Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Pyomyositis Secondary to Localized Cellulitis in a Dermatomyositis Patient: A Case Report and Review of Infectious Complications in Dermatomyositis
Received 9 May 2023
Accepted for publication 21 July 2023
Published 11 August 2023 Volume 2023:16 Pages 2201—2209
DOI https://doi.org/10.2147/CCID.S417772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xingwei Zhang,1,2 Xiaoyan Lyu1,2
1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Xiaoyan Lyu, Department of Dermatology, West China Hospital, Sichuan University, 37 Guoxue Road, Chengdu, 610041, People’s Republic of China, Tel +86 19938063616, Fax +86-028-85582994, Email [email protected]
Abstract: Dermatomyositis (DM) is an autoimmune disorder characterized by proximal muscle weakness and distinct cutaneous features. Unfortunately, infection is a frequent and potentially life-threatening complication in patients with DM. Here, we present a case of pyomyositis in a patient with DM resulting from localized cellulitis. The patient also presented with subcutaneous calcification nodules and dermatomyositis-associated lipodermatosclerosis nodules. To our knowledge, there have been no reports of pyomyositis in patients with DM to date. Furthermore, we reviewed the infectious complications related to DM and polymyositis (PM). We found that idiopathic inflammatory myopathy (IIM) patients exhibit a considerable infection-related mortality rate, ranging from 4.3% to 7.2%. In IIM, infections were identified as the primary cause of mortality in a substantial proportion of cases, accounting for 22.0– 83.3% of deaths. These findings have implications for the importance of identifying and managing infections in IIM patients and suggest the need for further research into infection-related complications in these patients.
Keywords: dermatomyositis, polymyositis, infection, mortality, case report
Introduction
Dermatomyositis is an autoimmune disease characterized by weakness in the proximal muscles and distinct skin manifestations. Pyomyositis is a subacute bacterial infection that primarily affects skeletal muscles and can arise from contiguous spread or hematogenous seeding. To date, there have been no documented cases of pyomyositis in individuals with DM. However, our case report suggests that pyomyositis may occur due to the presence of localized cellulitis in the muscles of patients with dermatomyositis. Early identification of pyomyositis is crucial for preventing muscle function loss or even death in patients with dermatomyositis. DM are associated with reduced long-term survival, particularly in cases of anti-MDA5+DM, with infections being a primary cause.1 However, there is a lack of comprehensive reviews of IIM-related infections across different anatomical locations and microbial agents, and reports are scattered and sporadic. Thus, we reviewed the infectious complications related to dermatomyositis/polymyositis.
Case Report
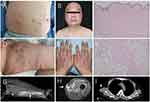
A 49-year-old male with a history of dermatomyositis and long-term use of glucocorticoids presented to our hospital with a painful indurated swelling in the anteromedial aspect of his thigh and a low-grade fever (maximum temperature of 37.9 degrees) persisting for 12 days (Figure 1). He did not achieve remission with 11 days of ceftazidime in another hospital. Physical examination revealed the presence of multiple subcutaneous nodules, scar tissue with hyperpigmentation, and local swelling and induration with redness and mild fluctuation in anteromedial thigh (Figures 1 and 2). Empirical treatment with cefoperazone-sulbactam was initiated, but the patient subsequently presented with crampy local pain and a “woody” texture of the anteromedial thigh muscle, making it difficult to straighten his left leg.
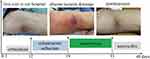 |
Figure 1 The time course of treatment and states of illness. |
Laboratory studies showed lymphocytopenia (white blood cell count 11.69*109/L, with 88.8% neutrophils) and evidence of inflammation/infection (procalcitonin 0.08ng/mL, C-reactive protein 7.58mg/L, interleukin-6 7.10pg/mL). An enhanced CT scan demonstrated skin and subcutaneous tissue swelling with irregular enhancement, soft tissue gas, focal fluid accumulation, and multiple calcifications in the left thigh. T1-weighted MR images demonstrated high signal intensity within the sartorius muscle, and culture of the lesion revealed Staphylococcus aureus. The ultimate diagnosis is stage I pyomyositis, resulting from abscess extension of the cellulitis. The patient’s condition markedly improved following ultrasonographic-guided percutaneous drainage and a two-week course of vancomycin. He subsequently received a two-week course of amoxicillin (Figure 1).
