Back to Journals » Journal of Pain Research » Volume 13
Quantification of Opioid Prescription Practice Changes Due to Hydrocodone Combination Product Rescheduling in an Academic Pain Clinic
Authors Ngo J, Parker D, Meroney M, Mitchell J, Veloz O, Lee O , Cunningham KA , Wilkes D
Received 2 March 2020
Accepted for publication 9 June 2020
Published 25 August 2020 Volume 2020:13 Pages 2163—2168
DOI https://doi.org/10.2147/JPR.S251386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
John Ngo,1 David Parker,2 Mathew Meroney,3 Jasmine Mitchell,2 Oscar Veloz,4 Oliver Lee,5 Katherine A Cunningham,2 Denise Wilkes1
1Department of Anesthesiology and Pain Management, University of Texas Medical Branch, Galveston, TX, USA; 2Center for Addiction Research, University of Texas Medical Branch, Galveston, TX, USA; 3Department of Anesthesiology and Pain Management, University of Florida, Gainesville, FL, USA; 4Department of Anesthesia, Mcgaw Northwestern University Medical Center, Chicago, IL, USA; 5Department of Anesthesia, University of Washington, Seattle, WA, USA
Correspondence: Denise Wilkes
Department of Anesthesiology and Pain Management, University of Texas, Medical Branch, 301 University Blvd, Galveston, TX 77555, USA
Tel +1 409-772-1221
Fax +1 409-747-0011
Email [email protected]
Purpose: To determine the effect of rescheduling on prescription practices in a large academic hospital-based multidisciplinary practice comprising anesthesiologist-trained pain physicians.
Patients and Methods: We examined the number of HCP prescriptions written and quantity of tablets prescribed during a 6-month period prior to rescheduling and compared this with a 6-month period 1 year after rescheduling. We also examined the changes in prescription of tramadol and acetaminophen with codeine from one period to the next.
Results: Our pain clinic conducted 3,320 office visits during the 6-month period prior to HCP rescheduling and 6,003 office visits in the 6-month period 1 year after rescheduling. The charted data from each of these visits were used for our analysis. The mean number of tablets of HCPs prescribed per patient decreased from 318.48 in the pre-period to 242.27 tablets in the post-period, while the mean number of HCP prescriptions per patient decreased from 2.24 to 1.84. The mean number of acetaminophen with codeine tablets prescribed per patient increased from 3.46 to 15.27 in the pre- and post-period. Similarly, the mean number of tramadol tablets per patient increased from 47.33 to 61.97 in the pre- and post-period. The mean number of acetaminophen with codeine and tramadol prescriptions per patient increased from 0.02 to 0.15 and 0.38 to 0.51 in the pre- and post-period, respectively. In the 6-month post-period, fewer new patients were started on opioids compared to the 6-month pre-period, 16% and 27%, respectively.
Conclusion: Our study showed a significant decrease in the mean number of HCP prescriptions written per patient, as well as a decrease in the mean number of HCP tablets prescribed. Pain physicians in our clinic increased the number of prescriptions for the non-HCPs. The number of acetaminophen with codeine and tramadol tablets prescribed significantly increased. Therefore, the rescheduling of HCPs has profoundly impacted practices within this academic pain clinic.
Keywords: hydrocodone, tramadol, acetaminophen, schedule, reschedule
Introduction
Prescription opioids were involved in nearly 17,000 overdose deaths in 2017.1 Initially, hydrocodone combination products (HCP), such as hydrocodone with either acetaminophen or ibuprofen, were classified as schedule III drugs when Congress passed the Controlled Substance act in 1970. HCPs became one of the most commonly prescribed opioids.2 As the opioid overdose crisis grew, the United States Drug Enforcement Agency (DEA) rescheduled HCPs to schedule II on October 6th of 2014 in an effort to curb opioid prescribing. Drugs are classified into 5 categories based mostly Medtronic embrace continuing review on abuse potential with category I being the most abused and category V being the least. This change produced a 22% decline in HCPs (schedule II) with a 5% increase in non-HCPs, such as acetaminophen with codeine (schedule III) and tramadol (schedule IV), in the following 12 months.3
Prescription rates also vary among different provider specialties. Primary care providers, including internal medicine, family medicine, and general practitioners, have and continue to prescribe a majority of the opioids.4 Since the rescheduling of HCPs, the number of opioid prescriptions from primary care providers has decreased from 128.3 to 77.7 million.4,5 In contrast, the number of opioid prescriptions written by pain medicine specialists have increased from 14.5 to 18.7 million, which might be due to increased referrals from primary care providers.4,5
Board-certified pain physicians complete a pain fellowship after a board certification in anesthesiology, neurology, preventative medicine, or psychiatry. Often, the type of specialty determines the type and scope of their pain practice. There are a wide variety of practices from those that provide a single service, such as medical management or procedures, to others that provide multiple services with a multidisciplinary approach. Little is known about how rescheduling has affected pain treatment practices. We aim to determine the effect of rescheduling on prescription practices in a large academic hospital-based multidisciplinary practice comprising anesthesiologist-trained pain physicians and to compare to schedule II to schedule III or IV prescription trends.
