Back to Journals » Patient Preference and Adherence » Volume 16
Qualitative Interviews to Support Development and Cognitive Debriefing of the Adelphi Adherence Questionnaire (ADAQ©): A Patient-Reported Measure of Medication Adherence Developed for Use in a Range of Diseases, Treatment Modalities, and Countries
Authors Bentley S, Morgan L , Exall E, Arbuckle R , Rossom RC, Roche N , Khunti K , Higgins V , Piercy J
Received 12 January 2022
Accepted for publication 26 July 2022
Published 15 September 2022 Volume 2022:16 Pages 2579—2592
DOI https://doi.org/10.2147/PPA.S358046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Sarah Bentley,1 Lucy Morgan,1 Elizabeth Exall,1 Rob Arbuckle,1 Rebecca C Rossom,2 Nicholas Roche,3 Kamlesh Khunti,4 Victoria Higgins,5 James Piercy5
1Patient-Centered Outcomes, Adelphi Values, Bollington, Cheshire, UK; 2HealthPartners Institute, University of Minnesota Medical School, Minneapolis, MN, USA; 3Respiratory Medicine, Cochin Hospital, APHP Centre University Paris Cité, Institut Cochin (UMR1016), Paris, France; 4Diabetes Research Centre, Leicester University, Leicester, UK; 5Adelphi Real World, Bollington, Cheshire, UK
Correspondence: Rob Arbuckle, Patient-Centered Outcomes, Adelphi Values, Bollington, Cheshire, UK, Tel +44 7720 880884, Email [email protected]
Purpose: The Adelphi Adherence Questionnaire (ADAQ©) is a newly developed generic patient-reported outcome (PRO) assessment of medication adherence. The aim was to assess its content validity by conducting cognitive debriefing (CD) interviews with patients prescribed medication(s) of various treatment modalities in a range of therapy areas.
Materials and Methods: Targeted literature/instrument review and concept elicitation interviews informed development of the ADAQ©. CD interviews were conducted with 57 adults from the United States of America (USA; n = 21), Spain (n = 18), and Germany (n = 18) who prescribed medication for hypertension, diabetes, depression, schizophrenia, asthma, multiple myeloma, psoriasis, and/or multiple sclerosis. Interviews were conducted in two rounds to explore the relevance and understanding of the item wording, instructions, recall period and response options. Verbatim transcripts were analysed in ATLAS.Ti using thematic analysis. Three expert clinicians provided guidance throughout the study.
Results: ADAQ© items/instructions were well understood and relevant to participants. Key modifications following round 1 included revising instructions to refer to current medication(s) for one condition to reduce cognitive burden, removing two items with lower relevance (specifically those assessing running out of medication and social discouragement), and adding a response option for participants to indicate if they had stopped taking a medication. Minor wording modifications were made following round 2. Subgroup differences in item relevance were explored based on clinical characteristics. Cost of medication was more relevant amongst US participants.
Conclusion: Content validity of the ADAQ© was confirmed in demographically and clinically diverse participants. Psychometric properties of the ADAQ© will be explored in future studies.
Keywords: patient-reported outcome development, qualitative research, adherence, cognitive debriefing
Introduction
Adherence to medications has been defined as the extent to which a patient takes medication as prescribed and in correspondence with agreed recommendations from the prescribing healthcare professional (HCP).1,2 Alternatively, and more specifically, it has been defined as the process by which patients take their medications as prescribed,3 including timing, dose and interval of medication intake.3–5 Unlike the outdated term compliance (which focuses on the patient taking medication as instructed by the HCP), the concept of medication adherence takes into account the influence of many interrelated factors on adherence, including patient factors (lifestyle, personality, attitudes, etc.) and the influence of HCPs, the healthcare system and the environment/society in general.5–7 Medication adherence is essential for optimising patient care, and achieving desired clinical goals and outcomes.1,8,9 Medication non-adherence can negatively impact a treatment’s beneficial effects, which may result in greater healthcare costs and mortality, and worsening of patients’ health.10 Despite these adverse outcomes, medication non-adherence is widespread; it is estimated that 20% of patients intentionally temporarily stop taking their prescription medication.1 Further, the World Health Organization estimates that up to 50% of patients prescribed medication for chronic conditions take their medication less than 80% of the time.1,11
Gaining an understanding of the extent of and reasons for non-adherence can enable HCPs to engage in effective communication with patients and efficient treatment planning to improve medication adherence, thereby improving patient care and health outcomes. Common forms of non-adherence reported in the literature include skipping a dose of medication, taking a different amount of medication than prescribed, and taking medication at a different time of day than prescribed.1,12–16 Previous research has indicated that non-adherence may arise for unintentional reasons, for example, individuals may simply forget to take medication (which may be because they are busy, do not feel sick or do not think their medication is important).15–20 In other instances, non-adherence may be intentional, with individuals choosing not to take their medication because (amongst other reasons): they experience or fear experiencing medication side effects, are discouraged from taking medication by family and/or friends, or think that medication is ineffective.15–24 The extent of medication adherence behaviours and drivers may vary based on condition (and the presence of concomitant conditions/multimorbidity leading to a burden of multiple medications), treatment modality and dosing schedule, alongside socio-economic, demographic, geographic, and cultural population characteristics.15 For example, non-adherence to medication for chronic diseases has been found to be greater than for acute conditions. This may be due to patients being symptom-free or not experiencing unpleasant symptoms when medication is missed.10
