Back to Journals » Clinical Interventions in Aging » Volume 15
Quality of Life and Frailty Syndrome in Patients with Atrial Fibrillation
Authors Sławuta A , Jacek P, Mazur G , Jankowska-Polańska B
Received 3 February 2020
Accepted for publication 9 April 2020
Published 29 May 2020 Volume 2020:15 Pages 783—795
DOI https://doi.org/10.2147/CIA.S248170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Agnieszka Sławuta,1 Polański Jacek,1 Grzegorz Mazur,1 Beata Jankowska-Polańska2
1Department of Internal Medicine, Occupational Diseases, Hypertension and Clinical Oncology, Wroclaw Medical University, Wrocław, Poland; 2Department of Clinical Nursing, Faculty of Health Science, Wroclaw Medical University, Wrocław, Poland
Correspondence: Beata Jankowska-Polańska
Department of Clinical Nursing, Wroclaw Medical University, K. Bartla 5, Wrocław 51-616, Poland
Tel +48 71 784 18 24
Fax +48 71 345 93 24
Email [email protected]
Introduction: Atrial fibrillation (AF) and frailty syndrome (FS) are a part of the aging process. Both are still of great importance in the assessment of quality of life (QoL). There is definitely a lack of research clarifying the association between FS and QoL in AF patients.
Objective: The aim of this study was to evaluate the influence of FS on QoL in AF patients.
Materials and Methods: The retrospective and observational study included 158 inpatients with mean age 69.8± 7.1 years, treated for AF in the cardiac department from 1 April 2019 to 31 June 2019. The following instruments were used: the Arrhythmia-Specific Questionnaire in Tachycardia and Arrhythmia (ASTA) and the Edmonton Frail Scale (EFS).
Results: The mean level of frailty in the study group was 8.5± 5.0. In 25.9% of patients, the level of frailty was mild, in 10.1% moderate, and in 17.1% severe. Patients were divided into two groups based on their frailty status. In comparative analysis of the QoL, there were significant differences between the groups: the frail group had more intense symptoms of arrhythmia than the non-frail group (14.9± 4.1 vs 11.9± 4.9; p< 0.001). In the analysis of the total score impact of arrhythmia on QoL, the frail group had a significantly higher score than the non-frail group (23.5± 5.2 vs 14.5± 5.5), which confirmed the stronger negative impact of arrhythmia on QoL. In the regression coefficient analysis, the independent predictor of symptom severity and QoL was FS. However, we observed a negative impact of diabetes, which increased the impact of arrhythmia on QoL, and physical activity, which improved QoL and decreased the impact of symptoms on everyday life.
Conclusion: Patients in the frail group have worse QoL and higher impact of arrhythmia on QoL in comparison to patients in the non-frail group. Frailty is an independent predictor of higher intensity of symptoms of arrhythmia and worse QoL. Diabetes and physical activity are predictors of QoL for patients with AF.
Keywords: atrial fibrillation, frailty, older age
Introduction
Population aging is associated with a progressive accumulation of cardiovascular risk factors, an increased prevalence of degenerative and involutional disorders, and alterations in the way the central nervous system, and consequently the entire body, is functioning.1 Atrial fibrillation (AF) and frailty syndrome (FS) are a part of this process,2 though these disorders by no means exhaust the long list of comorbidities.3 All these factors affect patients’ quality of life (QoL), but FS significantly modifies the diagnosis and treatment of other diseases, and AF is a perfect example of that. Diagnosis and multifaceted treatment of AF is considerably hindered in frail patients, which leads to greater complications such as QoL deterioration, thromboembolic incidents, heart failure, and premature death.4
