Back to Journals » Open Access Emergency Medicine » Volume 15
Pulmonary Thromboembolism in Pregnancy: A Case Report and Literature Review
Authors Urriago-Osorio GA , Melo-Burbano LÁ , López-Van Den Berghe J, Muñoz-Córdoba AM, Daza-Arana JE, Contreras-Zúñiga E
Received 11 February 2023
Accepted for publication 18 April 2023
Published 3 June 2023 Volume 2023:15 Pages 217—225
DOI https://doi.org/10.2147/OAEM.S404941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Gustavo Andrés Urriago-Osorio,1,2 Luis Álvaro Melo-Burbano,1,2 Juanita López-Van Den Berghe,2 Angela María Muñoz-Córdoba,2 Jorge Enrique Daza-Arana,1,3 Eduardo Contreras-Zúñiga2
1Department of Health, Internal Medicine Specialization Program, Universidad Santiago de Cali, Santiago de Cali, Colombia; 2Emergency Department, Clínica de Occidente S.A, Santiago de Cali, Colombia; 3Health and Movement Research Group, Universidad Santiago de Cali, Santiago de Cali, Colombia
Correspondence: Gustavo Andrés Urriago-Osorio, Department of Health, Internal Medicine Specialization Program, Universidad Santiago de Cali, Cl. 5 #No. 62 − 00, Pampalinda, Santiago de Cali, Valle del Cauca, 760035, Colombia, Tel +57 3163029030, Email [email protected]
Abstract: Data on the optimal diagnostic management of pregnant women with suspected pulmonary embolism are limited. Despite a lack of compelling evidence in some practices, clinical practice guidelines focus on the management of these patients. We present the case of a 24-year-old patient at 36 weeks of pregnancy in whom pulmonary thromboembolism (PTE) was diagnosed in a timely manner also with hemodynamic instability and echocardiographic images with clear involvement of the right cavities. She received thrombolytic therapy with alteplase 100 mg intravenously over 2 hours, which resulted in excellent outcomes for both the pregnant woman and fetus. Understanding the acute approach and management of these patients will improve our clinical practice; therefore, we reviewed a case report of a pregnant patient with high-risk PTE and compared it with current evidence. In conclusion, PE is a common disease with a high mortality rate during pregnancy. Therefore, having made a timely diagnosis using the relevant diagnostic aids and performing thrombolysis with rtPA increase the probability of survival in our patient, leading to successful results for both her and the fetus.
Keywords: pulmonary embolism, venous thromboembolism, pregnancy, thrombolysis, fibrinolytics
Introduction
Thromboembolic disease is part of a broad spectrum of diseases that includes deep vein thrombosis and pulmonary embolism (PE). PE is the obstruction of one or more of the pulmonary arteries by a liquid, solid, or gaseous component. In most cases, it is caused by a blood thrombus that originates in the deep venous system of the legs or pelvis and then migrates to the lungs. The annual incidence of PE is 60–120 per 100,000 people per year. Approximately 60,000–100,000 patients die of PE each year in the USA.1
The risk factors include all the conditions or alterations involved in Virchow’s triad (hypercoagulability, endothelial injury, and venous stasis). Examples of these conditions are previous venous embolism (OR > 10), autoimmune diseases (OR 2–9), pregnancy (OR < 2), and puerperium (OR < 2).2
The incidence of thromboembolic events in pregnant women is higher than in the general population3 due to the changes that pregnancy exerts on the coagulation system, thus increasing the synthesis of factors II, VII, and X and the fibrinolytic systems, causing a decrease in the titers of protein S and a resistance to activated protein C, and leading to a prothrombotic state with the ultimate aim of reducing the risk of bleeding during implantation, delivery, or placentation.4
Pulmonary thromboembolism (PTE) is still one of the leading causes of mortality during pregnancy.5 The worldwide incidence of VTE is approximately 1 in every 1000 pregnancies. This rate is five times that of the non-pregnant female population.3 Recently, it has been reported that the risk of presenting PTE is evenly distributed across the three trimesters. However, the risk is highest in the first 6 weeks after delivery, with a peak incidence at 3 weeks.6
So far, the indication for thrombolysis is high-risk PTE. Although it is a rare event, it is highly lethal. According to a systematic review, the performance of thrombolysis in patients with high-risk PTE has high maternal and fetal survival rates (94% and 88%).7 However, there is little evidence that thrombolysis is beneficial in low- or moderate-risk PTE.