There is a wealth of research regarding medication adherence behaviours and drivers, with a multitude of methods and measures employed for measuring (non-)adherence behaviours.25,26 While direct, objective methods of measuring non-adherence exist, each of these methods has its limitations. For example, prescription fill records or pill counts are frequently employed but do not provide certainty that every dose is taken – some will be lost, discarded or even diverted.27 Furthermore, many biological (eg blood and urine) assessments are unavailable for many medications and, when available, only provide a snapshot of a given day – without repeated sampling they cannot provide a representative picture of an individual’s medication (non-)adherence.27,28 Moreover, these methods are costly and hard to quickly complete on a large scale in routine clinical practice.27
Alternatively, indirect measures, which rely on self-reporting by the patient, can be used. Patient-reported medication adherence measures are quick, cost-effective, and often reliable and valid ways of gathering data on non-adherence, whilst offering an additional advantage of being able to explore reasons for non-adherence.29,30 However, many existing measures have been designed for a specific condition, and are not always appropriate for use across multiple conditions and treatment types.12,30–46 There are several that have been used across a range of conditions. These include the Morisky Medication Adherence Scale (MMAS-4/-8),47,48 Adherence to Refills and Medications Scale (ARMS),49 Self-efficacy for Appropriate Medication Use Scale (SEAMS),50 Hill-Bone Compliance Scale,51 and the Medication Adherence Rating Scale (MARS),52 to name a few. However, the content validity of these measures has not typically been confirmed in a range of disease populations, countries and medication types. Additionally, many of these measures only assess a limited range of the forms and drivers of non-adherence mentioned above. Only a few have been developed with evidence of content validity and with patient input throughout the development process, and not all are available for use.37,53,54
Given these limitations, we sought to develop a comprehensive, versatile, simple and pragmatic medication adherence measure that can be used across a variety of conditions in real-world studies and routine clinical practice and will have strong evidence of content validity. Best practice methods for the development and content validity testing of PRO measures typically start with review of the literature and/or qualitative concept elicitation research with the population of interest to ensure the measure assesses all relevant and important concepts.55–60 Such methods were employed to support the development of the Adelphi Adherence Questionnaire (ADAQ©), including conducting a literature review to identify important medication adherence concepts, reviewing existing medication adherence measures, and conducting concept elicitation (CE) interviews with patients with diverse conditions in Spain, Germany and the United States (US).61 The next step for the development of PRO measures is to perform qualitative cognitive debriefing (CD) interviews with individuals in the population(s) of interest to confirm that the measure assesses all important concepts of relevance using wording that is well understood and interpreted consistently by respondents. Given the aim that the ADAQ© can be used across disease populations and cultures, it was particularly important that these CD interviews included an appropriately diverse population. As such, this paper summarizes research aiming to evaluate the content validity of the ADAQ© by conducting CD interviews with patients prescribed with medication(s) in a range of therapy areas and using different treatment modalities.
Materials and Methods
The present study was a part of a wider process to develop the ADAQ©; a novel, representative, generic medication adherence measure. The study was designed in line with best practice methods for the development of PRO measures (PROMs).2,55–58 CD telephone interviews were conducted in July 2019 to March 2020 with 57 participants from the USA (n = 21), Germany (n = 18) and Spain (n = 18) who were currently receiving prescribed medications for their conditions (Figure 1). Countries were selected to provide representation of American and European cultures and differing healthcare systems. Clinician advisors with expertise in various therapeutic areas provided input at key stages of the study.
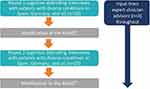 |
Figure 1 Study methodology. Abbreviations: ADAQ©, Adelphi Adherence Questionnaire; USA, United States of America. |
Participant Inclusion Criteria
Participants were recruited through partner recruitment agencies according to pre-defined inclusion and exclusion criteria. All participants were adults (18+ years) who were taking prescription medication for at least one of the following conditions: hypertension, diabetes, depression, schizophrenia, asthma, multiple myeloma, psoriasis, and/or multiple sclerosis, and had to be willing and able to participate in a telephone interview, as well as provide consent to taking part. Participants who were enrolled in a clinical trial at the time of recruitment were excluded.
Recruitment was purposefully targeted to ensure representation of participants with a range of chronic health conditions, treatment modalities (oral, topical and injected), and treatment schedules. Quotas were applied during recruitment to ensure a diverse group of participants were interviewed with representation of a range of medication types, demographics (including education level) and clinical characteristics.
Interview Methodology
Participants took part in 45-minute, qualitative, telephone interviews conducted by trained interviewers (who are authors of this paper). Interviews were conducted in two rounds to allow for modifications to the ADAQ© made following round one (n = 28) interviews to be tested in the second round (n = 29). Different groups of participants took part in round 1 and round 2 interviews. Interviews comprised a brief CE section, followed by detailed CD of the ADAQ© to support evaluation of its relevance and content validity.