Patients and Methods
Study Design
The study was a retrospective chart review of chronic pain patients seen at pain clinic at the University of Texas Medical Branch (UTMB) during two 6-month periods: pre and post 1 year after HCP scheduling. The pre-period was from April 2014 to October 2014, and the post-period was from October 2015 to April 2016, 1 year after HCP scheduling. The decision was made to wait 1 year after the rescheduling for the second 6-month period to take into account that many patients were likely to receive prescriptions with multiple refills up to October 2014. Therefore, 1 year would likely be a sufficient amount of time for these prescriptions to either be used or expire. Variables recorded included the number of tablets and prescriptions of hydrocodone-containing products, codeine-containing tablets, and tramadol. We also recorded age, gender, and the numerical pain score at the time of visit.
Statistical Analysis
IBM SSPS statistical program was used to analyze data and Kaleidagraph version 4.1.3 was used to produce graphics. Patient demographic and pre- and post-rescheduling groups were compared by t-test for continuous variables and chi-square test or Fischer exact test for categorical variables. Statistical significance represented by p<0.5. Means were reported as mean ± standard error of the mean (SEM).
Results
Participants
After the approval by the Institutional Review Board at the UTMB, a review of electronic medical records for all patients who attended the pain clinic during the “pre” and “post” time periods was conducted. The requirement to obtain informed consent was waived for this chart review study in accordance with 45 CFR 46.116(d). We excluded patients less than 18 years of age, as few patients in this age group are seen in our clinic. New patients were defined as the first appointment in the pain clinic in the last three years and follow-up patients had an appointment in the last three years.
UTMB Pain Clinic
The clinic is a multidisciplinary clinic that is associated with the university hospital. There are eight faculty members comprising seven anesthesiologists with six having completed a pain fellowship and one pain psychologist. The clinic is associated with anesthesiology residency and pain fellowship programs. There was no change in faculty or clinic location between the pre and post period.
Pain Clinic Standard of Care
The purpose of the new patient appointment was primarily evaluative; typically, medications are not prescribed at this visit. Physicians completed a thorough physical exam and history, reviewed medical records, Pharmacy Monitoring Program (PMP) records, examined pill bottles, obtained a pain clinic contract, and obtained a urine drug screen (UDS). UDS was done by liquid chromatography and mass spectrometry and obtained before starting prescriptions of opioids and 3–6months, thereafter. Medical cannabinoids were not legal in Texas at this time. A follow-up appointment was scheduled within a month at which results were reviewed and medications prescribed. The pain contract forbids patients from sharing medications with someone else, taking someone else’s pain medication or benzodiazepine, obtaining multiple prescriptions for pain medication from multiple providers, taking illicit drugs, consuming alcohol, selling medication, asking for early refills too many times (determined by treating physician), and/or abusive behavior towards clinic staff.
The maximum duration between patient visits was 6 months. The duration of follow-up was based on treating physician’s judgment. During the pre-period, patients were given a prescription with refills. In the post-period, patients were given a paper schedule II prescription for each month up to 3 months at one time. Electronic prescribing was not used during either period.
Participant Demographics
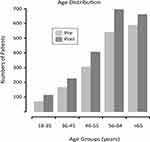
After rescheduling HCPs, the new patient appointments increased by 56% from the pre- to post-period with 435 and 680 patients seen in these periods, respectively (Figure 1). The total number of patient appointments increased dramatically from 3320 to 6003 between these two periods: an 81% increase. Similarly, the number of follow-up appointments increased by 85%. The increase in follow-up visits was due to a clinic policy change after rescheduling HCPs, in which the patients were required to visit the clinic monthly to pick up prescriptions. The increase in clinic patients did not change the distribution of age group seen in the pain clinic (Figure 1). There were no significant changes in the pain scores between the new and follow-up patients between these periods (Table 1).
 |
Table 1 Clinic Case Load with Associated Pain Scores |
Prescription Data Analysis
All clinic visits during the two 6-month periods were reviewed for prescription for HCPs, or acetaminophen with codeine or tramadol. A total of 3320 visits in the pre-period and 6003 visits in the post-period were reviewed. The prescription practice of the clinic was analyzed in three ways: number of prescribed tablets, numbers of prescriptions, and tablets per prescription. The mean number of tablets of HCPs per patient decreased from 318.48 ± 457.05 in the pre-period to 242.27 ± 453.18 (p<0.0001) tablets in the post period. The mean number of tablets of acetaminophen with codeine increased from 3.46 ± 52.71 to 15.27 ± 95.34 (p<0.0001) tablets in the pre- and post-period, respectively. Similarly, the mean number of tramadol tablets per patient increased from 47.33 ± 170.77 to 61.97 ± 219.22 (p<0.0215) in the pre- and post-period, respectively (Figure 2).
