Back to Journals » Integrated Blood Pressure Control » Volume 15
Pulmonary Hypertension in Pregnancy: Challenges and Solutions
Authors Afify H, Kong A , Bernal J, Elgendy IY
Received 3 January 2022
Accepted for publication 25 March 2022
Published 2 April 2022 Volume 2022:15 Pages 33—41
DOI https://doi.org/10.2147/IBPC.S242242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Hesham Afify,1,2 Alexander Kong,1,2 Jopher Bernal,1,2 Islam Y Elgendy3
1Department of Internal Medicine, University of Central Florida HCA Healthcare GME, Greater Orlando, FL, USA; 2Department of Internal Medicine, University of Central Florida College of Medicine, Orlando, FL, USA; 3Division of Cardiovascular Medicine, Gill Heart Institute, University of Kentucky, Lexington, KY, USA
Correspondence: Islam Y Elgendy, Division of Cardiovascular Medicine, Gill Heart Institute, University of Kentucky, Lexington, KY, USA, Email [email protected]
Abstract: Pulmonary hypertension (PH) is a heterogeneous disease characterized by an elevated mean pulmonary artery pressure of 20 mm Hg or above. PH is a prevalent condition among women of reproductive age and is linked with poor prognosis during pregnancy. Pregnancy is a stressful event and complicates the management and prognosis in patients with PH. In this review, we discuss the pathogenesis, clinical presentation as well as therapeutic options for PH during pregnancy. We also highlight knowledge gaps to guide future research.
Keywords: pulmonary hypertension, pulmonary arterial hypertension, pregnancy, women
Introduction
Cardiovascular diseases are one of the leading causes of preventable maternal morbidity and mortality in developed countries, including the United States (US).1 Pulmonary hypertension (PH) among pregnant women carries a high mortality risk for both the mother and fetus, with some data suggesting that the maternal mortality rate is as high as 30–56%.2 In recent years, studies have shown a decline in maternal mortality among pregnant women with well controlled PH, with mortality rates ranging from 9–25%.3–5 Primary pulmonary arterial hypertension (PAH) is the most prevalent type of PH among women of reproductive age, and it is 2–4 times more common among women compared with men,6,7 with a median survival rate of 2.8 years among untreated non-pregnant women.8,9 Societal guidelines recommend against pregnancy in patients with PH because of the high maternal mortality.10,11 Despite these recommendations, a number of women with PH decide to become pregnant while others might be diagnosed with PH for the first-time during pregnancy. In one study, 1 out of 4 women with PH associated with congenital heart disease (CHD) and 16% of primary PAH were diagnosed for the first time during pregnancy.12 In this review, we provide an overview for the pathogenesis, clinical presentation as well as therapeutic options for PH during pregnancy.
Classification
PH is a heterogenous disease that could be primary or secondary to other conditions. It is defined as a mean pulmonary arterial pressure (mPAP) ≥20 mmHg at rest.13,14 However, using a threshold value for PAP alone might not be sufficient to characterize PH. To better define PH, the values of both pulmonary capillary wedge pressure (PCWP) and pulmonary vascular resistance (PVR) are helpful, thus right heart catheterization (RHC) is usually indicated to confirm the diagnosis, especially among non-pregnant women. The 6th World Symposium on PH proposed a cut off value for PVR ≥3 Wood Units (WU) and PCWP >15 mmHg to further classify PH.13 Accordingly, PH is classified hemodynamically into pre-capillary, post-capillary, and combined pre- and post-capillary PH.
Clinically, PH is classified into 5 main groups: i) group 1: PAH; ii) group 2: PH secondary to left-sided heart disease; iii) group 3: PH secondary to lung disease and hypoxia; iv) group 4: PH due to PA obstruction; and v) group 5: PH with unclear mechanism.13 As such, pre-capillary PH would be characterized by mPAP ≥20 mmHg, PCWP ≤15 mmHg and PVR ≥3 WU corresponding clinically with groups 1, 3 and 4. Group 2 PH secondary to left-sided heart disease is distinguished by high PCWP >15mmHg together with elevated mPAP >20 mmHg. Depending on PVR, it can be further subclassified as isolated post-capillary PH or mixed pre- and post-capillary PH. Isolated post-capillary PH with PVR <3 WU and combined pre- and post-capillary PH with PVR ≥3 WU. Group 5 PH is a miscellaneous group of diseases with unclear multifactorial mechanisms that leads to PH which can manifest with either hemodynamic change (Table 1).
