Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Psoriatic Foot Involvement is the Most Significant Contributor to the Inconsistency Between PASI and DLQI: A Retrospective Study from China
Authors Yang J, Hu K, Li X, Hu J, Tan M , Zhang M, Chen J, Kuang Y
Received 8 December 2022
Accepted for publication 28 January 2023
Published 15 February 2023 Volume 2023:16 Pages 443—451
DOI https://doi.org/10.2147/CCID.S396997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jing Yang,1,2 Kun Hu,1,2 Xingyu Li,1,2 Jingjin Hu,1,2 Minjia Tan,1,2 Mi Zhang,1,2 Junchen Chen,1,2 Yehong Kuang1,2
1Department of Dermatology, Xiangya Hospital, Central South University, Changsa, Hunan, People’s Republic of China; 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsa, Hunan, People’s Republic of China
Correspondence: Yehong Kuang; Junchen Chen, Department of Dermatology, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan, 410000, People’s Republic of China, Tel +86-13574171102, Email [email protected]; [email protected]
Background: The Psoriasis Area and Severity Index (PASI) and the Dermatology Life Quality Index (DLQI) are important evaluation tools for assessing psoriasis severity and guiding treatment options. However, the scores of PASI and DLQI are often inconsistent.
Objective: This study aimed to identify the factors affecting the consistency between PASI and DLQI.
Methods: The retrospective study was based on 4125 patients. We collected the PASI, DLQI, demographic and clinical characteristics data.
Results: DLQI has a weak correlation with PASI (r=0.37; P< 0.001). For the DLQI > 10 groups, DLQI has almost no correlation with PASI (r=0.16; P< 0.001). There are 43.60% of mild-to-moderate patients (PASI< 10) in the DLQI> 10 groups. Our adjusted model showed that foot (OR=2.109; 95% CI:1.581– 2.815) involvement led to the greatest impairment of QoL except for PASI≥ 10 (OR=5.547; 95% CI:3.477– 8.845). Furthermore, DLQI impairment was associated with female (OR=1.336; 95% CI:1.071– 1.667); the age of 20– 39 subgroup (OR=1.795; 95% CI:1.100– 2.930); psoriatic arthritis (OR=1.718; 95% CI:1.208– 2.443); higher income (OR = 1.408; 95% CI: 1.067– 1.858); family history of psoriasis (OR=1.460; 95% CI:1.131– 1.885). Moreover, the influence of exposed lesions (such as scalp; face; neck; nails; and hands) were positively associated with severely impaired QoL.
Conclusion: Dermatologists should recognize the underestimated disease burden of psoriasis patients and actively identify and treat mild-to-moderate patients with high burden. In particular, the foot was a significant contributor to the burden.
Keywords: psoriasis, Dermatology Life Quality Index, Psoriasis Area and Severity Index, disease severity, retrospective study, foot
Introduction
Psoriasis is a chronic immune-mediated inflammatory disease characterized by silvery scaly plaques affecting over 60 million adults and children worldwide.1 This disease is not only associated with painful, debilitating, obvious physical symptoms but also with psychological impairments and increased prevalence of depression.2 The burden of psoriasis on Health-Related Quality of Life (HRQoL) is almost equivalent to cancer.3 Correct clinical treatment trade off decisions are necessary to mitigate the effects of psoriasis.
Clinical practice and clinical trials often assess psoriasis severity by the extent and intensity (erythema, scaling, and induration) of skin involvement, which is guided by the PASI.4 Psoriasis severity can also be measured by evaluating the quality of life (QoL), which can estimate the burden of psoriasis from the patients’ perspectives.5 Guidelines recommend that management and treatment of psoriasis require evaluation of PASI and DLQI.6 For example, the Rule of Tens describes “Current Severe Psoriasis” as if BSA involved > 10% or PASI > 10 or DLQI > 10.7 However, in light of existing literature, PASI was not a comprehensive indicator of disease severity, and the importance of the DLQI is underestimated.8 If the patient was assessed solely based on PASI, according to the “Rule of Tens”, patients were classified as “mild-to-moderate”. They only received topical therapy, even if their DLQI showed severe effects. Consistent with the gap, a survey from National Psoriasis Foundation revealed that 30% of “moderate psoriasis” received topical treatment solely, while 24–36% of patients felt they were untreated.9
In this retrospective study, DLQI, PASI, and demographic and clinical characteristics data were gathered from patients with psoriasis in China. The objectives of this study were to explore factors affecting the inconsistency between PASI and DLQI.