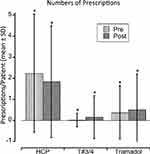
The mean number of prescriptions per patient decreased for HCP prescriptions from 2.24 ± 2.81 to 1.84 ± 2.65 (p<0.0001) in the pre- to post-period. The mean number of acetaminophen with codeine prescriptions increased from 0.02 ± 0.32 to 0.15 ± 1.02 (p<0.0001) in the pre- and post-period. The mean number of tramadol prescriptions per patient increased from 0.38 ± 1.26 to 0.51 ± 1.7 (p<0.0104) in the pre- and post-periods (Figure 3). In the post-period, fewer new patients were started on opioids in the 6-month period compared to the pre-period, 16% and 27%, respectively.
Discussion
We showed that the rescheduling of HCPs greatly changed our academic practice in the pain clinic. After rescheduling HCPs, clinic visits increased by 81% and new patient visits increased by 56%. Even though the clinic grew, the number of prescribed HCP tablets decreased. Concomitantly, there was an increase in acetaminophen with codeine and tramadol prescriptions. After rescheduling HCPs, new patients were less likely to receive opioids compared to before, 16% vs 27%, respectively.
The increase in clinic visits by 81% had a major administrative impact on our pain clinic and our patients. Patients were required to visit the clinic every month for a refill of their HCP prescription. In order to accommodate this increase, we developed a prescription refill appointment. During these appointments, our nurses identified the patient, recorded blood pressure, heart rate, and weight, and then gave the prescriptions to the patient. This involved increased administrative coordination of having the prescribing physicians write the prescription before the appointment as well as securing storage of the prescription, and delivery to the patient. If the appointment was missed, the prepared prescription was stored until the rescheduled appointment.
Long-term opioid therapy required complex schedules with prescription appointment monthly in between follow-up visits. For patients who were too ill to travel monthly, we allowed family members to pick up prescriptions with a letter, handwritten by the patient, giving permission to another person to pick up the prescription. The letter was signed by the patient and included a picture identification to ascertain the validity of the pick-up. The monthly clinic visits were also a burden on our patient population due to increased travel costs and time away from work and family.
Pain physicians see only 2% of chronic pain patients.6 Primary care physicians see the vast majority. The 56% increase in new patients most likely represents chronic pain patients, previously treated by their primary care provider, being referred to the pain clinic. A poll of Texas Medical Association physicians showed that 27% did not intend to prescribe HCPs after rescheduling.7 Therefore, the legislative change has substantively impacted the primary care practices as well.
Studies, examining the effects of rescheduling HCPs, have shown dramatic prescription practice changes. Retail pharmacies across the United States showed 1.1 billion fewer dispensed HCP tablets after the rescheduling.3 All physician specialties have shown a decrease in HCP prescriptions except pain physicians, which showed an increase.3 The increase was assumed to be due to increased referrals.
Several other studies have shown an increase in non-HCP prescriptions after rescheduling HCPs in agreement with our findings.3,8,9 A 4-year study, 2012–2015, showed a continuous gradual increase in tramadol prescriptions whereas acetaminophen with codeine showed a dramatic increase from 2014 to 2015.10 Tramadol has been considered to be safer than HCPs because they were shown to have fewer side effects and have less abuse potential.11,12 A more recent study tested the abuse potential of supra-therapeutic tramadol and found drug-liking effects similar to oxycodone and self-administration rates higher than oxycodone in prescription opioid abusers.13 This indicates that tramadol abuse potential was not as low as was once thought. This study also showed that codeine increased both drug liking and bad drug effects similarly and had less self-administration than tramadol or oxycodone.13
Tramadol abuse, trafficking, and overdoses are major problems in many countries. England and Wales have detected increase in tramadol overdoses.14 Tramadol has become one of the most illegitimately manufactured, trafficked, and abused drugs in Africa, India, and the Middle East. Recent attempts to interrupt the supply have generated large seizures such as one in Nigeria that reported seizures of 22.5 tons of high dose tramadol tablets in 2018.15 According to the surveys in the United States, legitimately manufactured tramadol continues to have lower risk compared to other prescribed pain medications. It is the illegitimate high dose tramadol fueling the tramadol crisis internationally report.16