According to the research, frailty affects approximately 7% of people aged 65 years or older and about 25–40% of people aged 80 years and more5, and the prevalence of frailty increases with age. It is also more likely to occur in women than men (8% vs 5%).3 The mechanisms of frailty are complex and are related to immune dysfunction, chronic inflammation, endocrine changes, permanent stress, and energy response systems.6 The number of chronic diseases in frail elderly people is about 1.5 times higher than in the non-frail population.7 Most often cardiovascular and chronic kidney disease occur, as well as diabetes mellitus and depressive symptoms.7–13 Frailty itself is a powerful predictor of mortality, mostly in cardiovascular patients, independent of age, underlying disease severity, comorbid conditions, and disability.14 Frail older adults are at a greater risk of multiple adverse outcomes, falls, disability, including procedural complications, adverse drug reactions, hospitalization, and shorter survival. Compared to the CHA2DS2-VASc score, the FI had a similar predictive power for the prediction of unplanned hospitalization, stroke, bleeding, and death.15
AF is the most common cardiac arrhythmia with an estimated prevalence of 2% in the general adult population of Europe and is responsible for approximately 365,000 hospital admissions annually, which is more than any other arrhythmia.16 Its prevalence also increases with age, ranging from 0.7% in people aged 55–59 years to almost 20% for those aged 85 years or older.17 Elderly patients suffering from AF, especially the frail ones, often suffer from numerous comorbidities and receive multiple medications.18 AF is associated with substantial morbidity and mortality from heart failure, stroke, and other thromboembolic complications.19 Frailty is associated with a higher left atrial volume,20 which is one of the main cardiac abnormalities related to the development of AF. Patients with AF had low gait speed, and low gait speed has been linked with impaired mood, cognition, and quality of life.21 The presence of AF influences negatively the quality of life, as it remains symptomatic in about two-thirds of patients despite them receiving medical treatment.22 Previous studies investigating the impact of AF on HRQoL found poorer HRQoL in AF patients compared to the general population.23
The major therapeutic goal in patients with AF is to restore and maintain sinus rhythm, which unfortunately is not possible for the long term in all cases. Thus, current management strategies focus on the heart rate and rhythm control, thromboembolism prevention, and treatment of underlying diseases. At present, there is not a single globally accepted definition of QoL in AF. The term is subjective, and it may be defined using one or many aspects such as symptoms, functional status, and patients’ health perceptions, experiences, and expectations. The subject of quality of life in atrial fibrillation is rarely presented. Few studies discuss both quality of life and frailty syndrome among patients with chronic diseases, but there are no papers that concern the group of patients with atrial fibrillation.
Purpose of the Study
The purpose of the study was to identify frailty syndrome (FS) between patients with atrial fibrillation (AF), and evaluate the influence of FS on QoL in patients suffering from AF.
Materials and Methods
The current research has a retrospective and observational study design. The study was conducted in the cardiology department. Participants’ recruitment was conducted from 1 April 2019 to 31 June 2019. Qualification for the study was carried out by a trained cardiologist or internal medicine doctors.
Inclusion criteria included a confirmed diagnosis of AF as per EHRA criteria, age ≥60 years, consent to participate, and cognitive function sufficient for unassisted completion of the questionnaire. Exclusion criteria were: age <60 years, lack of consent or withdrawal of consent during the study, cognitive impairment indicating dementia (Mini-Mental State Examination), and serious comorbidities during exacerbation that could affect the results of the quality of life study (eg HF – NYHA IV, angina – CCS IV, acute myocardial infarction, COPD during exacerbation).
All patients provided informed consent to participate in the study, and their clinical condition was stable. Patients were informed that the study was strictly anonymous, and that they could withdraw from it at any stage without providing a justification.
The study included 158 in patients aged above 60 years (mean age 69.8±7.1 years) treated for AF.
The study was approved by the Wroclaw Medical University Bioethics Committee. The study used a diagnostic survey, including the following instruments:
- The authors’ own questionnaire recording patients’ gender (F, M), age, education, marital status, and residence. Information concerning the clinical data comes from the clinic files.