The CE section was conducted to ensure that all pertinent forms and drivers of non-adherence were identified and fully understood.61 The CD section of the interview was used to determine whether concepts and items were understood by participants in the same way that the instrument developers intended.57–59 Specifically, the aim of the interviews was to assess relevance and understanding of item wording, instructions, recall period and response options of the ADAQ©. A “think-aloud” process was employed, where participants were asked to speak their thoughts aloud as they read all instructions and completed each item.60 All interviews were conducted in the participant’s native language.
Semi-structured interview guides were developed based on the literature review and input from expert clinician advisors. Verbatim transcripts were qualitatively analysed using thematic analysis methods and ATLAS.ti software.62 Patient quotes were highlighted and grouped by theme/topic, and frequency counts were generated per item and instruction of the ADAQ©.60,63 Subgroup comparisons identified patterns in instrument interpretation.
Three expert clinicians (RR [Geriatric Psychiatrist and Researcher in Mental Health and Cognition], NR [Professor of Respiratory Medicine in a University Tertiary Care Hospital], and KK [Primary Care Practitioner, Generalist, and Professor and International Researcher in Cardiometabolic Diseases]), with experience of treating patients with a range of different conditions and expertise in medication adherence provided advice and input at key time points. Specifically, they advised on the development of the study documentation and reviewed results from the interviews, advising on the development of and subsequent modifications to the ADAQ©.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and given an exemption by Western Independent Review Board, a centralized review board in the US. Participants provided written informed consent prior to any study activities and the collection of any data.
All participants provided written and verbal informed consent prior to taking part in an interview and were informed of their right to withdraw permission to use and disclose health information at any time. Participants were remunerated for taking part. They were given a unique identification code, and their names and other identifiable information were removed from all transcripts and other documents. Participant data were pseudonymized for internal analysis and anonymized for the purposes of external publication.
Results
Participant Demographic and Clinical Characteristics
Approximately half of the participants were female (56%), and half had been educated to a high school level or lower (54%). Over half of the participants were aged 36 to 60 years (54%), although participants aged 21 to 35 and 60 years or older were also represented. Half of the participants identified as white/Caucasian/of European heritage; participants identifying as mixed race, white Arabic/of north African heritage and black/African American/of African heritage were also represented. Participants were prescribed medication for a range of different conditions, with a variety of modes of administration. Primary conditions included hypertension, multiple myeloma, asthma, psoriasis, diabetes, depression, multiple sclerosis, and schizophrenia. While 68% of patients were receiving oral medication as part of their treatment plan, 67% of patients were receiving drugs using at least one other form of administration. Of note, some participants were taking more than one medication for their primary condition. Quotas relating to age, gender, race, education level and condition were all met (Table 1).
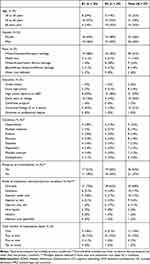 |
Table 1 Participant Demographic and Clinical Characteristics |
Development of the ADAQ©
A targeted literature review informed a preliminary conceptual model (which was later updated based on concept elicitation interview findings)61 and the development of an initial 17-item version of the ADAQ© (version [v] 1). Three expert clinician advisors provided feedback on the ADAQ© v1, which resulted in a number of modifications.
Specifically, an item about taking a different amount of medication was divided into two items, separately assessing taking more and taking less medication than prescribed. Items assessing non-adherence as a result of individuals not being in their normal routine (ie travelling, being busy or not having medication with them) were combined into one item. An item assessing non-adherence because individuals were asymptomatic was removed due to perceived conceptual overlap with an item assessing non-adherence because of low perceived need for medication. Finally, minor wording updates were made to improve comprehension.
This led to the development of the ADAQ© v2 in US English, which was translated into German and Spanish via forward-backward translation. This included 15 items assessing four forms of non-adherence, 10 drivers of non-adherence and an overall adherence to medication item.
Round 1 Interview Findings
Item Understanding
With the exception of items assessing frequency of taking less medication and global medication adherence (assessing frequency of adherence in consideration of all forms and drivers of medication non-adherence included in the ADAQ©), all items of ADAQ© v2 were well understood and interpreted consistently by more than two-thirds of the participants in round 1 interviews (Figure 2).
 |
Figure 2 Understanding of ADAQ© (v2) items in R1 interviews. Abbreviations: ADAQ©, Adelphi Adherence Questionnaire; R1, round 1; v2, version 2. |
Participants who misunderstood the item about taking less medication (item 4) predominantly considered times when they had skipped a dose of their medication and sometimes even considered medication breaks that were recommended by their prescriber. Participants with only a high-school education or less were more likely to misunderstand this item than those with further education (n = 3/8; 37.5% of participants with high-school education or less understood the item vs n = 15/20; 75.0% of participants with further education).
Let’s say ‘Rarely’, because every time I have to do the test I have an interval (stop) but then I have to keep on taking it … I have to rest for 3 days and get the test. (Spanish participant with multiple myeloma with high school education or less)
Participants who misunderstood the item assessing global medication adherence (item 15) mistakenly considered all questions asked during the interview (ie all probes covered within the interview guide) rather than only the ADAQ© items. It is anticipated that this is a circumstantial finding that would not be an issue in a real-world setting as the ADAQ© would not be completed as part of an interview.