Materials and Methods
Study Group
This was a single-center retrospective study in patients with chronic plaque psoriasis in China (4125 patients, 1523 women). All patients came from the specialized psoriasis clinic at Xiangya Hospital. At the time of each patient visit, patients were asked if they would be willing to provide demographic information and photographs of their skin lesions for retrospective analysis. After obtaining informed consent from patients, doctors and nurses would conduct structured interviews and take photographs. The patients: (i) first visited at the Department of Dermatology, Xiangya Hospital, Central South University, from May 2016 to July 2021, (ii) fulfilled the diagnosis of plaque psoriasis in adults, and (iii) signed written informed consents. The study followed the Declaration of Helsinki and was approved by the institutional research ethics boards of Xiangya Hospital.
Methods
The demographic characteristics (age, gender, education level, body mass index, marital status, income) and clinical characteristics (age at onset, disease duration, family history, psoriatic arthritis, and the locations of psoriasis) were collected in a face-to-face interview by the experienced physicians. The body mass index (BMI) was calculated as the weight (kg)/height (m)2. The income was divided into three levels: low (<3000 USD/year), medium (3000–11,400 USD/year), and high (>11,400 USD/year). Currently, China’s annual per capita income is 35,000 RMB/year (4400 USD/year).
Disease severity was assessed using PASI. The assessments are converted into a single score ranging from 0 to 72, with a higher score associated with maximal disease activity. Psoriasis severity was coded mild (<3 PASI), moderate (3–10 PASI), and severe (>10 PASI). Moreover, we further counted whether the nails were involved and recorded the distribution of psoriatic lesions. The nails were evaluated independently. And each patient was photographed after the PASI evaluation. For the photographs, a trained nurse instructs the patient to take the photo in the photography room next to the clinic each time, and the photo equipment is a Canon camera. Each patient was required to take a full body photo and scalp, face, neck, upper arms, fore arms, hands, trunk, perineum, hips, thigh, lower legs, foot. If the nails are involved, additional photographs are taken. The distribution of psoriatic lesions was assessed by the experienced physicians through photographs. The distribution of psoriatic lesions is evaluated by an experienced physician through photographs and recorded on a special form.
DLQI is a self-administered questionnaire to assess the QoL of patients suffering from skin diseases.5 And the Chinese version of DLQI is a reliable and valid measure to assess QoL on Chinese psoriasis patients.10 The questionnaire consists of 10 questions covering six aspects of life: daily activities, leisure, symptoms and feelings, personal relationships, work and school, and treatment of the disease. Each item is scored from 0 to 3. The sum of the individual items provides the total score used for evaluation: 0 to 1 = no effect on patient’s life, 2–5 = mild effect, 6–10 = moderate effect, 11–20 = severe effect, and 21–30 = significant effect.11 Patients’ QoL impairment was coded as severe if DLQI>10, so we divided to two groups by DLQI≤10 and DLQI>10. All patient information and photos are stored in the Dermatology Biospecimen Data Base at Xiangya Hospital.
Statistical Analysis
All statistical tests were performed in SPSS 23 (IBM, SPSS Statistics 23). Continuous variables were presented as means ± standard deviation (S.D.). Discontinuous variables were described using percentages of each modality and were analyzed using the Chi-square test. A correlation analysis was performed using Spearman rank order correlation test. Variables having a P-value <0.1 on the univariate analysis were included in multivariable analysis. We did not impute other variables because of the low rate of missing values. Odds ratio (OR) and 95% confidence interval were used to present the effect size of the associations. P < 0.05 was considered indicative of a statistically significant difference.
Results
A total of 4125 patients (1523 females, 36.9%) with psoriasis were included in the study. We completed PASI scores for 3791 patients and ultimately recorded the specific involvement sites of psoriasis for 3417 patients (Supplementary Table 1). The nail assessment was assessed for a total of 4004 patients (Table 1). The mean age of all participants was 40.16±15.71 years; the mean disease duration was 8.50±8.67 years; the mean PASI was 8.86±8.14 (Table 1 and Table 2). Approximately 50% of patients reported moderate to severe QoL impairment (DLQI>5) (Figure 1).
