Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
Psoriasis Patients with Specific HLA-Cw Alleles and Lower Plasma IL-17 Level Show Improved Response to Topical Lindioil Treatment
Authors Lin YK, Wang CY , Huang YH, Chang YC , Chen CB , Wang CW , Hui RCY, Chung WH
Received 27 November 2021
Accepted for publication 4 March 2022
Published 13 May 2022 Volume 2022:15 Pages 515—524
DOI https://doi.org/10.2147/PGPM.S351452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Yin-Ku Lin,1,2 Ching-Ya Wang,3,4 Yu-Huei Huang,3,4 Ya-Ching Chang,3,4 Chun-Bing Chen,3– 9 Chuang-Wei Wang,3,5– 7,* Rosaline Chung-Yee Hui,3,4,* Wen-Hung Chung3,5– 12,*
1Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Keelung, Taiwan; 2School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan; 4College of Medicine, Chang Gung University, Taoyuan, Taiwan; 5Cancer Vaccine and Immune Cell Therapy Core Laboratory, Chang Gung Memorial Hospital, Linkou, Taiwan; 6Chang Gung Immunology Consortium, Chang Gung Memorial Hospital and Chang Gung University, Linkou, Taiwan; 7Department of Dermatology, Xiamen Chang Gung Hospital, Xiamen, People’s Republic of China; 8Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan; 9Immune-Oncology Center of Excellence, Chang Gung Memorial Hospital, Linkou, Taiwan; 10Department of Dermatology, Beijing Tsinghua Chang Gung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 11School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 12Genomic Medicine Core Laboratory, Chang Gung Memorial Hospital, Linkou, Taiwan
*These authors contributed equally to this work
Correspondence: Wen-Hung Chung, Chang Gung Memorial Hospital, Linkou Branch, No. 5, Fusing St, Taoyuan, 333, Taiwan, Tel +886 3-3281200 #8495, Fax +886 3-3281200 #2206, Email [email protected]; [email protected]
Purpose: Lindioil, a medicine refined from indigo naturalis (a herb used in Chinese medicine), is effective in treating severe psoriasis; however, responses vary across individual patients. We aim to investigate genetic predispositions associated with treatment response to topical Lindioil among patients with psoriasis and correlations with plasma cytokine patterns.
Patients and Methods: We enrolled 72 psoriasis patients treated with Lindioil ointment and analyzed the human leukocyte antigen class C (HLA-Cw) genotypes and plasma cytokine expression patterns. We developed regression models of treatment response, defined as Psoriasis Area and Severity Index (PASI) 75, to examine correlations among HLA-Cw alleles, cytokine levels, and treatment response to Lindioil.
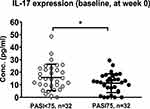
Results: Patients harboring HLA-Cw*06:02 were significantly more likely to respond to Lindioil (P = 0.02, odds ratio [OR]: 6.88), whereas Lindoil was ineffective in those harboring HLA-Cw*01:02 (P = 0.01, OR: 0.28). Patients who were HLA-Cw*06:02-positive or HLA-Cw*01:02-negative had better PASI scores and body surface area (BSA) improvement (73.3% vs 44.4%, P< 0.001) following an 8-week treatment period. Psoriasis patients achieving PASI 75 after 8 weeks presented with lower baseline plasma interleukin-17 (IL-17) levels than those who did not achieve PASI 75 (PASI 75: 11.28 pg/mL vs PASI < 75: 15.82 pg/mL, P = 0.05).
Conclusion: Our findings suggest that the presence of the HLA-Cw*06:02 or HLA-Cw*01:02 alleles and plasma IL-17 levels are predictive markers of treatment response to Lindioil ointment in patients with psoriasis.
Keywords: HLA-Cw*06:02, HLA-Cw*01:02, indigo naturalis, Lindioil, IL-17, psoriasis
Introduction
Psoriasis is a common, chronic, immune-mediated, inflammatory skin disease. Psoriasis results from an aberrant innate or adaptive immune response associated with T lymphocytes that leads to epidermal hyperplasia1 and is characterized by salmon-pink plaques with heavy silver scales.2 Psoriasis has negative effects on the patient’s health and quality of life, including psychosocial and economic impacts.3,4 The disease pathogenesis represents a complex interplay among genetic, environmental, and immunological factors. Certain alleles encoding human leukocyte antigen-Cw (HLA-Cw), such as HLA-Cw*06:02, have been identified as important genetic determinants of psoriasis development.5 HLA-Cw gene expression has been linked to psoriasis incidence, severity, phenotypic features, and treatment outcomes.6 The presence of HLA-Cw*06:02 has been suggested to serve as a predictive marker for the clinical response to ustekinumab (IL-12/23 antibody) and methotrexate treatment in psoriasis.7,8 Various therapies with different targets have been developed to alleviate psoriasis symptoms, including systemic therapy, phototherapy, topical therapy, and traditional Chinese medicine (TCM). The topical application of ointments developed from indigo naturalis a commonly used herb in TCM, has been proposed as an attractive treatment option with lower costs and reduced occurrence of long-term side effects compared with other anti-psoriasis treatments.9,10
Indigo naturalis is derived from a blue powder extracted from indigo plants, such as Baphicacanthus cusia.11 Indigo naturalis has been widely used in TCM for the treatment of inflammatory diseases and dermatosis for centuries.12 Indigo naturalis has been demonstrated to have anti-inflammatory and anti-proliferative properties through several mechanisms, mediated by its active components, including indirubin, indigo, and tryptanthrin.13 Indirubin, the important primary active component, inhibits the proliferation of epidermal keratinocytes through the inhibition of cyclin-dependent kinase14,15 and epidermal growth factor receptor (EGFR) activation.16,17 In addition, indirubin suppresses skin inflammation by inhibiting cytokine production, nuclear factor kappa B (NF-κB) activity, and mitogen-activated protein kinase signaling.18,19 Tryptanthrin also possesses anti-inflammatory properties that attenuate the T-helper 17 (Th17) signature12 and downregulate the IL-17 pathway.20 Another component, indigo, is less active than the other two,10 but both indigo and indirubin activate the aryl hydrocarbon receptor signaling pathway to modulate the immune response.21,22 Indigo naturalis in its crude form has also been shown to reduce T cell adhesion by suppressing tumor necrosis factor (TNF)-α–induced expression of vascular cell adhesion molecule 1 (VCAM-1).23