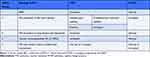 |
Table 1 Clinical and hemodynamic classification of pulmonary hypertension (PH) using the World Health Organization’s 6th Symposium classification system |
Burden of Disease and Underlying Etiology
The most common causes of PH during pregnancy are PH associated with CHD and PAH (ie, group 1). In a systematic review of 13 studies with 272 pregnancies with PH between 2008 and 2018, the common underlying etiologies were PH associated with CHD (64%), of which 30% had Eisenmenger syndrome, followed by PAH (22%).15 In another single tertiary center from China including 93 pregnant women with PH associated with CHD, 30 women had Eisenmenger syndrome, 51 had PH associated with systemic-to-pulmonary shunts, and 13 had PH with repaired CHD.16 In an analysis of a US administrative database, which included 1519 hospitalized pregnant patients with PH between 2003 and 2012, the majority (59.6%) had isolated PH, of which 5.8% were idiopathic and 3% associated with Eisenmenger syndrome, while the remaining (40.3%) had underlying cardiac disease, most commonly valvular heart disease (18.1%) and CHD (10.7%).8 However, this study was limited by the use of administrative codes which are subject to misclassification and the absence of PH classification. In developing countries, the epidemiology of PH in pregnant women is unknown due to lack of registries for pregnant women with PH. The cause of PH in such countries might differ from developed countries due to the higher prevalence of other conditions that are considered risk factors for PH.17 Unrepaired CHD and Eisenmenger syndrome account for most cases and contributes significantly to the overall disease burden of PH in developing countries, as opposed to PAH and valvular heart disease in developed countries.17
Pathophysiology of PH During Pregnancy and Delivery
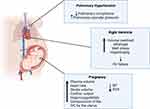
During pregnancy, pulmonary blood flow increases significantly. However, PAP remains unchanged secondary to hormonal changes and recruitment of the pulmonary microvasculature which leads to a reduction in PVR.18 These compensatory mechanisms are compromised in patients with pulmonary vascular disease, which in turn increases the risk of right ventricular (RV) failure. Furthermore, the increased levels of progesterone and estrogen during pregnancy, both of which have vasodilatory effects, exacerbate the decrease in systemic vascular resistance (SVR) and result in a significant drop in diastolic blood pressure.19 Besides the hormonal changes, there are other changes that occur as a result of the mechanical compression on the surrounding organs. For example, the gravid uterus compresses the inferior vena cava in supine position, reducing venous return.20,21 Accordingly, SVR drops during pregnancy to almost 10–30% below the pre-pregnancy level between 20–26 weeks.22,23 The reduction in blood pressure further leads to the activation of the renin-angiotensin-aldosterone system, resulting in salt and water retention and increased plasma volume.24 Collectively, this results in a 20–30% increase in stroke volume.23 Moreover, there is mild dilation and stretching of all cardiac chambers secondary to the increased plasma volume.25 As such, the LV mass increases slightly; however, the ratio of wall thickness to ventricular radius remains constant.26 This cardiac remodeling during pregnancy is believed to be secondary to an increase in myocardial angiogenesis without cardiac fibrosis.