Item Relevance
The majority of concepts assessed in the ADAQ© v2 were relevant to (ie had been experienced by) more than a third of the R1 participants (Figure 3).
 |
Figure 3 Relevance of ADAQ© (v2) items in R1 interviews. Abbreviations: ADAQ©, Adelphi Adherence Questionnaire; R1, round 1; v2, version 2. |
The following items assessed reasons for non-adherence that had been experienced by less than 30% of participants:
• Low perceived treatment efficacy (skipping medication[s] because the patient did not think it was working): relevant to only n = 6/22 (27.3%) participants asked.
• Treatment cost (skipping taking medication[s] or taking less of it, because of the cost): relevant to only n = 4/26 (15.4%) participants asked. This item was mostly relevant for the US participants asked (n = 3/4; 75.0%), though one German participant also found it relevant.
When I’ve had to pay out-of-pocket … and I haven’t had the full, the full amount to pay for it at that time and I had to sort of wait a little bit. So instead of taking the full amount just to make it last longer, I’ve taken less of it to last another day or two before I can afford to pay for it. (US participant with Diabetes)
• Social discouragement (avoiding taking medication[s] because family or friends encouraged the patient not to take it): relevant only to n = 2/26 (7.7%) participants asked.
Modifications to the ADAQ© Following Round 1 Interviews
The instructions were revised so that participants would be asked about their current medication(s) for one condition (rather than all prescription medications) to reduce the cognitive burden of thinking about numerous medications for multiple different conditions. For the purpose of this study, participants were asked to think about the condition for which they had been recruited.
Based on feedback from the expert clinician advisors, the definition of medication adherence used in the measure was updated to reflect that decisions about medication can be made collaboratively (ie by both the prescriber and patient) rather than unilaterally (ie by only the prescriber). This update was implemented throughout the items.
Based on feedback from participants, several items were amended or removed to form the ADAQ© v3 as follows:
• In order to improve understanding, examples were added to the item assessing whether participants take medication at a different time than prescribed.
• The item assessing global non-adherence was updated to include the phrasing “all of the above questions” to improve understanding.
• The items assessing non-adherence due to running out of medication and lack of social support were removed due to low relevance.
• The item assessing non-adherence due to medication cost was removed from the core questionnaire to be included for use in relevant countries only.
• A response option “I stopped taking my medication” was added to items where appropriate to allow participants to indicate that the items were not applicable to them because they had stopped taking medication altogether.
Although items assessing non-adherence due to side effects and social stigma/embarrassment were not highly relevant, they were retained in the main questionnaire for further testing at the recommendation of the expert clinician advisors, given their potential high importance to those patients for whom they are relevant.
Round 2 Interview Findings
Item Understanding
All 13 items of the ADAQ© v3 were well understood by participants. Participants’ demographic or clinical characteristics had no notable impact on understanding of the items (Figure 4).
 |
Figure 4 Understanding of ADAQ© (v3) items in R2 interviews. Abbreviations: ADAQ©, Adelphi Adherence Questionnaire; R2, round 2; v3, version 3. |
Over a third of participants (n = 10/28; 35.7%) in R2 interviews commented that the items assessing non-adherence due to low perceived treatment efficacy and necessity were conceptually similar, and responses to these items were highly correlated. In order to assess relevance and performance of the items in a larger cohort of participants, both items have been retained for psychometric testing.
Six and seven are the same questions it seems like … six is, how often do you skip a dose of your medication because you didn’t think it worked. Seven is, how often do you skip a dose of medication or take less because you didn’t think, think you needed it. So it’s—that’s, that question I would kind of—it would kind of be together. (US participant with hypertension)
Most participants with multiple medical conditions who were asked (n = 7/10; 70.0%) found it easy to respond to the questions thinking about medication taken for one condition only. Similarly, most participants taking multiple medications who were asked (n = 8/13; 61.5%) found it easy to respond to the items thinking about multiple medications taken for the same condition.
It’s been easy for me because I’ve focused on diabetes. I haven’t thought about hypertension. (Spanish participant with diabetes and hypertension, where diabetes was the primary condition)
Item Relevance
Relevance of the concepts in the ADAQ© was slightly lower in round 2 than in round 1 (Figure 5). This may be associated with the change in focus of the ADAQ©. While participants in round 1 may have recalled non-adherence for all medications (regardless of condition), participants in round 2 were asked to only think about non-adherence related to medications taken for one condition. Despite the slight impact on relevance, this change was required to make it easier for participants to accurately respond to the items by having to think about fewer medications.
Following round 2 interviews, minor wording updates were made to ensure consistency throughout the measure to form the ADAQ© v4. Further, as some participants (n = 4/26; 15.4%) equated the response option “I stopped taking my medication” with occasionally skipping a dose of medication for the reasons outlined, the term “completely” was added to the response option. A review copy of the ADAQ© can be obtained on request to the authors. However, it should be noted that the ADAQ© should not be used without permission of the developers.
ADAQ© Relevance: Subgroup Differences
Across round 1 and round 2 interviews, variation in item relevance was observed in participants with different clinical and demographic characteristics.
While all of the participants with asthma, psoriasis or diabetes reported forgetting to take their medication, only one participant with multiple myeloma did and none of the participants with multiple sclerosis did.