- The Arrhythmia-Specific Questionnaire in Tachycardia and Arrhythmia (ASTA) – Polish version for health-related Quality of Life (HRQoL), which evaluates the perceived impact of the disease on the patient’s QoL. Part I focuses on the arrhythmia experienced by the patients and the medication taken. Part II records the severity of the nine most common arrhythmia symptoms using the ASTA 9-item scale, along with their frequency and duration. The higher scores indicate a higher symptom burden. Part III evaluates the impact of arrhythmia on patients’ daily lives (ie HRQoL), and includes 13 items related to daily physical and psychological functioning. The total ASTA HRQoL score ranges between 0 (best possible HRQoL) and 39 (worst possible HRQoL), with higher scores indicating a greater negative impact of arrhythmia on HRQoL.24
- The Edmonton Frail Scale (EFS), which includes 10 domains related to cognitive function, mobility, balance, mood, social support, nutrition, health attitudes, QoL, medication, and functional independence. The geriatric condition evaluation is determined by three aspects: physical, psychological, and social. Each item is scored between 0 and 3 points. Overall, the maximum score is 17 and represents the highest level of frailty.25
For statistical analyses, the data collected in the study were recorded, processed, and analyzed using Statistica software. The statistical analyses of the survey data comprised the following stages. Qualitative variables measured on nominal (e.g. gender) and ordinal (e.g. education) scales were cross-tabulated, and the strength of the associations between the pairs of variables was assessed using the chi-squared test. When the expected count in at least one cell of a four-field table was lower than 5, Fisher’s exact test was used. For all quantitative variables, mean (M), standard deviation (SD), median (Me), lower quartile (Q1), upper quartile (Q3) values, and ranges (min and max) were calculated. For quantitative variables (e.g. age), the distribution normality was verified using the Shapiro–Wilk test. The homogeneity of variance was verified using Bartlett’s and Levene’s tests. The significance of differences between the mean values of variables with a normal distribution and homogeneous variances in two independent groups was verified using Student’s t-test. The significance of differences between the mean values of variables with a non-normal distribution or with heterogeneous variances in two groups was verified using the non-parametric Mann–Whitney U-test. The strength and direction of linear correlations between two continuous variables were determined using regression analysis based on the Pearson’s r linear correlation coefficient. Regression coefficient values were estimated using the least square method.
For all statistical tests, a significance threshold of p=0.05 was used. Statistical analysis results are shown in a graphical or table form. Calculations were performed using EXCEL spreadsheets and the STATISTICA v. 12 software package.
Results
The study included 158 patients (78 of whom were female – 49.4%) aged between 60 and 88 years (M=70.4±7.6 years) treated for AF. Over half of the respondents were single (51.9%), lived in urban places (58.2%), and were retired (62.7%). 53.2% were physically active (about 150 minutes of physical activity a week). The basic descriptive statistics are shown in Table 1.
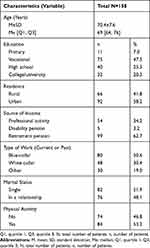 |
Table 1 General Social Characteristics of the Patients Studied |
The mean duration from the diagnosis of AF was 6.9±4.7 years. 79.7% of respondents had arterial hypertension, 57.6% diabetes, 53.8% respiratory disorders, and 51.3% ischemic heart disease. From the moment of diagnosis of AF, 30.4% of patients were hospitalized 3–4 times, and 27.2% of patients were hospitalized more than 10 times. Unfortunately, 41.1% of respondents were still active smokers. The basic descriptive statistics are shown in Table 2.
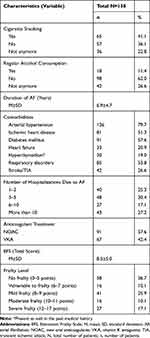 |
Table 2 Clinical Characteristics of the Study Group |
The mean level of frailty in the study group was 8.5±5.0. Frailty syndrome was not diagnosed in 36.7% of patients, 10.1% of patients were vulnerable to frailty, and frailty syndrome was revealed in the rest of the study group. In 25.9% of patients, the level of frailty was mild, in 10.1% moderate, and in 17.1% severe. For the quality of life analyses, patients were divided into subgroups based on the frailty status (Table 2):
Group 1 (frail): patients with mild, moderate, or severe frailty (8–17 points on the Edmonton Frail Scale).
Group 2 (non-frail): non-frail patients and vulnerable patients (0–7 points on the Edmonton Frail Scale).
In comparative analysis, the differences between the frail and non-frail groups as regards last experienced arrhythmia were determined. Patients from the frail group often had symptoms of arrhythmia, permanent (35.7%) or on and off every day (33.3%), whereas patients from the non-frail group rarely had the everyday symptoms of arrhythmia and often had the symptoms that appeared in the period “between 1 and 6 months ago”. All patients from the non-frail group were regularly treated, whereas only 89.2% of respondents in the frail group were treated. There were differences between the treatment of the patients observed. Patients from the frail group were often given antiarrhythmics, digoxin calcium channel blockers, and VKA in antithrombotic treatment. Patients from the non-frail group were often administered beta blockers and NOAC drugs (Table 3).
 |
Table 3 Arrhythmia-Specific Symptoms and QoL by ASTA Part I in Patients Divided by Frailty Status |
More frail than non-frail patients regularly took metoprolol (33.3% vs 58.1%; p=0.002), while less took verapamil (5.5% vs 23.9%; p=0.001). Moreover, more non-frail than frail patients were treated with antiarrhythmic medication – amiodarone + propafenone (21.4% vs 4.1%; p=0.001). This finding may have clinical implications but also indicates the therapeutic trend (Table 3).