These alterations might not be well tolerated among patients with PH. The normal pulmonary circulation is a low-pressure high compliance system. In PH, there is a reduction in compliance, leading to inability to adapt to the increased cardiac output and pulmonary blood flow.28 This results in an increase in RV afterload and end diastolic volume and ultimately RV dysfunction. The decrease in RV ejection fraction is considered the most important determinant of survival among patients with PH.29,30 The gradual increase in PAP allows the RV to adapt and leads to RV hypertrophy. However, the hemodynamic changes during pregnancy occur as early as 6–8 weeks of gestation, which might lead to the rapid deterioration of the RV function.31 Furthermore, acute hemodynamic changes are common during the delivery process. The associated stress and exertion during this period result in changes in blood pressure, heart rate, and cardiac output. During labor, 300–500 mL of blood circulates back from the placenta to the systemic circulation with each contraction.32,33 After delivery, stroke volume and cardiac output are enhanced due to the autotransfusion from the uteroplacental and a decrease in aortocaval compression.19 All these changes increase the risk of hemodynamic compromise and cardiogenic shock (Figure 1). PAH secondary to CHD has a better prognosis than other subtypes among non-pregnant women. This could be explained by the ability to maintain cardiac output with right to left shunting at the expense of systemic desaturation .27
During pregnancy, clotting factors, except for factors XI and XIII, are increased up to 50%.34,35 Moreover, fibrin degradation is halted, and anticoagulant factors including antithrombin III and protein S are reduced.34,35 This hypercoagulable state might predispose pregnant and post-partum women to thromboembolic events. Concurrent venous thromboembolism and PH are detrimental. PAH associated with CHD and connective tissue diseases has also been linked with increased risk of pre-eclampsia.5,36,37 Pregnant women with pre-eclampsia might have a mildly elevated RV systolic pressure, and evidence of RV diastolic dysfunction as well as a reduced strain pattern. In the background of PH, where the RV function is compromised, PH increases the likelihood of RV failure among women with pre-eclampsia.38
Diagnosis and Risk Stratification
Non-specific symptoms such as fatigue and dyspnea, which might occur with normal pregnancy, are common presentations among patients with PH and might contribute to the delay in diagnosis. Syncope might also be a manifestation of low cardiac output. Hepatomegaly, ascites, and ankle edema, which are signs of RV failure, might also be obscured by pregnancy.39
The World Health Organization (WHO) functional class (Table 2) is a helpful tool in guiding the prognosis of patients with PH, not only at the time of diagnosis, but also during follow-up. However; this tool has not been validated among pregnant women.40 A worsening functional class is considered a sign of disease progression.41,42 In pregnant women, functional classification may aid in risk stratification, as the majority of functional class I and II have favorable outcomes if well-controlled.4 In one study, the maternal mortality rate was 3.6%, and all deaths occurred in patients with functional class IV.43 Thus, symptoms and functional status should be monitored monthly in the first and second trimesters and then weekly during the third trimester.19 This could be further tailored if the woman is at higher risk of decompensation.
 |
Table 2 World Health Organization functional class description of patients with pulmonary hypertension |
Risk assessment tools such as the REVEAL risk score might be used to assess the prognosis. However; these tools have not been validated in pregnancy.44 Echocardiography is a safe tool during pregnancy for determining and monitoring the RV ejection fraction .45 In PH, the RV ejection fraction is a surrogate marker of RV function, although it might be difficult to measure . The tricuspid annular plane systolic excursion assesses the longitudinal component of RV contraction, and the RV fractional area change assesses both the longitudinal and transversal components.46 Additionally, RV tissue Doppler imaging is a non-invasive tool that allows the measurement of the peak systolic velocity and the RV myocardial performance index, allowing for the evaluation of RV dysfunction in PH.47 RHC is an invasive procedure that carries a small risk of radiation to the fetus and is not generally recommended during pregnancy.48 The role of BNP and NT-proBNP has been investigated in this setting since BNP/NT-proBNP levels are associated with myocardial dysfunction and could provide prognostic information both at the time of diagnosis and during follow-up.49 Since these assays are not specific to PH, they should be interpreted in the clinical context. Additionally, monitoring lab tests for end-organ damage such as creatinine and bilirubin levels might be helpful during follow up.50
Counseling
As previously noted, pregnancy is generally not recommended among women with PH due to the considerable mortality. Women with PH should be counseled on reasonable means of contraception. In women with cardiovascular disease, long-acting reversible contraceptives, such as intrauterine devices and progestin-only subcutaneous implants, are safe and effective, and generally carry a low risk of thromboembolism.51 Tubal ligation is a permanent procedure, but does come with some procedural risks.19 Women who wish to become pregnant are advised to have their condition as reasonably controlled as possible, with plans for close monitoring at a tertiary care center with a multidisciplinary team. Preconception counseling on the teratogenicity of some of the medications is also important. Echocardiography, and cardiopulmonary exercise stress tests are beneficial in establishing a baseline anatomical and functional status as part of the pre-pregnancy examination.52 Genetic counseling might be considered for patients with confirmed or suspected hereditary PAH as well as PAH associated with CHD, since some of these conditions have been linked with genetic disorders.53 Although society guidelines recommend offering pregnancy termination after shared decision making during the first trimester, some recent data suggests that maternal mortality among patients with PH is lower than previously observed.19 Maternal mortality is partly related to the underlying etiology of PH as well as the baseline functional capacity. Early clinical deterioration, severe RV dysfunction, elevated BNP, and functional class III or IV have been linked with worse outcomes.19 If a woman decides to proceed with the pregnancy, she needs to be counseled about the potential risks of pregnancy continuation.