Participants with multiple myeloma reported the lowest relevance across many items (including items assessing frequency of missing a dose of medication and not taking medication as prescribed due to embarrassment). Just one participant with multiple myeloma reported that each individual ADAQ© item was relevant to them.
No participants who had intravenous injections (n = 6/57; 10.5%) reported missing a dose of medication, taking more medication than prescribed, or skipping a dose of medication for reasons including low perceived treatment efficacy or necessity.
Participants who reported their conditions were severe tended to find fewer items relevant compared to participants who considered their condition to be of mild severity. Finally, no international differences in understanding or relevance were observed.
Additional Findings
Across both rounds of interviews, the instructions and response options were well understood. No important missing concepts were identified by participants, and participants thought that the instrument was easy to complete. Most participants who were asked estimated that the measure would take less than five (n = 6/14; 42.8%) or 10 (n = 5/14; 35.7%) minutes to complete (this is expected to be in line with time taken to complete other medication adherence measures).
When answering the ADAQ©, participants reported thinking about a range of recall periods, from current (n = 11) to multiple years (ranging between 2 and 20 years; n = 8). When asked what recall period would be most appropriate, most participants who were asked (n=21/26; 80.8%) suggested including a recall period of one month or more, and some (n = 3/26; 11.5%) noted that the ideal recall period would vary depending on the questionnaire item and the disease under consideration.
Discussion
The development of the ADAQ© followed best practice methods for development of PROMs.55,59,64 Input from participants across a variety of conditions, ethnicities, ages, educational levels, countries, cultures and healthcare systems ensured that the diverse factors associated with medication non-adherence are represented in this measure. Of equal importance, participants who used a range of modes of medication administration (eg injections, oral or topical), with varying dosing schedules, were interviewed to ensure the ADAQ© items are relevant for multiple medications and administration methods.
Medication adherence concepts reported during the interviews support and align with those cited in the seminal World Health Organization paper, and in more recent adherence literature.1,3–5 The concepts identified and included in the ADAQ© are consistent with those cited in health behaviour models as influencing adherence, such as patient knowledge and attitudes towards their condition and medication, perceived benefits of medication and barriers to taking it as instructed.65–68 Certain concepts included in the ADAQ© were found to be more relevant to certain conditions, modes of administration/dosing schedules, and countries than others. For example, fewer patients with multiple myeloma and multiple sclerosis reported forgetting their medication. This may reflect the relative severity of the conditions or greater implications for non-adherence (inadequate treatment could lead to mortality or serious complications) for these conditions. Additionally, this finding may also reflect the way in which the medications are taken (ie due to them being taken less frequently and administered via injection by a HCP). In contrast, the high incidence of medication non-adherence due to forgetfulness reported by participants with asthma, psoriasis, or diabetes may be associated with periods of asymptomatic disease. Had these groups of patients been excluded, the study results would have presented a skewed perspective of medication non-adherence behaviours.
In light of the diverse reasons for medication non-adherence across different subgroups, an inclusive approach was taken to the items retained in the ADAQ© for the measure to be suitable for use in varied patient populations. The majority of forms and drivers of medication non-adherence reported by five or more participants in the CE phase of the interviews, along with concepts most frequently reported in the literature, are represented in the ADAQ©.15–17,19,22,23,61 Therefore, the ADAQ© provides good conceptual coverage of the most relevant and important concepts associated with medication non-adherence. Further, the tool was developed and assessed in a multi-ethnic and diverse group of participants in multiple countries and languages. In addition, despite the broad conceptual coverage, the ADAQ© was reported to be quick to complete, making it feasible for this measure to be completed in everyday clinical practice.
As acknowledged in the introduction, there are several existing measures of medication adherence.47–52 However, despite the numerous medication adherence measures available, it has been suggested that no single gold standard generic medication adherence measure exists.31,69 Many legacy measures are often limited due to the development of items for a specific condition, medication or mode of action, limited assessment concepts, a lack of or limited evidence of content and psychometric validity, low internal consistency, or impractical scoring systems for clinical practice.31,69 Another common limitation is the focus on the assessment of missing a dose of medication over other important forms of medication non-adherence (such as taking a different amount of medication). The ADAQ© was developed to overcome these limitations and, importantly, to assess a variety of medication non-adherence behaviours and also capture the most common drivers of non-adherence, thereby providing greater insights than other available medication adherence measures.
The broad conceptual coverage of the measure, alongside the planned use of the ADAQ© across conditions and treatment modalities, prompted considerations around the most appropriate recall period for the measure. There is significant variation in the frequency with which medication is taken, for example, a maintenance inhaler and many pills are taken every day, whereas certain biologic medications may be taken once a month. In order to capture medication (non-)adherence behaviours in participants who take medication monthly, a recall period of more than a month would have to be employed. Indeed, most participants who were asked (80.8%) advocated that if a recall period was included, it would need to be a month or longer. However, it is widely accepted that recall periods of more than a week have lower accuracy; as such a recall period of more than a month would likely reduce the reliability of the measure.70,71 As such, and in order for the measure to be truly generic, the ADAQ© does not include a recall period. The only specification of time frame is that participants should be thinking about their current medication.