In comparative analysis of the quality of life (second part of the ASTA questionnaire), there were significant differences between the study groups. The patients from the frail group often had symptoms of arrhythmia, even pointing out “permanent arrhythmia” (35.7% vs 12.2), whereas most respondents from the non-frail group did not experience such symptoms or had them rarely, pointing out “fewer than 5 times during 3 years” (Table 4). Patients differ in terms of the period of duration of arrhythmia. Patients from the frail group claimed that arrhythmia lasts over 7 days (33.3%), whereas patients from the non-frail group felt arrhythmia between 1 and 7 hours (33.8%). In the frail group the last paroxysm of arrhythmia lasted over 7 days in 40% of respondents, but in the non-frail group between 24 and 48 hours in 52.7% of patients. What is interesting, respondents from the non-frail group more often had the symptoms of arrhythmia: tachycardia (67.6% vs 38.1%) and irregular heartbeat (70.3% vs 52.4%). Respondents from the frail group did not have such symptoms (31% vs 13.5%). What is more, a significant difference between both groups as regards the results of total ASTA II (symptom severity) was revealed, and the frail group had more intense symptoms than the non-frail group (14.9±4.1 vs 11.9±4.9; p<0.001). Moreover, patients from the frail group more often fainted due to arrhythmia than patients from the non-frail group (48.8% vs 13.5%) (Table 4).
 |
Table 4 Arrhythmia-Specific Symptoms Characteristic Between Frail and Non-Frail AF Patients |
The comparative analysis of the quality of life (third part of the ASTA questionnaire) showed that patients from the frail group more often could not work, could not spend time with their families or friends, and could not travel. Moreover, the respondents from the frail group more often could not engage in physical activity, could not concentrate, had negative emotions, had disturbed night sleep, and had a disturbed sex life. In analysis of the total score for the impact of arrhythmia on QoL, patients from the frail group had a significantly higher score than patients in the non-frail group (23.5±5.2 vs 14.5±5.5), which confirmed the stronger negative impact of arrhythmia on quality of life (Table 5).
 |
Table 5 QoL of the Patients Studied Between Fraily and Non-Frail AF Patients |
Single- and Multiple-Factor Analysis of the Impact of the Characteristics Analyzed and QoL (ASTA II and ASTA III)
In the analysis of correlation coefficients of chosen variables for symptom severity (ASTA II) and quality of life (ASTA III), there was observed a significant influence of elderly age, concomitant diabetes, and respiratory disorders, frequent hospitalizations, and frailty symptoms on the intensity of symptoms of arrhythmia and the impact of symptoms on quality of life. Physical activity decreases the intensity of symptoms of arrhythmia and decreases the impact of arrhythmia on QoL. Additionally, the low education level and the existence of comorbidities (heart failure, diabetes, previous stroke, or TIA) influence the impact of arrhythmia on QoL in ASTA III. In the analysis of correlation coefficients, professional activity and patients’ knowledge about the symptoms of arrhythmia and the therapy decreased the impact of arrhythmia on QoL in ASTA III. In the domain ASTA II, the lack of the symptoms of arrhythmia had significant association with QoL (Table 6).
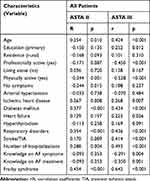 |
Table 6 Correlation Coefficients for Symptom Severity (ASTA II) and QoL (ASTA III), and the Characteristics Analyzed in the Study Group |
Independent predictors of symptom severity (ASTA II) and quality of life (ASTA III) included frailty degree. However, for ASTA III, the negative impact of diabetes, which increased the impact of arrhythmia on QoL, and the positive impact of physical activity, which improved QoL and decreased the impact of symptoms on everyday life, were revealed (Table 7).