Non-Pharmacological and Pharmacological Management
For women who decide to continue with the pregnancy, the overall treatment strategy is to optimize therapy, close monitoring, avoid teratogenic drugs, and incorporate a multidisciplinary team approach. Treatment is generally tailored to the individual patient, with an emphasis on preserving euvolemia and preventing systemic hypotension, hypoxemia, and acidosis.54 Women are advised to lie on their side whenever feasible to minimize obstruction by the inferior vena cava.
Pharmacological therapies are broadly categorized as anticoagulation and specific PH therapies. Anticoagulation with low molecular weight heparin or unfractionated heparin is recommended for idiopathic and hereditary PAH, as well as chronic thromboembolic pulmonary hypertension (CTEPH).10 Low-dose aspirin might also be considered to lower the risk of pre-eclampsia.55 In patients with a positive vasodilator test, long-term treatment with high dose calcium channel blockers (CCB) is associated with a sustained hemodynamic response and improved survival.56,57 CCBs are generally well-tolerated during pregnancy and are not associated with a teratogenic risk.58 The other medical therapies focus on targeting one of the following pathways: prostacyclins, endothelin-receptor antagonists, phosphodiesterase type-5 inhibitors (PDE5i), and guanylate cyclase stimulators. However, only prostacyclins and PDE5i can be considered during pregnancy since the other two classes are teratogenic.59 Furthermore, women who are chronically on medications with potential teratogenic effects prior to pregnancy are advised to switch to a different class. Prostaglandins are indicated for WHO functional class III or IV with reduced RV function because of the vasodilator effect, which in turn might improve the RV function. Parenteral prostaglandins are recommended for pregnant women with WHO functional class IV and severe RV dysfunction. While the use of intravenous epoprostenol in pregnant women has not been investigated, a few case reports have shown that this therapy can be used successfully.54,60,61 Inhaled iloprost62 and intravenous treprostinil combined with sildenafil could be used in pregnancy.63 Sildenafil, not tadalafil, has been shown in some case series to be effective in conjunction with prostaglandins.63,64
Delivery and Post-Partum Care
Ideally, patients would benefit from referral to specialized PH facilities with extracorporeal membrane oxygenation (ECMO) and transplant capabilities around the time of delivery.65 The timing of delivery should take into consideration both the maternal risk and fetal health, and planned before the expected delivery.66 Multidisciplinary teams involving maternal fetal medicine, neonatal intensive care, pulmonology, cardiology, obstetric and cardiac anesthesiologists, critical care medicine, pharmacy, and the ECMO team are recommended in the delivery planning. In the event of RV dysfunction and hemodynamic instability at any point, an emergency delivery should be considered. Elective delivery is generally recommended around 34–36 weeks of pregnancy.67 Cesarean section is considered the preferred mode of delivery.19 However, vaginal delivery is considered safe in some patients with PH associated with CHD .68 Vaginal delivery can also be considered in patients with functional class I or II who are well controlled. If vaginal delivery is planned, assisted vaginal delivery could be considered to shorten the delivery time and mitigate the risks of a protracted delivery.33 Valsalva, vasovagal response, and discomfort associated with vaginal birth, might contribute to cardiac decompensation. There is also a theoretical risk of paradoxical air embolism upon placental exposure during vaginal delivery or iatrogenic intravenous injections in patients with a patent right-to-left intracardiac shunt.54 If cesarean section is considered, epidural anesthesia is preferred due to the negative effects of spinal and general anesthesia on myocardial function and increased PVR .12,19
