4 Characteristics of Permanent Hair Dyes (n = 290) |
Estimation of PPD Levels in Permanent Hair Dyes After Mixing
The estimated mean of the PPD limit was 0.89 (95% CI [0.81–0.96]). Of the 290 tested hair dyes, 7.2% (n = 21) exceeded the recommended PPD concentration after mixing (2%) (Table 5).
 |
Table 5 Descriptive Statistics of PPD Limits in Hair Dyes After Mixing |
Table 6 displays the characteristics of the hair dyes that exceeded the recommended PPD concentration after mixing.
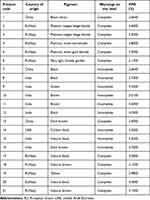 |
Table 6 List of Permanent Hair Dyes That Exceed the Recommended PPD Level After Mixing |
Comparison of the PPD Content According to the Country of Manufacture
A statistically significant association was found between the manufacturing countries and the PPD content. Permanent hair dyes manufactured in India and China were more likely to contain higher levels of PPD compared to those from other countries (P < 0.001) (Table 7).
 |
Table 7 PPD Content According to Country of Manufacture |
The prevalence of a PPD content exceeding 2% after mixing was significantly higher in hair dyes manufactured in India and China (P = 0.001). Moreover, hair dyes manufactured in India and the UAE were more likely to have incomplete descriptions of conditions of use and warnings on the labels (P = 0.002) (Table 8).
 |
Table 8 Compliance Behavior of Permanent Hair Dyes According to the Country of Manufacture |
Discussion
Hair dyes may produce allergic contact dermatitis in sensitive people and are also a substantial source of consumer exposure to sensitizing allergens. In the UAE, PPD is typically present in hair-coloring products available to consumers. Numerous incidents of contact allergies after the use of black henna have been documented in the UAE.27 The proper identification of the allergen and consequent avoidance of substances containing the allergen and potential cross-reactors are necessary for preventing allergic contact dermatitis as a result of the use of hair-coloring products.
The current study found that, after the mixing process was complete, the sampled permanent hair dyes had an average PPD concentration of 0.89%. This concentration was lower than that reported in a recent Saudi study (0.97–0.92%).6
Findings from the current study indicated that 7.2% of the hair dyes tested surpassed the acceptable PPD concentration after mixing (2%). This is especially concerning and contrasts with the Saudi investigation, where all tested samples met the PPD regulatory limits that were in effect at the time.6
According to a subsequent study, PPD was discovered in all of the black henna samples, with concentrations ranging from 0.38% to 29.5%.27 This finding is in line with that of other researchers, who discovered that the PPD level in henna tattoos was substantially higher than that in hair dye.28–31 However, the use of PPD directly on the skin, eyebrows, or eyelashes has been outlawed by the EU, the UAE, and the Food and Drug Administration (FDA) of the United States.23,24,28
Nevertheless, given that some users might leave the dye on their hair longer than advised to achieve a more vivid hue, the presence of such a high quantity of PPD after dying hair is concerning. Customers are at risk of exposure to unreacted PPD and/or other sensitizing intermediates as a result of this procedure, especially when washing and conditioning their hair.17,32 The calculated exposure period to the dye and the allergy caused by PPD-containing hair dyes were compared in a retrospective study. The frequency of dyeing revealed a positive link with allergy events in direct and indirect contact regions,33 even though the exposure time was not substantially correlated with the number of allergy events represented by skin lesions. Hair-dyeing procedures have been linked to mutagenic, carcinogenic, and hypersensitive occurrences.17–19,21
More intense colors are known to have higher PPD contents than less intense colors, so the link between color intensity and PPD concentration found in this study is unsurprising. Likewise, studies conducted in Saudi Arabia that examined dark-brown and light-brown colors for the presence of PPD found a positive relationship between color intensity and PPD levels.6
A PPD concentration beyond 2% after mixing was far more common in hair dyes made in China and India than in products manufactured elsewhere. Patients from northern European countries are known to have statistically lower rates of PPD positivity in patch tests than those from central and southern Europe.34 This proves that improving the legislative strategy is crucial. Furthermore, a global cosmetovigilance system must be urgently established. Such a comprehensive approach to public health can help obtain accurate information on the ingredients and safety of cosmetic products, as well as reduce the health hazards associated with their use.