Case Description
A 24-year-old female patient, gravidity 3 Parity 2, 26 weeks pregnant, was admitted to the emergency department after being found unconscious, diaphoretic, and cold, with subsequent partial recovery of consciousness, and after collapsing three times. Upon admission to the emergency department (Table 1), the patient was stuporous, diaphoretic, and cold, with a blood pressure of 60/28 mmHg and a heart rate of 155 bpm. Fluid replacement was started, and electrocardiogram revealed sinus rhythm, a Q-wave in DIII, and an inverted T-wave in derivative DIII (Figure 1). Point of care ultrasound (POCUS) was performed, which revealed right ventricular dilatation, and PTE was suspected. Therefore, it was decided to start low molecular weight heparin (enoxaparin 60 mg subcutaneously every 12 hours). The patient underwent computed axial tomography after initiating vasopressors centrally, which reported multiple opacification defects in the right and left branches of the pulmonary artery and segmental branches of both lower lobes, especially in relation to acute PE (Figure 2). An emergency echocardiogram revealed the following: a small left ventricle with normal wall thickness, no evident segmental alterations at rest, and preserved global systolic function. The ejection fraction calculated by Simpson was 59%. Severe dilation of the right ventricle with severe systolic dysfunction. Mild dilation of the right atrium. Normal valvular study. Severe pulmonary hypertension with signs of systemic venous hypertension and severe hemodynamic impact on the right cavities.
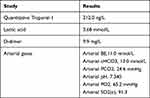 |
Table 1 Paraclinical Studies Requested Upon Admission |
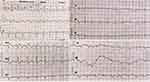 |
Figure 1 McGinn-White sign: Q-wave in DIII, and an inverted T-wave in derivative DIII. |
 |
Figure 2 Multiple opacification defects in the right and left branches of the pulmonary artery and segmental branches of both lower lobes. |
According to this report, dual vasopressor therapy (norepinephrine and vasopressin) was required in patients with obstructive shock who had a confirmed diagnosis of massive PTE. Thrombolysis with 100 mg of tissue plasminogen activator (Alteplase®) was performed within 2 hours. The patient was transferred to the intensive care unit with an embryonic heart rate and no hemorrhagic complications. A follow-up echocardiogram at 5 days revealed a non-dilated or hypertrophic left ventricle with no segmental alterations evident at rest or in scar areas and preserved global systolic function. The ejection fraction calculated by Simpson was 65%. Normal diastolic function. Mild dilation of the right cavities with preserved right ventricular systolic function. Normal valvular study. High probability of pulmonary hypertension within a moderate range with signs of systemic venous hypertension and mild hemodynamic impact on the right chambers. Pulmonary maturation was performed, and the patient was transferred to the Obstetric High-Risk Unit in a stable condition, without inotropic or vasopressor support.
Discussion
Pregnancy-related PE continues to be one of the main causes of maternal death in developed countries, regardless of the increased use of thromboprophylaxis in women at high risk of death.8 Pregnant women are four-to-five times more likely to have a PE compared to non-pregnant women of the same age.9 The exact incidence of PE is unknown, but it is estimated to be between 0.5 and 2 cases per 1000 pregnancies.10 Data from the United State showed that thrombosis and thromboembolism were the most frequent causes of direct maternal death, resulting 0.93 in 2003 to 1.96 in 2020 deaths per 100,000 live births.11
The risk is highest in the 3 weeks following a cesarean section, but it remains high between 3 and 6 weeks after birth and is the same as during pregnancy. From week 6, the risk is the same as that of a non-pregnant woman.12 Compared to non-pregnant women, most deep vein thromboses are left-sided (90% versus 55%) and mainly involve the iliac femoral vessels (72% versus 9%).13
Three pathophysiological mechanisms, either alone or in combination, are responsible for the high incidence of PE during pregnancy. These mechanisms are known as Virchow’s triad:
Vascular Injury
During childbirth, the pelvic veins may become distended and/or traumatized, especially if a cesarean section is performed.14
Venous Stasis
This problem begins in the third trimester of pregnancy and reaches a peak risk at approximately 26 weeks. It is caused by an increase in the circulating volume of progesterone, leading to a dilation of veins. Additionally, there is intermittent compression of the iliac arteries on the left iliac vein. This is known as the May–Thurner syndrome. Finally, the pregnant uterus causes pelvic compression.15
Hypercoagulable State
During pregnancy, there is an increase in some coagulation factors, namely factors II, VII, VIII, IX, and X. On the contrary, there is a decrease in the activation of fibrinolysis inhibitors PAI-1 and PAI-2, as well as the production of protein S16 (Table 2).