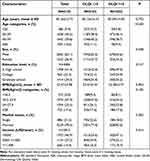 |
Table 1 DLQI Scores According to Demographic and Socioeconomic Factors in Patients with Psoriasis |
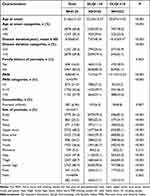 |
Table 2 DLQI Scores According to Clinical Factors in Patients with Psoriasis |
To detect correlations between PASI and DLQI, we performed two correlation analyses. Figure 2A shows that the correlation is moderate to low between DLQI and PASI (r=0.37, P<0.001, Figure 2A). However, the correlation between DLQI and PASI is almost irrelevant in the subjects who have severely impaired QoL (DLQI>10) (r=0.16, P<0.001, Figure 2B). In addition, we found there are 7.7% of mild patients (PASI<3) and 14.1% of moderate patients (PASI 3–10) reported DLQI>10 (Figure 1). Overall, these results indicate that the consistency between PASI and DLQI was poor. Taken together, we thought that PASI might not fully reflect the disease severity.
Clinical Features Affect the Consistency of PASI and DLQI
Even though PASI and DLQI are the indicators to assess the severity of psoriasis, they weigh different aspects of the disease.12 Firstly, to find the factors affecting DLQI, we divided all the subjects into two groups according to whether their DLQI scores were>10. We found no significant differences in age in the two groups, but the 20–39 subgroup is significantly higher in the DLQI>10 group after stratified analysis (46.1% vs 38.3%, P < 0.001). Compared to the DLQI≤10 group, the DLQI>10 group had a younger mean age at diagnosis (29.87±14.02 years vs 32.25±15.57 years, P < 0.001) and experienced a significantly longer duration of disease (7.87±8.34 years vs 10.42±9.37 years, P < 0.001). The DLQI >10 group showed significantly higher PASI scores than the DLQI≤10 group (13.15±10.23 vs 7.27±6.77, P < 0.001). Moreover, the percentage of family history of psoriasis and higher income and psoriasis arthritis, and marital status were slightly higher in the DLQI >10 group. No obvious differences were found in sex ratio, BMI, and education levels between the two groups (P>0.05, Table 1 and Table 2).
Secondly, the PASI is an objective evaluation standard, which has different weights according to the surface area of the four different body regions (head×0.1, arms×0.2, trunk×0.3, and legs×0.4). However, this weighting does not consider how psoriasis affects the small body areas (eg, foot, scalp, face, neck, hands, nails) and the burden of psoriasis on quality of life.13,14 We believe that this is the main reason for the consistency of PASI and DLQI. Hence, we compare the anatomical distribution of lesions in the two groups. It should be added that since some patients refused to be photographed. In the study, we performed a regional classification of lesions on a total of 3417 patients. For the sites of psoriasis, there were 708 patients were not rated and for nails 121 patients were not rated. We found that the proportion of small areas such as scalp (87.2% vs 79.4%, P < 0.001), face (34.7% VS 22.3%, P < 0.001), neck (22.6% vs 11.1%, P < 0.001), hands (36.6% vs 22.5%, P < 0.001), hips (63.0% vs 43.1%, P < 0.001), foot (22.2% vs 10.1%, P < 0.001) are significantly increased in the DLQI>10 group (Table 2).
Foot Involvements Had the Most Significant Impact on the Consistency Between PASI and DLQI
To verify the small body areas may increase the severity of psoriasis, which is less sensitive to change at PASI below 10. We used an adjusted model to examine the impact of small body areas on DLQI. The most striking finding was that foot most impacting DLQI (odds ratio [OR] 2.110; 95% confidence interval [CI]1.603–2.778). Moreover, the influence of exposed lesions (such as scalp, face, neck, nails, and hands) were positively associated with severely impaired QoL. In addition, female (OR=1.336, 95% CI:1.071–1.667), the age of 20–39 subgroup (OR=1.795, 95% CI: 1.100 −2.930), psoriatic arthritis (OR=1.718, 95% CI:1.208–2.443), higher income (OR = 1.408, 95% CI: 1.067–1.858), family history of psoriasis (OR=1.460, 95% CI:1.131–1.885) were associated with high impairment in DLQI (Table 3). In light of these data, we believe that small sites difficult to quantify by PASI do increase the severity of psoriasis, resulting in the inconsistency between PASI and DLQI. Moreover, the foot was the most important risk factor.