During delivery, routine PA monitoring is not generally recommended, but careful monitoring of vital signs, ECG, and pulse oximetry, preferably in an intensive care setting, is important. Complications during delivery include arrhythmias, heart failure, pre-eclampsia, eclampsia, preterm delivery, as well as fetal demise, obstetric and postpartum bleeding. Vasopressors and positive inotropes might be considered in the event of RV failure, although there has been no data on the appropriate inotrope in this setting. Oxytocin for the prevention of postpartum hemorrhage may precipitate a pulmonary hypertensive crisis with marked systemic hypotension due to the increased PVR and sudden reduction in SVR. A slow and low-dose oxytocin intravenous infusion or intrauterine injection is recommended when hemostasis is necessary, while other agents such as ergometrine or prostaglandins are contraindicated given their vasoconstrictive effects on the pulmonary vasculature.69
The first 24–36 hours of the post-partum period is a critical time, carrying the highest risk of maternal morbidity and mortality.5 Anticoagulation is usually preferred during the first postpartum week in PH patients, especially if they are confined to bed and non-ambulant. Those already on anticoagulation should resume therapy once hemostasis is achieved.70,71 If appropriate, medications with potential teratogenic effects such as endothelin-receptor antagonists may be re-introduced promptly after delivery. Breastfeeding is encouraged in the postpartum period. Breastfeeding while on sildenafil, treprostinil and bosentan has been anecdotally reported, however; strong safety data are lacking.72 Patients might benefit from follow up with PH specialists following hospital discharge.73
Knowledge Gaps and Future Directions
The true prevalence of pregnant PH patients remains unknown, and further epidemiological studies are needed to better understand the prevalence and mortality related to pregnancy in PH. It is important to encourage centers from developing countries to contribute to such registries to better understand the underlying causes and outcomes in these countries to develop better management strategies. Given the recent advances in PH therapies, future research should focus on risk stratifying women with PH in order to better identify the subgroup of women with PH who can safely endure pregnancy. PH treatment has evolved in recent years with the introduction of newer agents, however; the safety of these medications during pregnancy and breastfeeding should be further investigated. Future studies are also encouraged to evaluate the safety and effectiveness of some of the novel therapeutic drugs that target PA remodeling, such as inflammatory pathways, endothelial cell development and death, and platelet-derived growth factor signaling.74
Summary
PH is an uncommon yet potentially fatal disease in pregnant women. Screening and early identification of PH among pregnant women is crucial due to the non-specific nature of the symptoms. Advancements in non-invasive imaging modalities such as echocardiography are helpful in the early diagnosis of PH among pregnant women. Society guidelines advise against pregnancy and recommend considering termination if a PH woman becomes pregnant. A multidisciplinary decision-making approach regarding the optimal treatment options and alternatives to teratogenic medications should be considered if the women decides to continue with the pregnancy.
Disclosure
Dr Islam Y Elgendy reports grants from Caladrius Biosciences, Inc, outside the submitted work. The authors report noconflicts of interest in this work. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
1. Petersen EE, Davis NL, Goodman D, et al. Vital Signs: pregnancy-Related Deaths, United States, 2011-2015, and Strategies for Prevention, 13 States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423–429.
2. Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998;31(7):1650–1657.
3. Kiely DG, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG. 2010;117(5):565–574.
4. Corbach N, Berlier C, Lichtblau M, et al. Favorable Pregnancy Outcomes in Women With Well-Controlled Pulmonary Arterial Hypertension. Front Med. 2021;8:689764.