 |
Table 2 Summary of the Main Pro-Coagulant Changes That Occur During Pregnancy |
Diagnosis
In North America, PE is diagnosed in only 1 in 20 patients who are evaluated for it when they visit the emergency department.17
The clinical signs of PE are difficult to assess because the majority of healthy pregnant women present with lower limb edema, which becomes more common with increasing gestational age. According to some studies, up to 70% of pregnant women experience respiratory distress as a result of an increased abdominal perimeter. The diagnosis requires a high level of clinical suspicion based on predisposing factors and triggers.18
A clinical probability that the clinical picture is due to a PE should be established. Based on the clinical history and physical examination, the risk can be calculated using the Wells scale and the Geneva score (Table 3). Unfortunately, these scales have not been validated in pregnant women. One study found three variables that predicted the risk of deep vein thrombosis in pregnant women: left leg symptoms, the first trimester of pregnancy, and a calf circumference difference of >2 cm. If none of these variables is present, the negative predictive value is 100% (95% CI: 92–100%). However, the positive predictive value of only one of these variables is low.19
 |
Table 3 The Wells Scoring System |
Clinical Probability
0–1 points: low
2–6 points: intermediate
>7 points: high
During pregnancy, D-dimer levels are normally elevated, and this is especially noticeable in the late third trimester and early puerperium. Additionally, there is an increase in conditions such as placental abruption and pre-eclampsia. Normal D-dimer levels have been reported in patients with confirmed PE.8
Chest x-ray should be taken to rule out other pathologies, such as pneumonia or pneumothorax, which may cause symptoms similar to PE. Chest X-ray may be normal in up to 50% of cases. Among the most common findings associated with PE are pleural effusion, basal atelectasis, pulmonary edema, and focal opacities. If these abnormalities are found, computed tomography pulmonary angiogram (CTPA) should be performed. However, CTPA may fail to identify up to 30% of peripheral PEs. Additionally, its reliability may be reduced by hyperdynamic circulation during pregnancy. Ventilation/perfusion scintigraphy or perfusion scintigraphy alone is a safer alternative to CTPA because it exposes both the mother and fetus to less radiation.20,21
Ionizing radiation can cause death, ocular malformations, intrauterine growth retardation, and/or carcinogenic and mutagenic effects in the fetus depending on two main factors: gestational age (the fetus is more vulnerable between the 2nd and 8th week) and absorbed radiation dose. Unfortunately, the excessive fear of harming the fetus has limited this type of study. Fetal malformations have been associated with radiation doses of more than 100–200 mGy. A dose of >250 mGy has been associated with a 0.1% risk of fetal malformation (Table 4).20
 |
Table 4 Summary of Estimated Fetal Exposure According to Type of Study |
Clinical assessment of the venous system in pregnant women can be challenging owing to the peripheral edema that most of them experience. The presence of deep vein thrombosis in pregnant women is an indication to initiate anticoagulation. Unfortunately, up to 70% of patients with PE do not have deep venous thrombosis at the time of diagnosis (Figure 3).22
 |
Figure 3 Diagnostic algorithm for patients with suspected hemodynamically stable pulmonary embolism. |
Echocardiography is a critical diagnostic tool in patients with hemodynamic instability and suspected PE. It can be performed at the patient’s bedside. If an acute dysfunction of the right ventricle is absent, other probable causes of hemodynamic instability can be evaluated, such as cardiac tamponade, acute coronary syndrome, aortic dissection, valvular dysfunction, or hypovolemia (Figure 4).22
 |
Figure 4 Diagnostic algorithm for patients with suspected hemodynamically unstable pulmonary embolism. |
Treatment
Heparins, including LMWH, such as unfractionated heparin (UFH), can be safely used during pregnancy. Treatment is usually with LMWH for at least 3 months and up to 6 weeks postnatally.23 This is the treatment most commonly used by physicians according to descriptive studies of pregnant patients with PE.24