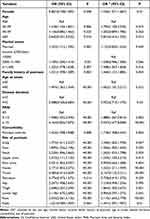 |
Table 3 Factors Affecting Quality of Life in Patients with Psoriasis |
Discussion
As mentioned earlier, many countries often used assessment indicators PASI and DLQI to assess the severity and monitor disease response to treatment, and guide reimbursement decisions.15–17 For example, European systemic treatment guidelines define moderate-to-severe psoriasis as PASI >10.15 Those with lower PASI would likely be treated with topical medications only even DLQI indicating severe disease. Hence, PASI may not fully measure the impact of disease severity, resulting in undertreatment. Moreover, there is limited research on the possible factors for the inconsistency of PASI and DLQI. Our study summarized the possible features that impact the inconsistency. Importantly, we found foot was the most important risk factor.
Consistent with former findings,18 our study also reported the inconsistency of PASI and DLQI by revealing a weak correlation between PASI and DLQI (r=0.37, P<0.001). Besides, we found that PASI and DLQI have almost no correlation between values of DLQI >10 from our real-world data (r=0.16, P<0.001), which in accordance with one study reported that patients with DLQI >10 also reported PASI mostly lower than 10.8 However, there are also some studies that reported a good correlation between PASI and DLQI. The possible reason for this is that only patients with PASI >10 were allowed to enter clinical trials.19,20 Additionally, in a Swedish registry study, decisions for biologic treatment were more strongly associated with PASI than DLQI, which demonstrated that the relevance of the DLQI may be underestimated in clinical practice.21
Our studies also supported that women, higher income, psoriatic arthritis, family history of psoriasis, and special sites (foot, scalp, face, neck, nails, hands) lead to a higher burden of disease is consistent with former studies.22–24 However, if they had a PASI lower than 10, they could easily be considered mild rather than moderate to severe psoriasis. The DLQI outperforms the PASI in terms of indicating patient suffering and social costs.25 Although the direct overall impression of the disease activity is predominated by the visual impression of skin disease as reflected by the PASI. It is remarkable that special sites were not sensitive to PASI can bring a high impact on functional or psychosocial well-being.26–28 Besides, the impact of palmoplantar psoriasis on QoL can be enormous when it affects walking or working. It may be due to the fact that the foot is a weight-bearing area, associated with daily activities, and that the plantar aspect of the foot is more dependent on topical medications.28 To rescue psoriasis patients with underestimated disease burden, we need to see the individual patient in their own specific setting. Thus, evaluation scales were developed that specifically measure disease impact in these areas such as Nail Psoriasis and Severity Index (NAPSI).29 Furthermore, new scoring systems that consider both the severity of psoriatic lesions and indicators of quality of life may provide better guidance in determining the required treatment options such as the Brigham Scalp Nail Inverse Palmoplantar Psoriasis Composite Index30 and Delphi Consensus.31
Several limitations of this study must be considered. Our results are based on an analysis of retrospective study, which does not permit longitudinal assessment of disease severity. However, our findings are from a cohort of more than four thousand patients with psoriasis.
Conclusion
In general, we should be aware that defining severe psoriasis is fraught. Our data showed the inconsistency between PASI and DLQI. Existing assessment criteria are not sufficient to measure patient burden. Data has also shown some features that need to be focused on the patients with lower PASI. Significantly, the foot is probably the most influential factor for inconsistency.
Ethics Approval
This study was approved by the institutional research ethics boards of Xiangya Hospital, Central South University, Changsha, China (Registration number:2018121106).
Acknowledgments
The authors would like to thank that Yehong Kuang Ph.D. initiated the study.
Author Contributions
All authors made significant contributions to the reported work, whether in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas; participated in drafting, revising or critically reviewing the article; gave final approval for the published version; agreed on agreed to submit the manuscript to the journal; and agreed to take responsibility for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (82073447,82003362,82003354), National key R & D program (2018YFC0117004).