 |
Table 7 Regression Coefficients for Symptom Severity (ASTA II) and Quality of Life (ASTA III) and the Characteristics Analyzed in the Study Group |
Discussion
The main message of our study is frailty being an independent predictor of higher intensity of symptoms of arrhythmia and worse QoL in older patients with atrial fibrillation. AF is a common arrhythmia in elderly patients whereas old age and underlying disease in this patient group are associated with a greater mortality and risk of other complications.20,26–28 In our study, the mean age was 69.8 years, similar to other published studies.20,26-29 On the other hand, old age and AF increase the risk of frailty and significantly contribute to decreased physical and cognitive capabilities.30
In the present study, FS was found at different severity levels in more than half of the studied patients. Only a few of the papers determined the correlation between FS and AF.22 The research shows that frailty affects 4.4–75.4% of AF patients, while AF is found in 48.2–75.4.% of the frail patients.22 In a study by Młynarska et al, there was also a strong significant correlation between EHRA score and FS severity.26 In the study by Wojaszel et al, AF was related to significantly higher scores of FS, independently from age, gender, multimorbidity, and polypharmacy.31
In our study, the regression analysis identified FS as a statistically significant independent predictor of increased arrhythmia symptom severity in ASTA II and decreased QoL in ASTA III. The issue of QoL is now often addressed in medicine. Studies on the QoL of AF patients are available,32 but to the best of the authors’ knowledge, none of these investigated correlations between FS and QoL.
Freeman et al33 demonstrated a strong negative impact of AF symptom severity on patients’ QoL. In a study by Dorian et al34, patients who had not had an arrhythmic episode for more than 3 months had a better QoL than in those with documented recurrences. In symptom-free patients, HRQoL may depend on factors unrelated to symptoms, diagnosis, or treatment, for instance, financial troubles, medication side effects, or restrictions in professional activity.33 In the present study, “no symptoms” in AF in the HRQoL questionnaire (ASTA III) were significantly correlated with frailty. Lomper et al reported that although AF is not a life-threatening condition, a frequent occurrence of symptoms may have an impact on patients’ functioning and significantly reduce their HRQoL.29 Patients’ QoL is often affected by the frequency of arrhythmic episodes, their duration, and severity of arrhythmia-specific symptoms; frequent episodes were found to be particularly damaging to QoL.35
In our studies, in a correlation coefficient analysis the number of hospitalizations due to arrhythmia correlated with the worsening of symptoms of arrhythmia and its unfavorable impact on quality of life. It should be supposed that hospitalized patients had a higher level of EHRA and arrhythmia symptoms were found to have a significant negative impact on QoL. It is highly likely that life quality, as it is currently assessed, will be highly dependent on the patient’s symptomatic status at the time of the assessment, especially if the symptoms are severe. Maryniak et al reported that circumstances of AF episodes and the associated disruption of activities significantly affect QoL in this patient group.36 Similar findings were reported by Freeman et al, observing a strict correlation between QoL and symptom severity, and, consequently, the risk of hospitalization.33
We previously demonstrated that AF, the severity of specific symptoms, and the discomfort associated with these symptoms led to numerous hospitalizations, disrupting patients’ daily activities and social life, and reducing their HQRoL.37 The frail patients in question experienced arrhythmia interfering with already limited activities of daily living. The illness had a negative impact on their physical ability, sexual life, and ability to concentrate. Respondents in the present study also reported trouble with sleeping, breathlessness, and anxiety – all of which were twice more common in frail than in non-frail patients. Similar findings have been reported by other authors, showing that atrial fibrillation, regardless of its type, significantly restricts patients’ sex life, professional activity, and household chores.38
In the present study, frail patients were more commonly treated with VKA anticoagulants. In the literature it was shown that elderly patients with AF and FS are less likely to be treated with anticoagulants.39 There is, however, no evidence to support such an approach; on the contrary, their use in patients over 75 years old is beneficial and associated with lower stroke risk.40 In a study by Młynarska et al26, patients with FS were treated with VKAs and were at a significantly higher risk of thromboembolic events than non-frail patients. Both the discomfort associated with the treatment and the presence of FS may significantly affect adherence to treatment and increase the risk of adverse events.41 This is why treatment schemes should be simplified, especially for frail patients, which was done in our study group.39 It is very interesting in our study that patients in the frail group often were given oral antiarrhythmic and calcium channel blockers, whereas patients in the non-frail group often were administered beta blockers. This particular finding, not reported by other authors, could have different clinical explanations. The most probable is that the non-frail population could be treated differently for the goal of maintaining the sinus rhythm indicating the paroxysmal nature of the disease and the frail patients would receive the medication for a rate-control strategy. This could also be a subconscious effect of doctors' attitudes toward the frail and non-frail population – less effort in the sicker population. In particular, the last conclusion could be of important clinical implication as it is potentially dangerous. As these were not the goals of our study we could not draw unequivocal conclusions, but this particular issue would deserve a systematic study in a larger population, clearly divided in terms of paroxysmal and permanent AF.