Vitamin K antagonists (VKA) and direct anticoagulants cross the placental barrier. When VKA are used early in pregnancy, congenital malformations affecting the central nervous system have been reported, especially between the 6th and 12th weeks. Embryopathy associated with VKA occurs in 5–6% of pregnancies due to the inhibition of protein production necessary for cartilage and bone formation. During childbirth, there is an increased risk of cerebral hemorrhage in the fetus. Its use should be considered only in patients who have mechanical heart valves.25,26
Recently, direct oral anticoagulants have replaced VKAs as the first-line treatment for PE. Currently, there is little evidence on their safety during pregnancy. Animal models have documented its crossing into the placenta, and it has been found in breast milk (Table 5).27
 |
Table 5 Characteristics of Anticoagulants |
Inferior Vena Cava Filter
It is indicated in patients with recurrent PE despite adequate anticoagulation and in cases where anticoagulation is contraindicated. Experience with pregnant patients is limited, and a temporary filter should be chosen. Among the risks are the likelihood of migration (20%), fracture (5%), perforation (5%) and, finally, death (0.12–0.3%).28
Systemic Thrombolysis
Thrombolysis involves the administration of a fibrinolytic drug to fragment and dissolve the clot. Although pregnancy is a relative contraindication for thrombolysis, guidelines from the main scientific societies support its use in patients with hemodynamic instability due to massive PE.
Given their size, all fibrinolytic agents should theoretically have a low capacity for crossing the placental barrier. There is no evidence of teratogenicity associated with the use of fibrinolytic drugs or of their excretion through breast milk. Alteplase, the main agent used, is administered at a dose of 100 mg over 2 hours. The main complications are bleeding, postpartum vaginal bleeding, and intraabdominal hemorrhage. These occur mainly within 72 hours of fibrinolysis.19,29
However, recent systematic reviews have been carried out which found no statistically significant differences between the dose of alteplase 50 mg in 2 hours or 100 mg in 2 hours.30
Extracorporeal Membrane Oxygenation (ECMO)
ECMO aims to temporarily restore hemodynamic and circulatory stability, but it is not reperfusion therapy. It is a modified cardiopulmonary bypass circuit that can help patients with hemodynamic instability. The level of evidence remains low and is based on a case series. It has been used to treat refractory hypoxemia in pregnant women as well as adult respiratory distress syndrome. PE is still a rare occurrence (approximately 5% of all cases).19
Childbirth
Once labor approaches, LMWH is usually discontinued and a UFH infusion is started 36 hours prior to delivery, especially if neuroaxial anesthesia is planned. Finally, UFH should be discontinued 4–6 hours prior to delivery. The resumption of anticoagulation will be determined by several factors, including the delivery route and thrombotic and hemorrhagic risks. Ideally, the multidisciplinary team in charge of the patient makes this decision. In the case of epidural catheter removal, LMWH should be restarted at least 4 hours later.29
Conclusion
PE is a common disease with a high mortality rate during pregnancy. Therefore, having made a timely diagnosis using the relevant diagnostic aids and performing thrombolysis with rtPA increase the probability of survival in our patient, leading to successful results for both her and the fetus.
Acknowledgments
This research was funded by the General Direction of Research of the Universidad Santiago de Cali under Call No. 02-2023. The activities of the research team for conducting the study were sponsored by Clínica de Occidente S.A. and Universidad Santiago de Cali. However, the authors declare that a total level of autonomy for the development of the study in all phases of the study.
Disclosure
The authors do not declare conflicts of interest.
References
1. Freund Y, Cohen-Aubart F, Bloom B. Acute pulmonary embolism: a review. JAMA. 2022;328:1336–1345. doi:10.1001/jama.2022.16815
2. Spanish Society of Cardiology. Guía ESC. 2019 para el diagnóstico y tratamiento de la embolia pulmonar aguda [ESC guide. 2019 for the diagnosis and treatment of acute pulmonary embolism]. Rev Española Cardiol. 2020.
3. Kane EV, Calderwood C, Dobbie R, et al. A population-based study of venous thrombosis in pregnancy in Scotland 1980–2005. Eur J Obstet Gynecol Reprod Biol. 2013;169:223–229. doi:10.1016/j.ejogrb.2013.03.024
4. Bates SM. Pulmonary embolism in pregnancy. Semin Respir Crit Care Med. 2021;42:284–298. doi:10.1055/s-0041-1722867
5. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Heal. 2014;2:e323–e333.