Of note, the patient presented with two distinct types of subcutaneous nodules: fibrocollagenous nodules, confirmed by pathology, and multiple calcifications, demonstrated on enhanced CT imaging (Figure 2). The diagnosis of deep morphea was ruled out. The existence of fibrocollagenous nodules in dermatomyositis patients is insufficiently reported and could represent the fibrotic/late phase of dermatomyositis panniculitis. This could be related to the presence of membranous cystic changes and mild lymphocytic inflammation in the adipose lobules of skin biopsy (Figure 2).
Discussion
This report describes a case of pyomyositis in a patient with dermatomyositis, which may be related to the long-term use of glucocorticoids. For a period of 5 years, the patient’s condition of dermatomyositis was effectively managed with a combination of methylprednisolone and methotrexate. Methylprednisolone was initially prescribed at a daily dosage of 30mg and gradually tapered over time. In the last year leading up to admission, the dosage was reduced to 5mg once daily, and six months before admission, it was further decreased to 2.5mg once daily. The occurrence of pyomyositis can be observed in an immunosuppressed patient. This case is expected to be distinguished from necrotizing fasciitis and clostridial myonecrosis. Timely recognition and treatment of pyomyositis are crucial to prevent severe complications and mortality in patients with dermatomyositis. However, early identification can be challenging. Healthcare providers should be vigilant in monitoring and promptly identifying signs and symptoms of pyomyositis in this population.
In addition, we reviewed the clinical studies related to infection in patients with DM/PM. We conducted a comprehensive literature search on PubMed and Web of Science. For the literature screening process, two reviewers independently conducted the screening, and any disagreements were resolved through consensus. Figure 3 illustrates the literature screening flowchart employed in this study. Tuberculosis and COVID-19 were excluded from our study due to prior systematic reviews. Overlap syndrome is also ruled out. As of March 20th, 2023, this study has included a total of 40 studies and 10,694 patients. 33 (82.5%) of the studies are retrospective. The studies were predominantly retrospective in design and exhibited considerable variability in follow-up duration, with mean times ranging from 1 to 13 years. Although the heterogeneity in infection-related mortality was relatively low, the disparate timeframes precluded quantitative synthesis of the data. This literature review strengthens the evidence base and enhances understanding of the link between inflammatory myositis and infections.
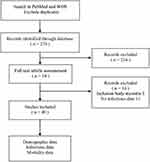 |
Figure 3 The literature screening flowchart. |
Our review revealed a wide range of overall mortality proportions (4–45%) among patients with idiopathic inflammatory myopathy. Among these patients, infection-related mortality rates were identified to range from 2.2% to 7.2% (Table 1). Notably, a significant proportion of deaths (22.0–83.3%) in patients with idiopathic inflammatory myopathy were primarily attributed to infections. Our study also provides a summary of infection rates observed in different IIM subtypes and infection types (Table 2 and Table 3). The most common infection agents are cytomegalovirus, Herpes Simplex Virus/Varicella Zoster Virus, Candidiasis, pneumocystis Carinii pneumonia (Table 3). Among all patients, those with melanoma differentiation associated gene 5 antibody-positive dermatomyositis (Anti-MDA-5+DM) have the highest susceptibility to infections, with an infection rate ranging from 42.1 to 83.3% (Table 2).
 |
Table 1 The Clinical Data and Mortality Rates in Patients with IIM |
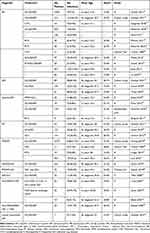 |
Table 2 The Clinical Data and Prevalence of Infections in Patients with Different IIM Subtypes |
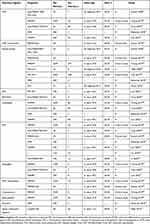 |
Table 3 The Clinical Data and Prevalence of Infections in Patients with Different Infection Types |
Conclusion
Our case report highlights the potential for pyomyositis as a complication of DM resulting from localized cellulitis, while our review underscores the importance of identifying and managing infections in reducing infection-related mortality in IIM patients. These findings have important implications for the clinical management of DM and IIM and underscore the need for further research into infection-related complications in these patient populations. A better understanding of the relationship between infections and DM/IIM could help inform the development of more targeted interventions aimed at improving patient outcomes.