More and more attempts are being made to identify factors that affect perceived QoL. However, the number of studies and publications addressing QoL in AF patients, especially the elderly ones, remains insufficient to date.33,42 A few available publications demonstrate differences in FS severity among AF patients, depending on their gender, age, and comorbidities. In our own study, the patient’s age was found to increase the severity of AF-specific symptoms and to reduce QoL only in the analysis of correlation coefficients, which is obvious, but in the regression analysis age was not an independent predictor. Reynolds et al confirmed the association between age and quality of life, but they believed that the effect potentially depended on the assessment tool used. In general, older patients report lower generic QoL scores, particularly on scales related to physical functioning.43 The empiric evidence suggests that older patients might have decreased arrhythmia symptoms compared to younger patients, so the younger patients often show improved QoL after the interventions of rhythm control compared to older patients.44
In older patients the symptoms of AF are confused with chest discomfort and dyspnea accompanying arrhythmia, which can mimic CAD complaints. The symptoms of arrhythmia in older patients (>65 years) are not so specific as in younger patients.45 This can be the explanation for amore invasive diagnostic approach and more diagnoses of CAD in patients with AF.
Older patients with AF are burdened with multimorbidity, and its consequence is polytherapy. In the present study we proved the following variables influence QoL – except for frailty: concomitant comorbidities, professional activity, and knowledge on disease and therapy. It should be underlined that in correlation analysis – except for frailty – we found two independent predictors significantly influencing QoL. Diabetes in regression analysis negatively increased the impact of symptoms on worsening of QoL (ASTA III), and, on the contrary, physical activity improved QoL and decreased the impact of symptoms on QoL (ASTA III). Hagens et al showed that the presence of coronary artery disease and diabetes were found to predict worsened QoL.44 The authors point out the importance of the specific questionnaires in the case of occurrence of chronic comorbidities due to the possibility of altered perception of the efficacy of the AF treatment.44
The association of physical activity with QoL is well documented in the literature.46 It is widely known that physical activity represents one of the foremost interventions capable ofreducing the health burden of cardiovascular disease. Furthermore, the benefits of moderate-intensity physical activity have been established both in young and elderly subjects.47 Our study group was not analyzed in terms of other treatment methods, including electrotherapy, which could establish regularization of the heart rate, influencing profoundly the quality of life and exercise capacity.48 It should be also pointed out that the relationship between AF and physical activity can be bilateral – AF can negatively influence the ability to undertake physical activity.49
At the end of the discussion there comes an important question regarding the impact of frailty and AF on QoL and their relation in frail patients. On the basis of our results there is no clear answer for such a question, in particular for the question of whether the change of QoL results from FS or AF. The results of this study suggest the important need to project and carry out extensive research focusing on this topic, especially with division to patients with persistent and non-persistent type of AF. Interdependence between FS and AF, resulting from even the advanced age of a population of patients, is enough evidence that would justify conducting such a new study.
Conclusions
- Patients in the frail group have higher symptom severity than patients in the non-frail group.
- Patients in the frail group have worse QoL and higher impact of arrhythmia on QoL in comparison to patients in the non-frail group.
- Frailty is an independent predictor of higher intensity of symptoms of arrhythmia and worse QoL.
- Among the independent determinants, diabetes worsens QoL and decreases the symptoms of arrhythmia, and physical activity improves QoL and decreases the intensity of symptoms of AF.
Study Limitations
This study has its limitations, the most important one being the medium sample size and its single-center character, both limiting the generalizability of study findings. Another important limitation is the lack of analysis depending on the method of therapy of different types of AF. The authors focused only on the presence of AF as a diagnosis. Different results might probably be obtained if the patients had clearly distinguished paroxysmal and permanent AF as suggested in the Discussion, even though some literature reports do not confirm it.
Implications for Practice
FS in elderly patients with AF is a serious issue that requires the introduction of routine screening, thus allowing for early identification of patients at particular risk of lower QoL and agreater arrhythmic symptom severity, and for the implementation of appropriate interventions to alleviate the consequences of arrhythmia and those of frailty.