6. Dado CD, Levinson AT, Bourjeily G. Pregnancy and pulmonary embolism. Clin Chest Med. 2018;39:525–537. doi:10.1016/j.ccm.2018.04.007
7. Martillotti G, Boehlen F, Robert‐Ebadi H, et al. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. 2017;15:1942–1950. doi:10.1111/jth.13802
8. Greer IA, Solomon CG. Clinical Practice. Pregnancy complicated by venous thrombosis. N Engl J Med. 2015;373:540–547. doi:10.1056/NEJMcp1407434
9. Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697. doi:10.7326/0003-4819-143-10-200511150-00006
10. Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can. 2009;31:611–620. doi:10.1016/S1701-2163(16)34240-2
11. Farmakis LT, Braekkan SK, Connors JM, et al. Maternal mortality related to pulmonary embolism in the United States, 2003–2020. Am J Obstet Gynecol. 2023;5:100754.
12. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. 2008;6:905–912. doi:10.1111/j.1538-7836.2008.02961.x
13. Nelson-Piercy C. Handbook of obstetric medicine. Handb Obstet Med. 2020. doi:10.1201/9780429330766
14. Aird WC. Vascular bed-specific thrombosis. J Thromb Haemost. 2007;5(Suppl 1):283–291. doi:10.1111/j.1538-7836.2007.02515.x
15. Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997;104:191–197. doi:10.1111/j.1471-0528.1997.tb11043.x
16. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074. doi:10.1016/S0140-6736(06)68397-9
17. Germini F, Zarabi S, Eventov M, et al. Pulmonary embolism prevalence among emergency department cohorts: a systematic review and meta-analysis by country of study. J Thromb Haemost. 2021;19:173–185. doi:10.1111/jth.15124
18. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–3197. doi:10.1093/eurheartj/ehr218
19. Blondon M, de Tejada M, Glauser B, Righini, M F, Robert-Ebadi H. Management of high-risk pulmonary embolism in pregnancy. Thromb Res. 2021;204:57–65. doi:10.1016/j.thromres.2021.05.019
20. Prasad M, Gupta R, Patthi B; A. S and diagnostic & 2016, U. Imaging more imagining less: an insight into knowledge, attitude and practice regarding radiation risk on pregnant women among dentists of Ghaziabad–a; 2018. Available from: ncbi.nlm.nih.gov.
21. Blanco-Molina Á, Rota L, Micco PD, et al. Venous thromboembolism during pregnancy, postpartum or during contraceptive use. Thromb Haemost. 2010;103:306–311. doi:10.1160/TH09-08-0559
22. Konstantinides SV. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019;41:543–603.
23. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e691S–e736S. doi:10.1378/chest.11-2300
24. Jerjes-Sánchez C, Rodriguez D, Farjat AE, et al. Pregnancy-associated venous thromboembolism: insights from GARFIELD-VTE. TH Open. 2021;05:e24–e34. doi:10.1055/s-0040-1722611
25. Xu Z, Fan J, Luo X, et al. Anticoagulation regimens during pregnancy in patients with mechanical heart valves: a systematic review and meta-analysis. Can J Cardiol. 2016;32:1248.e1–1248.e9. doi:10.1016/j.cjca.2015.11.005
26. Vitale N, De Feo M, De Santo LS, et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. 1999;33:1637–1641. doi:10.1016/S0735-1097(99)00044-3
27. Lameijer H, Aalberts JJJ, van Veldhuisen DJ, Meijer K, Pieper PG. Efficacy and safety of direct oral anticoagulants during pregnancy; a systematic literature review. Thromb Res. 2018;169:123–127. doi:10.1016/j.thromres.2018.07.022
28. Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol. 2016;27:354–360.e8. doi:10.1016/j.jvir.2015.11.024
29. Romualdi E, Dentali F, Rancan E, et al. Anticoagulant therapy for venous thromboembolism during pregnancy: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2013;11:270–281. doi:10.1111/jth.12085
30. Rodriguez D, Jerjes-Sanchez C, Fonseca S, et al. Thrombolysis in massive and submassive pulmonary embolism during pregnancy and the puerperium: a systematic review. J Thromb Thrombolysis. 2020;50:929–941. doi:10.1007/s11239-020-02122-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