Abbreviations
ARS, Anti-Aminoacyl-tRNA Synthetase; ASS, Antisynthetase syndrome; BT, Biologic therapy; CMV, Cytomegalovirus; CYC, Cyclophosphamide; DM, dermatomyositis; GCs, Glucocorticosteroid; HSV, Herpes Simplex Virus; IIM, idiopathic inflammatory myositis; ILD, interstitial lung diseases; ISD, Immunosuppressive drug; IVIG, Intravenous immunoglobulin; MDA5, melanoma differentiation associated gene 5; MMF, mycophenolate mofetil; NTM, Non-tuberculosis mycobacteria; OM, overlap myositis; P, Prospective; PCP, Pneumocystis carinii pneumonia; PM, polymyositis; R, Retrospective; RTX, Rituximab; TAC, Tacrolimus; TIF1-γ, Transcription Intermediary Factor 1 Gamma; TOF, Tofacitinib; VZV, Varicella-Zoster Virus.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Perspective
The use of Vancomycin has been instrumental in my recovery, and I have noticed a significant improvement in my symptoms since starting the medication.
Ethical Approval and Consent to Participate
The Institutional Review Board (IRB) has approved our research application. The patient provided informed consent for the publication of their case.
Acknowledgments
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Svensson J, Holmqvist M, Lundberg IE, Arkema EV. Infections and respiratory tract disease as risk factors for idiopathic inflammatory myopathies: a population-based case-control study. Ann Rheum Dis. 2017;76(11):1803–1808. doi:10.1136/annrheumdis-2017-211174
2. Amaral Silva M, Cogollo E, Isenberg DA. Why do patients with myositis die? A retrospective analysis of a single-centre cohort. Clin Exp Rheumatol. 2016;34(5):820–826.
3. Nuño L, Joven B, Carreira P, et al. [Multicenter registry on inflammatory myositis from the Rheumatology Society in Madrid, Spain: descriptive analysis] Registro de pacientes con miopatía inflamatoria de la Sociedad Madrileña de Reumatología: análisis descriptivo. Reumatol Clin. 2017;13(6):331–337. Spanish. doi:10.1016/j.reuma.2016.07.010
4. Nuño-Nuño L, Joven BE, Carreira PE, et al. Mortality and prognostic factors in idiopathic inflammatory myositis: a retrospective analysis of a large multicenter cohort of Spain. Rheumatol Int. 2017;37(11):1853–1861. doi:10.1007/s00296-017-3799-x
5. Taborda AL, Azevedo P, Isenberg DA. Retrospective analysis of the outcome of patients with idiopathic inflammatory myopathy: a long-term follow-up study. Clin Exp Rheumatol. 2014;32(2):188–193.