From the clinical perspective, frailty should be assessed in order to optimize the process of monitoring elderly patients with AF and enable the introduction of appropriate pharmacological and therapeutical modifications to enhance treatment outcomes and quality of life.
Compliance with Ethical Standards
All participants provided written informed consent. All procedures performed in studies involving human participants conform to the standards of the institutional and national ethics committees, as well as to the 1964 Helsinki Declaration and subsequent relevant ethics.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):643–659. doi:10.1016/j.bpobgyn.2013.02.005.
2. Fumagalli S, Potpara TS, Larsen TB, et al. Frailty syndrome: an emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace. 2017:1–7. doi:10.1093/europace/eux288.
3. Wojszel ZB, Magnuszewski Ł, Świętek M, et al. Frailty syndrome and functional correlates of atrial fibrillation in patients admitted to the geriatric ward. Gerontol Pol. 2019;27:11–15.
4. Sankaranarayanan R, Kirkwood G, Visweswariah R, Fox DJ. How does chronic atrial fibrillation influence mortality in the modern treatment era? Curr Cardiol Rev. 2015;11(3):190–198. doi:10.2174/1573403x10666140902143020
5. Strandberg TE, Pitkälä KH. Frailty in elderly people. Lancet. 2007;369(9570):1328–1329. doi:10.1016/S0140-6736(07)60613-8
6. Limpawattana P, Putraveephong S, Inthasuwan P, et al. Frailty syndrome in ambulatory patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1193–1198. doi:10.2147/COPD.S134233
7. Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2011;27(1):39–52. doi:10.1016/j.cger.2010.08.003
8. Artz AS. Anemia and the frail elderly. Semin Hematol. 2008;45(4):261–266. doi:10.1053/j.seminhematol.2008.06.002
9. Pérez-Tasigchana RF, León-Muñoz LM, Lopez-Garcia E, et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing. 2017;46(5):807–812. doi:10.1093/ageing/afx023
10. Caterina T, Veronese N, Maggi S, et al. Factors influencing transitions between frailty states in elderly adults: the progetto veneto anziani longitudinal study. J Am Geriatr Soc. 2017;65:179–184. doi:10.1111/jgs.14515
11. Veronese N, Stubbs B, Trevisan C, et al. Results of an observational cohort study of hyperuricemia as a predictor of poor physical performance in the elderly. Arthritis Care Res (Hoboken). 2017;69(8):1238–1244. doi:10.1002/acr.23118
12. Castell MV, van der Pas S, Otero A, et al. Osteoarthritis and frailty in elderly individuals across sixEuropean countries: results from the European Project on OSteo Arthritis (EPOSA). BMC Musculoskelet Disord. 2015;16:359.
13. Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi:10.1016/j.arr.2017.03.005
14. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103(11):1616–1621. doi:10.1016/j.amjcard.2009.01.375
15. Gugganig R, Aeschbacher S, Leong DP, et al. Frailty to predict unplanned hospitalization, stroke, bleeding, and death in atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2020;1–10.
16. Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56-e528.
17. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi:10.1016/j.amjcard.2009.07.022
18. Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi:10.1016/s0735-1097(00)01107-4
19. Freestone B, Lip GYH. Epidemiology and costs of cardiac arrhythmias. In: Lip GYH, Godtfredsen J, editors. Cardiac Arrhythmias: A Clinical Approach. Edinburgh: Mosby; 2003:3–24.
20. Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol a Biol Sci Med Sci. 2001;56:M158–66.
21. Marino FR, Lessard DM, Saczynski JS, et al. Gait speed and mood, cognition, and quality of life in older adults with atrial fibrillation. J Am Heart Assoc. 2019;8(22):e013212.
22. Villani ER, Tummolo AM, Palmer K, et al. Frailty and atrial fibrillation: a systematic review. Eur J Intern Med. 2018;56:33–38. doi:10.1016/j.ejim.2018.04.018
23. Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448 e1–19.
24. Walfridsson U, Stromberg A, Arestedt K. Development and validation of an arrhythmia-specific scale in tachycardia and arrhythmia with focus on health-related quality of life. J Cardiovasc Nurs. 2015;30:98–108. doi:10.1097/JCN.0000000000000149
25. Rolfson DB, Majumdar SR, Taher A, Tsuyuki RT. Development and validation of a new instrument for frailty. Clin Invest Med. 2000;23:336.