Disconcertingly, this study found that the cautions and restrictions of use were not fully stated on the labels of 19% of the hair dyes tested. The investigated hair dyes are marketed directly to consumers, many of whom might not properly follow the instructions because they lack any hair-dyeing experience. Customers who self-apply the product without wearing gloves, for instance, run the risk of developing hand dermatitis.35
Emphasizing the prioritization of Personal Protective Equipment (PPE) is paramount when assessing hairdressers’ exposure to hair color compounds, particularly p-phenylenediamine (PPD), with the aim of mitigating potential health risks. The issue of hairdresser exposure is a significant concern, as inadequate use of PPE, including gloves and masks, can elevate the risk of skin contact and inhalation. It is crucial to note that despite its minimal volatility at room temperature, PPD still poses an inhalation hazard, particularly during procedures such as hair drying. Furthermore, the diverse chemical compositions within hair color formulations can influence the rate at which PPD penetrates the skin. Additives, preservatives, and permeation agents may impact skin absorption, potentially leading to increased absorption and discomfort. Addressing these risks necessitates strict adherence to recommended PPE guidelines, providing hairdressers with comprehensive awareness training, and enhancing salon ventilation. In the pursuit of minimizing the risk of skin absorption without compromising effectiveness, the industry should explore alternative formulations. This proactive approach will ultimately create a safer working environment for hairdressers and ensure the well-being of both professionals and clients.
Hair dyes should not be left on the skin for an extended period of time because this could result in chemical burns or irritant contact dermatitis. Long-term skin contact might make sensitization more likely. Cases in India and Sudan have even drawn attention to the practice of ingesting commercially available hair dyes as a means of committing suicide.1,36 PPD and other hair dye ingredients can also induce contact allergies and allergic contact dermatitis in hairdressers.37
In addition, warnings and conditions of use are more likely to be poorly written on the labels of hair dyes made in India and the UAE. The inadequate use of these products may be connected to the allergic contact dermatitis induced by PPD, which can appear in the form of hand eczema in hairdressers and scalp, generalized facial, perioral, or periorbital dermatitis in customers.
A case of note was also documented in the UAE. Following a laboratory test, a patient was determined to have henna poisoning, which caused vomiting and stomach pain. Furthermore, the patient had a hereditary condition called glucose-6-phosphate dehydrogenase (G6PD) enzyme deficiency, which is prevalent in the Gulf region.38 Hemolytic anemia, or abnormal red blood cell rupture (breakage), is typically the result of G6PD deficiency. There is evidence that numerous drugs have the potential to be toxic to people who lack G6PD, who may experience severe hemolysis. These drugs include sulfonamides, antimalarial drugs like primaquine and chloroquine, sulfamethoxazole, and sulfanilamide. Due to their structural similarity, PPD may have a comparable effect on G6PD-deficient individuals.
In addition, henna has been linked to the development of hemolytic crises in G6PD-deficient newborns.39,40 In a study conducted in the UAE, Raupp and colleagues reported that topical henna application caused a hemolytic crisis in four G6PD-deficient children, among whom a female neonate recovered after receiving a transfusion, while a male infant died despite receiving a transfusion.41
The findings of this study have substantial ramifications for regional hair dye producers. Manufacturers must specifically address the inadequate phrasing of the cautions and restrictions of use on the label. To address the absence of quality control and compliance among producers, reevaluating the effectiveness of the current UAE rules in this area is required. The appropriate authorities should ensure that these products adhere to UAE market labeling and packaging regulations.
Even when the manufacturers added clear instructions regarding the time of dye application and users followed them rigorously, high PPD levels are likely to be found. Unconsumed PPD has to be further assessed by regulatory organizations in light of the health hazards connected to this compound, as well as the possible carcinogenicity of PPD-related compounds. Consumers should ideally be exposed only to negligible amounts of dangerous chemicals included in hair dyes to color their hair as desired. To determine the safe quantities of sensitizing compounds in hair dyes (notably PPD), cooperation between industry, regulatory authorities, and health-related decision-makers is vital.6
The quest for safer alternatives to p-phenylenediamine (PPD) in hair dyes has intensified due to concerns regarding allergic reactions and potential health risks associated with PPD exposure. To address these issues while ensuring the delivery of vibrant and effective hair color, several alternative compounds have been thoroughly explored. Notable among these alternatives are resorcinol, p-aminophenol, and para-toluenediamine (PTD).