Physicians should still consider the side effect profiles when switching from HCP to tramadol or acetaminophen with codeine. Codeine-containing products are extensively metabolized by the CYP2D6 to morphine. Genetic studies have shown that some people lack CYP2D6 and will experience no analgesic effect while others can have fast metabolizer morphology and have an increased risk of overdose.17 Tramadol has a mixed mechanism of action at opioid, serotonin, and norepinephrine receptor, which can increase the risk for drug interactions compared to HCP. Tramadol also increases the risk of seizures, even at low doses.18,19
The limitations of this study relate to its retrospective nature. The reasons, such as patient preference or insurance denial, for the changes in prescription rates cannot be extrapolated from this retrospective review. We were also not able to determine the conversion factors used when changing from one opioid to another or the physician-patient discussion at the time of prescription change. Another limitation is that this study only reflects a single academic site in the greater Galveston-Houston area and may not reflect the practices elsewhere in the region or in Texas. Regional areas of the US that have higher percentages of non-Hispanic whites, prevalence of diabetes and arthritis, and rates of unemployment and Medicaid status have been noted to have higher HCP prescription rates.4
Conclusion
Our study showed an increase in new patient visits, but a decreased in both the total number of HCP tablets prescribed and the percentage of new patients receiving HCPs. Pain physicians in our clinic reduced the number of HCP tablets prescribed, while increasing the number of HCP prescriptions, which means that more patients were prescribed a reduced number of tablets on each prescription. Similar to other specialties, pain physicians in our clinic increased the number of prescriptions for the non-HCPs. Therefore, the rescheduling of HCPs has profoundly impacted practices within this academic pain clinic.
Acknowledgments
Thank you to Dr. Yong-Fang Kuo for her expertise in statistics. This study was partially sponsored by NIH NIDA 5T32DA007287-24.
Disclosure
Dr. Denise Wilkes discloses that she has investigator-initiated research with Medtronic, reports personal fees from medtronic embrace study, outside the submitted work; and has apatent US patent 8,212,104 with royalties paid by Medtronic. No disclosures by other authors declared at this time. Further, the authors have not received any reimbursement or honorarium in any other manner.
References
1. Abuse NIoD. Overdose Death Rates, (ed 1/19). Available from: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
2. Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy (Basel). 2018;6.
3. Jones CM, Lurie PG, Throckmorton DC. Effect of US drug enforcement administration’s rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med. 2016;176(3):399–402. doi:10.1001/jamainternmed.2015.7799
4. Guy GP
5. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med. 2015;49:409–413. doi:10.1016/j.amepre.2015.02.020
6. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738–747. doi:10.1097/SMJ.0b013e3181e74ede
7. Fleming ML, Driver L, Sansgiry SS, et al. Physicians’ intention to prescribe hydrocodone combination products after rescheduling: a theory of reasoned action approach. Res Social Adm Pharm. 2017;13:503–512. doi:10.1016/j.sapharm.2016.07.001
8. Schultz S, Chamberlain C, Vulcan M, Rana H, Patel B, Alexander JC. Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J Opioid Manag. 2016;12(2):119–122. doi:10.5055/jom.2016.0323
9. Oehler EC, Day RL, Robinson DB, Brown LH. Has the rescheduling of hydrocodone changed ED prescribing practices? Am J Emerg Med. 2016;34(12):2388–2391. doi:10.1016/j.ajem.2016.09.002
10. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. doi:10.1007/s11916-019-0777-x
11. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi:10.2165/00003088-200443130-00004
12. Radbruch L, Grond S, Lehmann KA. A risk-benefit assessment of tramadol in the management of pain. Drug Saf. 1996;15(1):8–29. doi:10.2165/00002018-199615010-00002
13. Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129(1–2):116–124. doi:10.1016/j.drugalcdep.2012.09.018
14. Handley SA, Flanagan RJ. Drugs and other chemicals involved in fatal poisoning in England and Wales during 2000–2011. Clin Toxicol (Phila). 2014;52(1):1–12. doi:10.3109/15563650.2013.872791
15. Control BIN. Report for the International Narcotics Control Board for 2018. New York; 2018.
16. Report USNaW. How Tramadol, Touted as Safer Opioid, Became 3rd World Peril. Press A, (ed);2019.
17. Nafziger AN, Barkin RL. Opioid therapy in acute and chronic pain. J Clin Pharmacol. 2018;58:1111–1122. doi:10.1002/jcph.1276
18. Hassamal S, Miotto K, Dale W, Tramadol: DI. Understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(1382):e1381–1382 e1386. doi:10.1016/j.amjmed.2018.04.025
19. Beyaz SG, Sonbahar T, Bayar F, Erdem AF. Seizures associated with low-dose tramadol for chronic pain treatment. Anesth Essays Res. 2016;10(2):376–378. doi:10.4103/0259-1162.177181
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.