26. Mlynarska A, Mlynarski R, Golba KS. Frailty syndrome in patients with heart rhythm disorders. Geriatr Gerontol Int. 2017;17(9):1313–1318.
27. Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26):1726–1731. doi:10.1093/eurheartj/ehu197
28. Hogan DB, MacKnight C, Bergman H, et al. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29.
29. Lomper K, Sławuta A, Dudek K, et al. Psychometric evaluation of the Polish version of the Arrhythmia-Specific Questionnaire in Tachycardia and Arrhythmia: a new tool for symptom and health-related quality of life assessment. Kardiol Pol. 2019;77(5):541–552. doi:10.5603/KP.a2019.0046
30. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm-issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737.
31. Witassek F, Springer A, Adam L, et al. Swiss-AF study investigators. Health-related quality of life in patients with atrial fibrillation: the role of symptoms, comorbidities, and the type of atrial fibrillation. PLoS One. 2019;14(12):e0226730. doi:10.1371/journal.pone.0226730
32. Aliot E, Botto GL, Crijns HJ, Kirchhof P. Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace. 2014;16:787–796. doi:10.1093/europace/eut369
33. Freeman JV, Simon DN, Go AS. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes. 2015;8:393–402. doi:10.1161/CIRCOUTCOMES.114.001303
34. Dorian P, Paquette M, Newman D. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143:984–990. doi:10.1067/mhj.2002.122518
35. Heidt ST, Kratz A, Najarian K, et al. Symptoms in atrial fibrillation: a contemporary review and future directions. J Atr Fibrillation. 2016;9(1):1422.
36. Maryniak A, Walczak F, Bodalski R, et al. Atrial fibrillation onset circumstances and their relation to patients’ quality of life. Kardiol Pol. 2006;64(10):1102–1108.
37. Jankowska-Polańska B, Kaczan A, Lomper K, et al. Symptoms, acceptance of illness and health-related quality of life in patients with atrial fibrillation. Eur J Cardiovasc Nurs. 2018;17(3):262–272. doi:10.1177/1474515117733731
38. Patel D, Mc Conkey ND, Sohaney R, et al. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol. 2013;2013:159850.
39. Madhavan M, Holmes DN, Piccini JP, et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J. 2019;211:77–89. doi:10.1016/j.ahj.2019.01.005
40. Oqab Z, Pournazari P, Sheldon RS. What is the impact of frailty on prescription of anticoagulation in elderly patients with atrial fibrillation? A systematic review and meta-analysis. J Atr Fibrillation. 2018;10(6):1870.
41. Palmer K, Marengoni A, Russo P, et al. Frailty and Drug Use. J Frailty Aging. 2016;5(2):100–103. doi:10.14283/jfa.2016.84
42. Jankowska-Polańska B, Uchmanowicz I, Dudek K, et al. Sex differences in the quality of life of patients with acute coronary syndrome treated with percutaneous coronary intervention after a 3-year follow-up. Patient Prefer Adherence. 2016;10:1279–1287. doi:10.2147/PPA.S106577
43. Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19(7):762–768. doi:10.1111/j.1540-8167.2007.01091.x
44. Hagens VE, Ranchor AV, Van Sonderen E, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43(2):241–247. doi:10.1016/j.jacc.2003.08.037
45. Reynolds MR, Lavelle T, Essebag V, et al. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097–1103. doi:10.1016/j.ahj.2006.08.011
46. Berg J, Lindgren P, Nieuwlaat R, et al. Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res. 2010;19(3):381–390. doi:10.1007/s11136-010-9591-y
47. Santulli G, Ciccarelli M, Trimarco B, Iaccarino G. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the beta adrenergic system. Front Physiol. 2013;4:209.
48. Sławuta A, Mazur G, Małecka B, Gajek J. Permanent His bundle pacing – An optimal treatment method in heart failure patients with AF and narrow QRS. Int J Cardiol. 2016;214:451–452. doi:10.1016/j.ijcard.2016.04.022
49. Magnani JW, Wang N, Benjamin EJ, et al. Atrial fibrillation and declining physical performance in older adults: the health, aging, and body composition study. Circ Arrhythm Electrophysiol. 2016;9(5):e003525.
50. Walfridsson U, Arestedt K, Stromberg A, Development and validation of a new Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA) with focus on symptom burden. Health Qual Life Outcomes. 2012;10:44.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