Particularly, PTD has gained prominence as a substitute for PPD due to its lower propensity to cause allergies while retaining the ability to provide vibrant hair color. Moreover, advancements in cosmetic science have led to the development of oxidative hair dye formulations incorporating innovative compounds. One such compound is ME-PPD (methyl ethyl p-phenylenediamine), which is marketed as having reduced allergenic properties. Ongoing research aims to strike a delicate balance between safety and performance, with a crucial consideration being how effectively these substitutes produce the desired color outcomes.
Consumer preferences play a pivotal role in determining the success of these alternatives, encompassing factors such as color brilliance, durability, and coverage. Maintaining this delicate equilibrium between safety and efficacy is essential in meeting the diverse needs of consumers while mitigating potential adverse effects associated with conventional PPD-containing hair dyes.42–45
This study faced some limitations. First, the amount of unreacted PPD in the dyeing materials was not examined. Because unreacted PPD is thought to be the primary cause of PPD-related allergies and/or sensitizations, estimating its concentration is crucial. Second, the unconsumed PPD is measured using hair dyes rather than actual hair samples, which may not accurately reflect the presence of the dye in the hair. Hence, additional research should be performed to generalize the present findings because data regarding the effects of unconsumed PPD are lacking in the literature.
Conclusion
The study underscores the vital importance of integrating global best practices, drawn from successful models in other regions, into the legislative framework. By scrutinizing regulatory approaches in countries with similar cosmetic standards, valuable insights can be gleaned to enhance and reinforce the existing regulatory framework in the UAE.
Moreover, the imperative of instituting stringent guidelines for the production of hair dyes is underscored through the advocacy for the adoption of Good Manufacturing Practices (GMPs). This involves diligent monitoring of PPD (p-Phenylenediamine) concentrations and ensuring the overall quality and safety of the products. Encouraging manufacturers to adhere to established protocols can significantly mitigate potential risks associated with the use of hair colorants. The study also advocates for comprehensive training, extensive research initiatives, and systematic reporting of adverse reactions. Incorporating consumer education and robust research programs can elevate consumer awareness and facilitate ongoing enhancements in hair color formulation. The establishment of a systematic adverse reaction reporting system facilitates timely attention to safety concerns and fosters a culture of accountability within the industry. The study underscores the imperative of implementing a holistic strategy to ensure the safety and quality of hair dyes in the United Arab Emirates. This encompassing approach involves enacting specialized regulations, incorporating global best practices, enforcing GMPs, conducting research projects, offering instructional programs, and establishing a systematic framework for reporting adverse reactions. By addressing these multifaceted issues comprehensively, the cosmetics sector can contribute to creating a more transparent and secure environment for consumers.
Data Sharing Statement
All data will be provided upon request. Further inquiries can be directed to the corresponding authors (Ammar Abdulrahman Jairoun and Moyad Shahwan).
Ethical Approval
Approval for this study was obtained from the Institutional Review Board of An-Najah National University (reference number Int.R.March.2021/14).
Funding
This Paper was supported by Ajman University Internal Research Grant No. [2023-IRG-PH-6]. The research findings presented in this Paper are solely the responsibility of the author(s).
Disclosure
All authors declare that they have no conflict of interest.
References
1. Chisvert A, Miralles P, Salvador A. Chapter 8—hair dyes in cosmetics: regulatory aspects and analytical methods. In: Salvador A, Chisvert A, editors. Analysis of Cosmetic Products.