With regard to limitations of the study, most participants reported being very adherent to their medication and as a result many of the concepts included in the ADAQ© had low relevance. This may be a product of volunteer bias, as participants who were more adherent may have been more likely to agree to take part in the study.72,73 Due to this, several concepts which had relatively low relevance were retained in the ADAQ© until psychometric testing of the measure in a larger number of participants can confirm their wider relevance. Furthermore, as this was a qualitative study, subgroup comparisons are not definitive and are based on relatively small sample sizes.
While completing the ADAQ©, participants are asked to think about medication for one condition. Where individuals take multiple medications for one condition, the accuracy of the data relies on their ability to generate an aggregate response for each item. While most participants who were asked (61.5%) found it easy to consider multiple medications for one condition, it is unclear whether their level of medication adherence was captured in the same way as participants who only had to think about one medication. However, adding further specification to the instructions would increase their complexity and likely decrease patient understanding of the instructions. Moreover, there is some indication that adherence to one medication will often correlate with adherence to another medication.74 Finally, the need for individuals to complete the ADAQ© multiple times (once for each medication of interest) would drastically increase participant burden and reduce the feasibility of administering the instrument in clinical practice. Future research aims to assess the psychometric properties of the ADAQ© across multiple countries, conditions and dosing schedules, including, but also going beyond those conditions, countries, etc., included in the qualitative research conducted to date. During psychometric validation, the responses of participants who take multiple medications for one condition will be compared to participants who take only one medication to confirm whether there are any notable differences.
While one of the strengths of the content validity testing is that the participants interviewed included a range of conditions, ethnicities, age groups and countries, there are, of course, many health conditions and countries that could not feasibly be included. Further testing in other countries (particularly including countries in Asia, South America and Africa) and among people with other health conditions would add even greater confidence in the relevance across patient populations.
Conclusion
This paper outlines a qualitative CD interview study conducted to generate evidence of the content validity of the ADAQ© across multiple different conditions, disease severities, modes of medication administration, dosing schedules, countries, ages, races and education levels. The ADAQ© is a well-understood, 13-item (including one optional item), patient-reported medication adherence measure, which captures a wide range of forms and drivers of non-adherence. The ADAQ© addresses an unmet need for a generic medication adherence measure for use across a variety of conditions in real-world studies and routine clinical practice with evidence of content validity. Collection of data for the psychometric validation of the ADAQ© in a large and diverse patient population is currently underway.
Disclosure
SB, RA, VH, and JP are employees of Adelphi, and EE and LM were employees of Adelphi when the research was conducted. RR, NR, and KK were contracted by Adelphi to provide clinical advice throughout this project. KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. NR reports grants and personal fees from Boehringer Ingelheim, Novartis, Pfizer, GlaxoSmithKline and personal fees from Teva, AstraZeneca, Chiesi, Sanofi and Zambon. RR reports research grants from the National Institutes of Health, Otsuka and BioXcel. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003.
2. FDA. Are you taking medication as prescribed?; 2009 Available from: https://www.fda.gov/consumers/consumer-updates/are-you-taking-medication-prescribed.
3. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
4. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi:10.1111/j.1524-4733.2007.00213.x
5. Gast A, Mathes T. Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):1–17. doi:10.1186/s13643-019-1014-8
6. Steiner JF, Earnest MA. The language of medication-taking. Ann Intern Med. 2000;132(11):926–930. doi:10.7326/0003-4819-132-11-200006060-00026
7. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
8. Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract. 2019;73(6):e13350. doi:10.1111/ijcp.13350
9. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi:10.1016/j.ypmed.2017.03.008
10. Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155. doi:10.5001/omj.2011.38
11. Fitzpatrick C, Gillies C, Seidu S, et al. Effect of pragmatic versus explanatory interventions on medication adherence in people with cardiometabolic conditions: a systematic review and meta-analysis. BMJ open. 2020;10(7):e036575. doi:10.1136/bmjopen-2019-036575
12. Dima AL, van Ganse E, Laforest L, Texier N, de Bruin M; ASTRO-LAB Group T. Measuring medication adherence in asthma: development of a novel self-report tool. Psychol Health. 2017;32(10):1288–1307. doi:10.1080/08870446.2017.1290248