Disclosure
The authors have no conflict of interest to declare in this work.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/S0140-6736(20)32549-6
2. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi:10.1111/jdv.13854
3. de Arruda LH, De Moraes AP. The impact of psoriasis on quality of life. Br J Dermatol. 2001;144(Suppl 58):33–36. doi:10.1046/j.1365-2133.2001.144s58033.x
4. Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51(4):563–569. doi:10.1016/j.jaad.2004.04.012
5. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
6. Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. doi:10.1007/s00403-010-1080-1
7. Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152(5):861–867. doi:10.1111/j.1365-2133.2005.06502.x
8. Golbari N, van der Walt JM, Blauvelt A, et al. Psoriasis severity: commonly used clinical thresholds may not adequately convey patient impact. J Eur Acad Dermatol Venereol. 2021;35(2):417–421. doi:10.1111/jdv.16966
9. Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013;149(10):1180–1185. doi:10.1001/jamadermatol.2013.5264
10. He Z, Lu C, Basra MKA, et al. Psychometric properties of the Chinese version of Dermatology Life Quality Index (DLQI) in 851 Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2013;27(1):109–115. doi:10.1111/j.1468-3083.2011.04371.x
11. Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi:10.1111/j.0022-202X.2005.23621.x
12. Drummond MF, Schwartz JS, Jönsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24(3):244–258. doi:10.1017/S0266462308080343
13. McKenna KE, Stern RS. The outcomes movement and new measures of the severity of psoriasis. J Am Acad Dermatol. 1996;34(3):534–538. doi:10.1016/S0190-9622(96)90469-7
14. Jacobson CC, Kimball AB. Rethinking the Psoriasis Area and Severity Index: the impact of area should be increased. Br J Dermatol. 2004;151(2):381–387. doi:10.1111/j.1365-2133.2004.06035.x
15. Nast A, Gisondi P, Ormerod AD, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. doi:10.1111/jdv.13354
16. Egeberg A, See K, Garrelts A, et al. Epidemiology of psoriasis in hard-to-treat body locations: data from the Danish skin cohort. BMC Dermatol. 2020;20(1):3. doi:10.1186/s12895-020-00099-7
17. Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. Part 1: on efficacy and choice of treatment. Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104(8):694–709. doi:10.1016/j.ad.2013.04.003
18. Amy de la Breteque M, Beauchet A, Quiles-Tsimaratos N, et al. Characteristics of patients with plaque psoriasis who have discordance between Psoriasis Area Severity Index and dermatology life quality index scores. J Eur Acad Dermatol Venereol. 2017;31(5):e269–e272. doi:10.1111/jdv.14021
19. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled Phase 3 trial. Lancet. 2021;397(10273):487–498. doi:10.1016/S0140-6736(21)00125-2
20. Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi:10.1016/S0140-6736(15)60125-8
21. Hagg D, Sundström A, Eriksson M, et al. Decision for biological treatment in real life is more strongly associated with the Psoriasis Area and Severity Index (PASI) than with the Dermatology Life Quality Index (DLQI). J Eur Acad Dermatol Venereol. 2015;29(3):452–456. doi:10.1111/jdv.12576
22. Scala E, Kaczmarczyk R, Zink A, et al. Sociodemographic, clinical and therapeutic factors as predictors of life quality impairment in psoriasis: a cross-sectional study in Italy. Dermatol Ther. 2022;35(8):e15622. doi:10.1111/dth.15622
23. Hawro T, Zalewska A, Hawro M, et al. Impact of psoriasis severity on family income and quality of life. J Eur Acad Dermatol Venereol. 2015;29(3):438–443. doi:10.1111/jdv.12572
24. Guillet C, Seeli C, Nina M, et al. The impact of gender and sex in psoriasis: what to be aware of when treating women with psoriasis. Int J Womens Dermatol. 2022;8(2):e010. doi:10.1097/JW9.0000000000000010
25. Schmitt JM, Ford DE. Work limitations and productivity loss are associated with health-related quality of life but not with clinical severity in patients with psoriasis. Dermatology. 2006;213(2):102–110. doi:10.1159/000093848
26. Augustin M, Sommer R, Kirsten N, et al. Topology of psoriasis in routine care: results from high-resolution analysis of 2009 patients. Br J Dermatol. 2019;181(2):358–365. doi:10.1111/bjd.17403
27. Carrascosa JM, Plana A, Ferrandiz C. Effectiveness and safety of psoralen-UVA (PUVA) topical therapy in palmoplantar psoriasis: a report on 48 patients. Actas Dermosifiliogr. 2013;104(5):418–425. doi:10.1016/j.ad.2012.12.009
28. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49(2):271–275. doi:10.1067/S0190-9622(03)01479-8
29. Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–212. doi:10.1067/S0190-9622(03)00910-1
30. Patel M, Liu SW, Qureshi A, et al. The Brigham Scalp Nail Inverse Palmoplantar Psoriasis Composite Index (B-SNIPI): a novel index to measure all non-plaque psoriasis subsets. J Rheumatol. 2014;41(6):1230–1232. doi:10.3899/jrheum.140177
31. Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122. doi:10.1016/j.jaad.2019.08.026
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