2. Morel OJX, Christie RM. Current trends in the chemistry of permanent hair dyeing. Chem Rev. 2011;111(4):2537–2561. doi:10.1021/cr1000145
3. Guerra-Tapia A, Gonzalez-Guerra E. Hair cosmetics: dyes. Actas Dermosifiliogr. 2014;105(9):833–839. doi:10.1016/j.ad.2014.02.004
4. Gavazzoni Dias MF. Hair cosmetics: an overview. Int J Trichol. 2015;7(1):2–15. doi:10.4103/0974-7753.153450
5. Kumar AB, Shamim H, Nagaraju U. Premature graying of hair: review with updates. Int J Trichol. 2018;10(5):198–203. doi:10.4103/ijt.ijt_47_18
6. Al-Enezi MH, Aldawsari FS. Study of P-phenylenediamine (PPD) concentrations after hair dye mixing: a call for safety reassessment. Cosmetics. 2022;9(2):41. doi:10.3390/cosmetics9020041
7. Bolduc C, Shapiro J. Hair care products: waving, straightening, conditioning, and coloring. Clin Dermatol. 2001;19(4):431–436. doi:10.1016/S0738-081X(01)00201-2
8. Draelos ZK. Hair cosmetics. Dermatol Clin. 1991;9(1):19–27. doi:10.1016/S0733-8635(18)30429-7
9. Ahn HJ, Lee WS. An ultrastructural study of hair fiber damage and restoration following treatment with permanent hair dye. Int J Dermatol. 2002;41(2):88–92. doi:10.1046/j.1365-4362.2002.01375.x
10. Larsen WG, Jackson EM, Barker MO, et al. A primer on cosmetics. AAD advisory board, CTFA task force on cosmetics. J Am Acad Dermatol. 1992;27(3):469–484. doi:10.1016/S0190-9622(08)80890-0
11. Da França SA, Dario MF, Esteves VB, Baby AR, Velasco MVR. Types of hair dye and their mechanisms of action. Cosmetics. 2015;2(2):110–126. doi:10.3390/cosmetics2020110
12. Harrison S, Sinclair R. Hair colouring, permanent styling and hair structure. J Cosmet Dermatol. 2003;2(3–4):180–185. doi:10.1111/j.1473-2130.2004.00064.x
13. Puri N, Puri A. ”A study on contact dermatitis to hair dye and henna”. Nasza Dematologia. 2013;4(4):545–548.
14. HSDB (Hazardous Substances Data Bank). Department of Health and Human Services. Bethesda, MD, USA: National Toxicology Information Program, National Library of Medicine; 1993.
15. Ezendam J, Salverda-Nijhof JGW. Hair Dye Allergy in Consumers; RIVM Report;. Utrecht, The Netherlands: National Institute for Public Health and the Environment; 2011.
16. Scientific Committee on Consumer Products (SCCP). Skin Penetration of Oxidative Hair Dyes Formed by the Coupling of Precursors and Couplers Under Simulated Conditions of Hair Dyeing. Luxembourg: European Commission, Health & Consumer Protection Directorate-General; 2006.
17. Scientific Committee on Consumer Products (SCCP). Opinion on Exposure to Reactants and Reaction Products of Oxidative Hair Dye Formulations. Luxembourg: European Commission, Health & Consumer Protection Directorate-General; 2005.
18. Chong HP, Reena K, Ng KY, Koh RY, Ch N, Chye SM. Para phenylenediamine containing hair dye: an overview of mutagenicity, carcinogenicity and toxicity. J Environ Anal Toxicol. 2016;6:5.
19. Shakoor Z, Al-Mutairi AS, Al-Shenaifi AM, Al-Abdulsalam AM, Al-Shirah BZ, Al-Harbi SA. Screening for skin-sensitizing allergens among patients with clinically suspected allergic contact dermatitis. Saudi Med J. 2017;38(9):922–927. doi:10.15537/smj.2017.9.19864
20. Almogren A, Shakoor Z, Rab MOG, Adam MH. Pattern of patch test reactivity among patients with clinical diagnosis of contact dermatitis: a hospital-based study. Ann Saudi Med. 2012;32(4):404–407. doi:10.5144/0256-4947.2012.404
21. Kim K-H, Kabir E, Jahan SA. The use of personal hair dye and its implications for human health. Environ Int. 2016:89–90, 222–227.
22. Thyssen JP, White JM, Thyssen JP, White JM. European society of contact dermatitis. epidemiological data on consumer allergy to p-phenylenediamine. Contact Dermatitis. 2008;59(6):327–343. doi:10.1111/j.1600-0536.2008.01427.x
23. Regulation (EC) No 1223/2009 of the European parliament and of the council ofon cosmetic products [cited 20/05/2022]; Available from: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf.