5. Sliwa K, van Hagen IM, Budts W, et al. Pulmonary hypertension and pregnancy outcomes: data from the Registry Of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail. 2016;18(9):1119–1128.
6. Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139(1):128–137.
7. Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030.
8. Thomas E, Yang J, Xu J, Lima FV, Stergiopoulos K. Pulmonary Hypertension and Pregnancy Outcomes: insights From the National Inpatient Sample. J Am Heart Assoc. 2017;6(10):343.
9. D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349.
10. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–2294.
11. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975.
12. Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. Feb. 2009;30(3):256–265.
13. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):3234.
14. Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894.
15. Low TT, Guron N, Ducas R, et al. Pulmonary arterial hypertension in pregnancy-a systematic review of outcomes in the modern era. Pulm Circ. 2021;11(2):20458940211013671.
16. Li Q, Dimopoulos K, Liu T, et al. Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease. Eur J Prev Cardiol. 2019;26(10):1067–1076.
17. Hasan B, Hansmann G, Budts W, et al. Challenges and Special Aspects of Pulmonary Hypertension in Middle- to Low-Income Regions: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(19):2463–2477.
18. Robson SC, Hunter S, Boys RJ, Dunlop W. Serial changes in pulmonary haemodynamics during human pregnancy: a non-invasive study using Doppler echocardiography. Clin Sci (Lond). 1991;80(2):113–117.
19. Hemnes AR, Kiely DG, Cockrill BA, et al. Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ. 2015;5(3):435–465.
20. Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89–94.
21. Kim DR, Wang E. Prevention of supine hypotensive syndrome in pregnant women treated with transcranial magnetic stimulation. Psychiatry Res. 2014;218(1–2):247–248.
22. Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol. 1993;169(6):1382–1392.
23. Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv. 1994;49(12 Suppl):S1–14.
24. Lopes van Balen VA, van Gansewinkel TAG, de Haas S, et al. Maternal kidney function during pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54(3):297–307.
25. Katz R, Karliner JS, Resnik R. Effects of a natural volume overload state (pregnancy) on left ventricular performance in normal human subjects. Circulation. 1978;58(3 Pt 1):434–441.
26. Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 2002;283(4):H1627–1633.
27. D’Alto M, Mahadevan VS. Pulmonary arterial hypertension associated with congenital heart disease. Eur Respir Rev. 2012;21(126):328–337.
28. Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13(2):276–284.
29. van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58(24):2511–2519.
30. Huston JH, Maron BA, French J, et al. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol. 2019;4(11):1112–1121.
31. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–1008.
32. Robson SC, Dunlop W, Boys RJ, Hunter S. Cardiac output during labour. Br Med J. 1987;295(6607):1169–1172.
33. Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317–329.
34. Hellgren M, Blomback M. Studies on blood coagulation and fibrinolysis in pregnancy, during delivery and in the puerperium. I. Normal condition. Gynecol Obstet Invest. 1981;12(3):141–154.
35. Rodger M, Sheppard D, Gandara E, Tinmouth A. Haematological problems in obstetrics. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):671–684.
36. Ateka-Barrutia O, Nelson-Piercy C. Connective tissue disease in pregnancy. Clin Med. 2013;13(6):580–584.
37. Schlichting LE, Insaf TZ, Zaidi AN, Lui GK, Van Zutphen AR. Maternal Comorbidities and Complications of Delivery in Pregnant Women With Congenital Heart Disease. J Am Coll Cardiol. 2019;73(17):2181–2191.
38. Vaught AJ, Kovell LC, Szymanski LM, et al. Acute Cardiac Effects of Severe Pre-Eclampsia. J Am Coll Cardiol. 2018;72(1):1–11.
39. Pieper PG, Hoendermis ES. Pregnancy in women with pulmonary hypertension. Neth Heart J. 2011;19(12):504–508.
40. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417–1431.
41. Barst RJ, Chung L, Zamanian RT, Turner M, McGoon MD. Functional class improvement and 3-year survival outcomes in patients with pulmonary arterial hypertension in the REVEAL Registry. Chest. 2013;144(1):160–168.
42. Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–172.