6. Limaye V, Hakendorf P, Woodman RJ, Blumbergs P, Roberts-Thomson P. Mortality and its predominant causes in a large cohort of patients with biopsy-determined inflammatory myositis. Intern Med J. 2012;42(2):191–198. doi:10.1111/j.1445-5994.2010.02406.x
7. Guimarães F, Yildirim R, Isenberg DA. Long-term survival of patients with idiopathic inflammatory myopathies: anatomy of a single-centre cohort. Clin Exp Rheumatol. 2023;41(2):322–329. doi:10.55563/clinexprheumatol/486yh4
8. Johnson NE, Arnold WD, Hebert D, et al. Disease course and therapeutic approach in dermatomyositis: a four-center retrospective study of 100 patients. Neuromuscul Disord. 2015;25(8):625–631. doi:10.1016/j.nmd.2015.04.013
9. Truzzi NCC, Hoff LS, Borges IBP, de Souza FHC, Shinjo SK. Clinical manifestations, outcomes, and antibody profile of Brazilian adult patients with dermatomyositis: a single-center longitudinal study. Adv Rheumatol. 2022;62(1):41. doi:10.1186/s42358-022-00276-x
10. Sartori Vieira C, de Rezende RPV, Mendes Klumb E, Cardoso Mocarzel LO, Altenburg Gismondi R. Mortality profile related to the spectrum of systemic connective tissue diseases: a retrospective, population-based, case-control study. Lupus. 2019;28(12):1498–1500. doi:10.1177/0961203319876993
11. Tani K, Tomioka R, Sato K, et al. Comparison of clinical course of polymyositis and dermatomyositis: a follow-up study in Tokushima University Hospital. J Med Invest. 2007;54(3–4):295–302. doi:10.2152/jmi.54.295
12. Lao M, Ouyang H, Li N, et al. Clinical characteristics and associated factors for infection and in-hospital mortality in inpatients with polymyositis/dermatomyositis in China: a retrospective study. Infect Drug Resist. 2023;16:289–299. doi:10.2147/idr.S392585
13. Chan SCW, Chung HY, Lau CS, Li PH. Epidemiology, mortality and effectiveness of prophylaxis for Pneumocystis jiroveci pneumonia among rheumatic patients: a territory-wide study. Ann Clin Microbiol Antimicrob. 2021;20(1):78. doi:10.1186/s12941-021-00483-2
14. Naveen R, Rathore U, Agarwal V, Gupta L. Characteristics and outcomes of overlap myositis: a comparative multigroup cohort study in adults from the MyoCite cohort. Rheumatol Int. 2021;41(3):551–563. doi:10.1007/s00296-020-04779-y
15. Bae S, Charles-Schoeman C. Oral cyclophosphamide in treatment of patients with refractory idiopathic inflammatory myopathies: a retrospective observational study. Clin Rheumatol. 2018;37(8):2113–2123. doi:10.1007/s10067-018-4174-3
16. Redondo-Benito A, Curran A, Villar-Gomez A, et al. Opportunistic infections in patients with idiopathic inflammatory myopathies. Int J Rheum Dis. 2018;21(2):487–496. doi:10.1111/1756-185x.13255
17. Xiao Y, Luo H, Zhou B, et al. Comparison of soluble urokinase plasminogen activator receptor, soluble triggering receptor expressed on myeloid cells 1, procalcitonin and C-reactive protein in distinguishing concurrent bacterial infection from idiopathic inflammatory myopathy. Rheumatol Int. 2017;37(4):585–592. doi:10.1007/s00296-016-3609-x
18. Couderc M, Gottenberg JE, Mariette X, et al. Efficacy and safety of rituximab in the treatment of refractory inflammatory myopathies in adults: results from the AIR registry. Rheumatology. 2011;50(12):2283–2289. doi:10.1093/rheumatology/ker305
19. Conticini E, d’Alessandro M, Grazzini S, et al. Rituximab-induced hypogammaglobulinaemia in patients affected by idiopathic inflammatory myopathies: a multicentre study. Clin Exp Rheumatol. 2023;41(2):285–290. doi:10.55563/clinexprheumatol/790ihy
20. Cronin ME, Miller FW, Hicks JE, Dalakas M, Plotz PH. The failure of intravenous cyclophosphamide therapy in refractory idiopathic inflammatory myopathy. J Rheumatol. 1989;16(9):1225–1228.