24. Government of Dubai (2022). Apply for Consumer Products Registration (MONTAJI). Available from: https://hub.dm.gov.ae/link/servicedetails?servicecode=3560
25. Venkatesan G, Dancik Y, Sinha A, et al. Development of novel alternative hair dyes to hazardous para-phenylenediamine. J Hazard Mater. 2021;402:123712. doi:10.1016/j.jhazmat.2020.123712
26. Goebel C, Troutman J, Hennen J, et al. Introduction of a methoxymethyl side chain into p-phenylenediamine attenuates its sensitizing potency and reduces the risk of allergy induction. Toxicol Appl Pharmacol. 2014;274(3):480–487. doi:10.1016/j.taap.2013.11.016
27. Al-Suwaidi A, Ahmed H. Determination of para-phenylenediamine (PPD) in henna in the United Arab Emirates. Int J Environ Res Public Health. 2010;7(4):1681–1693. doi:10.3390/ijerph7041681
28. Brancaccio RR, Brown LH, Chang YC, Fogelman JP, Mafong EA, Cohen DE. Identification and quantification of para-phenylenediamine in a temprorary black henna tattoo. Am J Contact Dermat. 2002;13(1):15–18. doi:10.1053/ajcd.2002.30466
29. Nawaf AM, Joshi A, Nour-Eldin O. Acute allergic contact dermatitis due to para-phenylenediamine after temporary henna painting. J Dermatol. 2003;30(11):797–800. doi:10.1111/j.1346-8138.2003.tb00480.x
30. Wolf R, Wolf D, Matz H, Orion E. Cutaneous reactions to temporary tattoos. Dermatol Online J. 2003;9:3.
31. Di Prisco MC, Puig L, Alomar A. Contact dermatitis due to para-phenylenediamine (PPD) on a temporal tattoo with henna: cross reaction to azoic dyes. Invest Clin. 2006;47(3):295–299.
32. Rastogi SC, Sosted H, Johansen JD, Menne T, Bossi R. Unconsumed precursors and couplers after formation of oxidative hair dyes. Contact Dermat. 2006;55(2):95–100. doi:10.1111/j.0105-1873.2006.00887.x
33. Lee SE, Lee SH. Skin Barrier and Calcium. Ann Dermatol. 2018;30(3):265–275. doi:10.5021/ad.2018.30.3.265
34. P TJ, E AK, Bruze M, et al. p -Phenylenediamine sensitization is more prevalent in central and Southern European patch test centres than in Scandinavian: results from a multicentre study. Contact Dermatitis. 2009;60(6):314–319. doi:10.1111/j.1600-0536.2009.01547.x
35. Gass JK, Todd PM. PPD: is this a connubial dermatitis? Contact Dermatitis. 2006;55(5):309. doi:10.1111/j.1600-0536.2006.00897.x
36. Chandran J, Manners R, Agarwal I, Ebenezer K. Hair dye poisoning in a paediatric patient. Case Rep Pediatr. 2012;2012:931463. doi:10.1155/2012/931463
37. Lyons G, Roberts H, Palmer A, Matheson M, Nixon R. Hairdressers presenting to an occupational dermatology clinic in Melbourne, Australia. Contact Dermatitis. 2013;68(5):300–306. doi:10.1111/cod.12016
38. Al-Suwaidi M Henna additive health warning. The National Newspaper 2009, February.
39. Seyedzadeh A, Hemmati M, Gheiny S. Henna—induced severe hemolysis: in glucose 6- phosphate dehydrogenase deficiency. Pak J Med Sci. 2007;23:119–121.
40. Soker M, Devecioglu C, Haspolat K, Kikicl B, Dogru O. Henna Induced acute hemolysis in a G6PD deficient patient: a case report. Int Pediatric. 2000;15:114–116.
41. Raupp P, Hassan JA, Varughese M, Kristiansson B. Henna cause’s life threatening haemolysis in glucose-6-phosphate dehydrogenase deficiency. Arch Dis Child. 2001;85(5):411–412. doi:10.1136/adc.85.5.411
42. Blömeke B, Brans R, Coenraads PJ, et al. Para-phenylenediamine and allergic sensitization: risk modification by N-acetyltransferase 1 and 2 genotypes. British J Dermat. 2009;161(5):1130–1135. doi:10.1111/j.1365-2133.2009.09352.x
43. Rietschel SM, Herrera-Acosta E, López-Navarro N, et al. p-Phenylenediamine and other hair dye sensitizers in Spain. monitoring the years 2005-2011 by the Spanish contact dermatitis and skin allergy research group. Actas Dermo-Sifiliograficas. 2012;103(2):120–126. doi:10.1016/j.ad.2011.04.010
44. Vano-Galvan PA, Dogu F, Tuygun N, et al. Systemic contact dermatitis from para-phenylenediamine in hair dye with tolerance to azo dyes. J Invest Allergol Clin Immunol. 2011;21(5):401–502.
45. Kind F, Scherer K, Bircher AJ . Contact dermatitis to para‐phenylenediamine in hair dye following sensitization to black henna tattoos–an ongoing problem. J Dtsch Dermatol Ges. 2012;10(8):572–578. doi:10.1111/j.1610-0387.2011.07882.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.