43. Suwanrath C, Thongphanang P, Pinjaroen S, Suwanugsorn S. Validation of modified World Health Organization classification for pregnant women with heart disease in a tertiary care center in southern Thailand. Int J Womens Health. 2018;10:47–53.
44. Farber HW, Miller DP, Poms AD, et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148(4):1043–1054.
45. Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6(5):711–721.
46. Augustine DX, Coates-Bradshaw LD, Willis J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):G11–G24.
47. Simon MA, Rajagopalan N, Mathier MA, Shroff SG, Pinsky MR, Lopez-Candales A. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest Heart Fail. 2009;15(6):271–276.
48. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1494–1563.
49. Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32(2):503–512.
50. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation. 2020;141(8):678–693.
51. Apter D, Briggs P, Tuppurainen M, et al. A 12-month multicenter, randomized study comparing the levonorgestrel intrauterine system with the etonogestrel subdermal implant. Fertil Steril. 2016;106(1):151–157 e155.
52. Clapp MA, Bernstein SN. Preconception Counseling for Women With Cardiac Disease. Curr Treat Options Cardiovasc Med. 2017;19(9):67.
53. Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):843.
54. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241.
55. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–622.
56. Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327(2):76–81.
57. Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111.
58. Jais X, Olsson KM, Barbera JA, et al. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40(4):881–885.
59. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436.
60. Bildirici I, Shumway JB. Intravenous and inhaled epoprostenol for primary pulmonary hypertension during pregnancy and delivery. Obstet Gynecol. 2004;103(5 Pt 2):1102–1105.
61. Garabedian MJ, Hansen WF, Gianferrari EA, et al. Epoprostenol treatment for idiopathic pulmonary arterial hypertension in pregnancy. J Perinatol. 2010;30(9):628–631.
62. Elliot CA, Stewart P, Webster VJ, et al. The use of iloprost in early pregnancy in patients with pulmonary arterial hypertension. Eur Respir J. 2005;26(1):168–173.
63. Wang T, Lu J, Li Q, et al. Rapid Titration of Intravenous Treprostinil to Treat Severe Pulmonary Arterial Hypertension Postpartum: a Retrospective Observational Case Series Study. Anesth Analg. 2019;129(6):1607–1612.
64. Duarte AG, Thomas S, Safdar Z, et al. Management of pulmonary arterial hypertension during pregnancy: a retrospective, multicenter experience. Chest. 2013;143(5):1330–1336.
65. Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151(4):1154–1160.
66. Luo J, Shi H, Xu L, Su W, Li J. Pregnancy outcomes in patients with pulmonary arterial hypertension: a retrospective study. Medicine. 2020;99(23):e20285.
67. Kiely DG, Condliffe R, Wilson VJ, Gandhi SV, Elliot CA. Pregnancy and pulmonary hypertension: a practical approach to management. Obstet Med. 2013;6(4):144–154.
68. Asfour V, Murphy MO, Attia R. Is vaginal delivery or caesarean section the safer mode of delivery in patients with adult congenital heart disease? Interact Cardiovasc Thorac Surg. Jul. 2013;17(1):144–150.
69. Wang J, Lu J. Anesthesia for Pregnant Women with Pulmonary Hypertension. J Cardiothorac Vasc Anesth. 2021;35(7):2201–2211.
70. Roldan T, Landzberg MJ, Deicicchi DJ, Atay JK, Waxman AB. Anticoagulation in patients with pulmonary arterial hypertension: an update on current knowledge. J Heart Lung Transplant. 2016;35(2):151–164.
71. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2Suppl):e691S–e736S.
72. Ballard W, Dixon B, McEvoy CA, Verma AK. Pulmonary Arterial Hypertension in Pregnancy. Cardiol Clin. 2021;39(1):109–118.
73. Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of Pregnancy in Patients With Complex Congenital Heart Disease: a Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135(8):e50–e87.
74. Qaiser KN, Tonelli AR. Novel Treatment Pathways in Pulmonary Arterial Hypertension. Methodist DeBakey Cardiovasc J. 2021;17(2):106–114.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.