13. Kosilov K, Loparev S, Kuzina I, Shakirova O, Zhuravskaya N, Lobodenko A. Self-assessment of treatment compliance with antimuscarinic drugs and lower urinary tract condition among women with urinary incontinence. Int Urogynecol J. 2017;28(11):1663–1669. doi:10.1007/s00192-017-3333-4
14. Sidorkiewicz S, Tran V-T, Cousyn C, Perrodeau E, Ravaud P. Development and validation of an instrument to assess treatment adherence for each individual drug taken by a patient. BMJ open. 2016;6(5):e010510. doi:10.1136/bmjopen-2015-010510
15. Ahmad A, Sorensen K. Enabling and hindering factors influencing adherence to asthma treatment among adolescents: a systematic literature review. J Asthma. 2016;53(8):862–878. doi:10.3109/02770903.2016.1155217
16. Hanghøj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health. 2014;54(2):121–138. doi:10.1016/j.jadohealth.2013.08.009
17. Huang W-C, Chen C-Y, Lin S-J, Chang C-S. Medication adherence to oral anticancer drugs: systematic review. Expert Rev Anticancer Ther. 2016;16(4):423–432. doi:10.1586/14737140.2016.1159515
18. Macdonald M, Martin-Misener R, Helwig M, et al. Experiences of adults with cystic fibrosis in adhering to medication regimens: a qualitative systematic review. JBI Database Syst Rev Implement Rep. 2016;14(5):258–285. doi:10.11124/JBISRIR-2016-002362
19. McSharry J, McGowan L, Farmer A, French D. Perceptions and experiences of taking oral medications for the treatment of type 2 diabetes mellitus: a systematic review and meta‐synthesis of qualitative studies. Diabetic Med. 2016;33(10):1330–1338. doi:10.1111/dme.13152
20. Rebafka A. Medication adherence after renal transplantation—a review of the literature. J Ren Care. 2016;42(4):239–256. doi:10.1111/jorc.12181
21. Rezaei M, Valiee S, Tahan M, Ebtekar F, Gheshlagh RG. Barriers of medication adherence in patients with type-2 diabetes: a pilot qualitative study. Diabetes Metab Syndr Obes. 2019;12:589. doi:10.2147/DMSO.S197159
22. Dockerty T, Latham S, Smith T. Why don’t patients take their analgesics? A meta-ethnography assessing the perceptions of medication adherence in patients with osteoarthritis. Rheumatol Int. 2016;36(5):731–739. doi:10.1007/s00296-016-3457-8
23. Lin C, Clark R, Tu P, Bosworth HB, Zullig LL. Breast cancer oral anti-cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat. 2017;165(2):247–260. doi:10.1007/s10549-017-4317-2
24. Vitalis D. Factors affecting antiretroviral therapy adherence among HIV-positive pregnant and postpartum women: an adapted systematic review. Int J Std AIDS. 2013;24(6):427–432. doi:10.1177/0956462412472807
25. Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intestinal Res. 2017;15(4):434. doi:10.5217/ir.2017.15.4.434
26. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(80 3):S11. doi:10.1097/MLR.0b013e31829b1d2a
27. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:1–12. doi:10.1155/2015/217047
28. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090. doi:10.1016/S0149-2918(99)80026-5
29. Nguyen TMU, Caze AL, Cottrell N. What are validated self‐report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77(3):427–445. doi:10.1111/bcp.12194
30. Kim C-J, Schlenk EA, Ahn J-A, Kim M, Park E, Park J. Evaluation of the measurement properties of self-reported medication adherence instruments among people at risk for metabolic syndrome: a systematic review. Diabetes Educ. 2016;42(5):618–634. doi:10.1177/0145721716655400
31. Culig J, Leppée M. From Morisky to Hill-bone; self-reports scales for measuring adherence to medication. Coll Antropol. 2014;38(1):55–62.
32. Gagné M, Boulet LP, Pérez N, Moisan J. Patient‐reported outcome instruments that evaluate adherence behaviours in adults with asthma: a systematic review of measurement properties. Br J Clin Pharmacol. 2018;84:1928–1940. doi:10.1111/bcp.13623
33. Pérez-Escamilla B, Franco-Trigo L, Moullin JC, Martínez-Martínez F, García-Corpas JP. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer Adherence. 2015;9:569. doi:10.2147/PPA.S76139
34. Mansberger SL, Sheppler CR, McClure TM, et al. Psychometrics of a new questionnaire to assess glaucoma adherence: the glaucoma treatment compliance assessment tool (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2013;111:1.
35. Boas LCG-V, Lima MLSAP, Pace AE. Adherence to treatment for diabetes mellitus: validation of instruments for oral antidiabetics and insulin. Rev Lat Am Enfermagem. 2014;22(1):11–18. doi:10.1590/0104-1169.3155.2386
36. Bou Serhal R, Salameh P, Wakim N, et al. A new Lebanese medication adherence scale: validation in Lebanese hypertensive adults. Int J Hypertens. 2018;2018:3934296. doi:10.1155/2018/3934296
37. Cahir C, Dombrowski SU, Kennedy MJ, Sharp L, Bennett K. Developing and validating a theoretical measure of modifiable influences on hormonal therapy medication taking behaviour in women with breast cancer. Psychol Health. 2017;32(10):1308–1326. doi:10.1080/08870446.2017.1296151
38. de Oliveira Marsicano E, da Silva Fernandes N, Colugnati F, et al. Transcultural adaptation and initial validation of Brazilian-Portuguese version of the Basel assessment of adherence to immunosuppressive medications scale (BAASIS) in kidney transplants. BMC Nephrol. 2013;14(1):108. doi:10.1186/1471-2369-14-108
39. Demirtaş A, Akbayrak N. Development of an assessment scale for treatment compliance in type 2 diabetes mellitus in Turkish population: psychometric evaluation. Int J Nurs Sci. 2017;4(3):244–251. doi:10.1016/j.ijnss.2017.06.002
40. Lehane E, McCarthy G, Collender V, Deasy A, O’Sullivan K. The reasoning and regulating medication adherence instrument for patients with coronary artery disease: development and psychometric evaluation. J Nurs Meas. 2013;21(1):64. doi:10.1891/1061-3749.21.1.64
41. Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D. Out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102(2):96–104. doi:10.1016/j.diabres.2013.09.010
42. Plaza V, Fernández-Rodríguez C, Melero C, et al. Validation of the ‘Test of the Adherence to Inhalers’(TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi:10.1089/jamp.2015.1212
43. Robinson-Papp J, George MC, Wongmek A, et al. The quantitative analgesic questionnaire: a tool to capture patient-reported chronic pain medication use. J Pain Symptom Manage. 2015;50(3):381–386. doi:10.1016/j.jpainsymman.2015.03.013
44. Rodrigues MTP, Moreira TMM, Andrade D. Elaboration and validation of instrument to assess adherence to hypertension treatment. Rev Saude Publica. 2014;48(2):232–240. doi:10.1590/S0034-8910.2014048005044
45. Schatz M, Zeiger RS, Yang S-J, et al. Development and preliminary validation of the adult asthma adherence questionnaireTM. J Allergy Clin Immunol Pract. 2013;1(3):280–288. doi:10.1016/j.jaip.2013.03.001
46. Teixeira A, Oliveira C, Teixeira M, et al. Development and validation of a novel Questionnaire for Adherence with Topical Treatments in Psoriasis (QATOP). Am J Clin Dermatol. 2017;18(4):571–581. doi:10.1007/s40257-017-0272-2
47. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
48. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi:10.1111/j.1751-7176.2008.07572.x
49. Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi:10.1111/j.1524-4733.2008.00400.x
50. Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the Self-efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J Nurs Meas. 2007;15(3):203. doi:10.1891/106137407783095757
51. Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone compliance to high blood pressure therapy scale. Prog Cardiovasc Nurs. 2000;15(3):90–96. doi:10.1111/j.1751-7117.2000.tb00211.x
52. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (Mars) for the psychoses. Schizophr Res. 2000;42(3):241–247. doi:10.1016/S0920-9964(99)00130-9
53. Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11(1):1–9. doi:10.1186/1471-2288-11-149
54. Unni EJ, Olson JL, Farris KB. Revision and validation of Medication Adherence Reasons Scale (MAR-Scale). Curr Med Res Opin. 2014;30(2):211–221. doi:10.1185/03007995.2013.851075
55. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–977. doi:10.1016/j.jval.2011.06.014
56. EMEA. Reflection Paper on the Regulatory Guidance for the Use of Health-Related Quality of Life (HRQL) Measures in the Evaluation of Medicinal Products. London: European Medicines Agency; 2005.
57. FDA Draft Guidance. Patient-focused drug development: methods to identify what is important to patients guidance for industry, food and drug administration staff, and other stakeholders [DRAFT]. US Food and Drug Administration; 2019.
58. FDA Guidance. Patient-focused drug development: collecting comprehensive and representative input; guidance for industry, food and drug administration staff, and other stakeholders. US Food and Drug Administration; 2020.
59. FDA Guidance. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. US Food and Drug Administration; 2009.
60. Willis GB. Cognitive Interviewing: a tool for improving questionnaire design; 2004.
61. Morgan L, Exall E, Bentley S, et al. Qualitative interviews to explore drivers and behaviours associated with medication non-adherence across a range of diseases, treatment modalities, and countries International Society of Patient Reported Outcomes. (ISPOR) EU; 2020.
62. ATLAS. Scientific software development GmbH version 8 [computer program]; 2020.
63. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
64. FDA Draft Guidance. Patient-focused drug development guidance: methods to identify what is important to patients and select, develop or modify fit-for-purpose clinical outcome assessments [DRAFT]. US Food and Drug Administration; 2018.
65. Munro S, Lewin S, Swart T, Volmink J. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health. 2007;7(1):1–16. doi:10.1186/1471-2458-7-104
66. Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130(1):65S–72S. doi:10.1378/chest.130.1_suppl.65S
67. Adefolalu AO. Cognitive-behavioural theories and adherence: application and relevance in antiretroviral therapy. South Afr J HIV Med. 2018;19(1):1–7. doi:10.4102/sajhivmed.v19i1.762
68. Rimer BKGK Theory at a glance: application to health promotion and health behaviour. National Cancer Institute. Available from: http://www.cancer.gov/cancertopics/cancerlibrary/theory.pdf.
69. Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2011;51(1):90–94. doi:10.1331/JAPhA.2011.09154
70. Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25(4):929–942. doi:10.1185/03007990902774765
71. Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63(2):217–235. doi:10.1177/1077558705285298
72. Jordan S, Watkins A, Storey M, et al. Volunteer bias in recruitment, retention, and blood sample donation in a randomised controlled trial involving mothers and their children at six months and two years: a longitudinal analysis. PLoS One. 2013;8(7):e67912. doi:10.1371/journal.pone.0067912
73. Sackett DL. Bias in analytic research. In: The Case-Control Study Consensus and Controversy. Elsevier; 1979:51–63.
74. Krigsman K, Nilsson JLG, Ring L. Adherence to multiple drug therapies: refill adherence to concomitant use of diabetes and asthma/COPD medication. Pharmacoepidemiol Drug Saf. 2007;16(10):1120–1128. doi:10.1002/pds.1433
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