21. Ramos-Casals M, García-Hernández FJ, de Ramón E, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28(4):468–476.
22. Cheng L, Li Y, Wu Y, et al. Risk of early infection in idiopathic inflammatory myopathies: cluster analysis based on clinical features and biomarkers. Inflammation. 2023;46:1036–1046. doi:10.1007/s10753-023-01790-w
23. Nuño-Nuño L, Joven BE, Carreira PE, et al. Overlap myositis, a distinct entity beyond primary inflammatory myositis: a retrospective analysis of a large cohort from the REMICAM registry. Int J Rheum Dis. 2019;22(8):1393–1401. doi:10.1111/1756-185x.13559
24. Rouster-Stevens KA, Morgan GA, Wang D, Pachman LM. Mycophenolate mofetil: a possible therapeutic agent for children with juvenile dermatomyositis. Arthritis Care Res. 2010;62(10):1446–1451. doi:10.1002/acr.20269
25. Prasad S, Misra R, Agarwal V, Lawrence A, Aggarwal A. Juvenile dermatomyositis at a tertiary care hospital: is there any change in the last decade? Int J Rheum Dis. 2013;16(5):556–560. doi:10.1111/1756-185x.12053
26. Ruperto N, Pistorio A, Oliveira S, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. 2016;387(10019):671–678. doi:10.1016/s0140-6736(15)01021-1
27. Bader-Meunier B, Decaluwe H, Barnerias C, et al. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol. 2011;38(7):1436–1440. doi:10.3899/jrheum.101321
28. Uchino M, Yamashita S, Uchino K, et al. Long-term outcome of polymyositis treated with high single-dose alternate-day prednisolone therapy. Eur Neurol. 2012;68(2):117–121. doi:10.1159/000338474
29. Hsu CY, Ko CH, Wang JL, Hsu TC, Lin CY. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. 2019;21(1):211. doi:10.1186/s13075-019-1997-5
30. Cherin P, Piette JC, Wechsler B, et al. Intravenous gamma globulin as first line therapy in polymyositis and dermatomyositis: an open study in 11 adult patients. J Rheumatol. 1994;21(6):1092–1097.
31. Unger L, Kampf S, Lüthke K, Aringer M. Rituximab therapy in patients with refractory dermatomyositis or polymyositis: differential effects in a real-life population. Rheumatology. 2014;53(9):1630–1638. doi:10.1093/rheumatology/keu024
32. Takada K, Katada Y, Ito S, et al. Impact of adding tacrolimus to initial treatment of interstitial pneumonitis in polymyositis/dermatomyositis: a single-arm clinical trial. Rheumatology. 2020;59(5):1084–1093. doi:10.1093/rheumatology/kez394
33. Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008;63(1):53–59. doi:10.1136/thx.2006.069237
34. Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–498. doi:10.1002/art.41105
35. Shirai T, Machiyama T, Sato H, Ishii T, Fujii H. Intensive induction therapy combining tofacitinib, rituximab and plasma exchange in severe anti-melanoma differentiation-associated protein-5 antibody-positive dermatomyositis. Clin Exp Rheumatol. 2023;41(2):291–300. doi:10.55563/clinexprheumatol/8kulbf
36. Billet AC, Barba T, Coutant F, et al. Infection risk in patients with dermatomyositis associated with anti-MDA5 antibodies: a historical cohort study. Biomedicines. 2022;10(12). doi:10.3390/biomedicines10123176
37. Matsuda KM, Yoshizaki A, Kuzumi A, et al. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. J Dermatol. 2020;47(5):483–489. doi:10.1111/1346-8138.15274
38. Tsai SY, Lin CL, Wong YC, et al. Increased risk of herpes zoster following dermatomyositis and polymyositis: a nationwide population-based cohort study. Medicine. 2015;94(28):e1138. doi:10.1097/md.0000000000001138
39. Liu L, Zhang Y, Liu S, et al. Compound sulfamethoxazole improved the prognosis of dermatomyositis patients positive with anti-melanoma differentiation-associated gene 5. Rheumatology. 2023. doi:10.1093/rheumatology/kead034
40. Li J, Wang S, Zheng J, Li Q, Li J, Lu L. Clinical characteristics of and risk factors for Pneumocystis jirovecii pneumonia in anti-melanoma differentiation-associated gene 5 (Anti-MDA5) antibody-positive dermatomyositis patients: a single-center retrospective study. Clin Rheumatol. 2023;42(2):453–462. doi:10.1007/s10067-022-06403-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.