Lindioil is a refined form of indigo naturalis ointment powder, with the original blue color removed to prevent staining (staining of skin and clothes was the reason for poor patient compliance with crude indigo naturalis ointment); Lindoil remains a safe and effective treatment.10 Our Clinical trial11 showed that 200 µg/g of indirubin in Lindioil ointment was the most effective concentration for topical psoriasis treatment. However, treatment response varied across individual patients, and the underlying mechanisms that lead to differential responses remain unclear. Although the HLA-Cw allele and the IL-17/IL-23 mediated pathway are regarded as important factors in the response to psoriasis treatment, the associations among HLA allele status, plasma cytokine levels, and response to Lindioil treatment have not yet been thoroughly explored. Therefore, to improve our ability to provide precise and effective treatment options, we attempted to identify useful biomarkers for predicting the treatment response to Lindioil ointment among psoriasis patients.
Materials and Methods
Study Population
Between November 2012 and April 2014, 72 patients were identified for study inclusion who were diagnosed with plaque psoriasis for at least 1 year, with <20% of their body surface area (BSA) affected by psoriasis, and a Psoriasis Area and Severity Index (PASI) sCore <20. Washout periods of at least 4 weeks following the end of systemic therapy or phototherapy regimens and at least 2 weeks following the end of topical therapies were required prior to the initiation of study medication. All patients were previously enrolled in our clinical trial study, which is registered with ClinicalTrials.gov (identifier NCT01735864), and were treated with Lindioil for at least 8 weeks. After completion of the clinical trial, we used the remaining DNA and plasma samples from enrolled patients to perform HLA-Cw genotyping and evaluate cytokine expression, respectively. All participants provided written informed consent and agreed to the use of their samples for this study (IRB No. 201507317B0). The study was conducted at Chang Gung Memorial Hospital, Linkou, and Taipei branches. The protocol was approved by the institutional review boards of Chang Gung Memorial Hospital in Taiwan. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices.
Human Leukocyte Antigen Genotyping
All 72 patients were genotyped for HLA-Cw alleles using SeCore HLA sequence–based typing (SBT) (Invitrogen, Life Technologies, Carlsbad, CA). The HLA-Cw genotyping data for 507 individuals in the general Chinese population with no history of severe skin disorders was obtained as described previously.24
Cytokine Level Analysis
The 64 patients with psoriasis were analyzed for cytokine expression patterns, including IL-6, IL-17, IL-22, IL-23, and TNF-α. The concentrations of these cytokines in plasma were determined by using the Bio-Plex pro assays (Bio-Rad Laboratories, Irvine, California), according to the manufacturer’s instructions. All samples were analyzed in duplicate.
Definition of Treatment Response
PASI scores were recorded at baseline and weeks 8, 12, and 20 following initiation of treatment. The mean percentage of PASI score improvement at weeks 8, 12, and 20 were calculated, and the proportions of patients achieving at least 50% reduction (PASI 50), 75% reduction (PASI 75), and 90% reduction (PASI 90) at each time point were compared between HLA genotypes. Positive treatment response was defined as at least PASI 75 relative to baseline. Patients achieving PASI 75 were considered good responders, whereas those who did not achieve PASI 75 were classified as poor responders. We evaluated the mean percentage of improvement in PASI and BSA scores at weeks 8, 12, and 20.
Statistical Analyses
Statistical analysis was performed using SPSS for Windows software version 21.0 (IBM Corp, Armonk, NY). Data are presented as the number (percentage) for categorical variables and as the mean ± standard deviation (SD) for continuous variables. Differences were evaluated using Fisher’s exact test for categorical variables, whereas an independent two-tailed t-test was used to evaluate differences in continuous variables. Only those variables with P<0.05 in univariate analyses were included in the multiple regression analysis for treatment response. A two-tailed P-value <0.05 was considered significant.
Results
Table 1 shows the demographic and clinical characteristics of the 72 patients with psoriasis (including 43 men and 29 women) treated with Lindioil included in this study. The mean age of the patients was 35.3±8.2 years, and the mean body mass index was 24.6±4.1 kg/m2. The mean duration of psoriasis was 11.2±7.5 years, and all patients (100%) had received prior treatment, including 2 patients treated with the biologic etanercept. In addition, 98.6% used topical therapy, and 76.4% of patients used Chinese herbs in either oral or topical forms. The mean PASI score and BSA% affected by psoriasis at baseline were 11.8±4.2 and 10.6%±6.0%, respectively. The mean duration of Lindioil treatment was 17.0±5.6 weeks. After 8 weeks of treatment, 51.4% had achieved at least PASI 50, and 47.2% of patients had achieved PASI 75. However, 5 patients (6.9%) experienced treatment-related adverse events (AEs). Treatment-related AEs in our study included acute psoriasis flare-ups, contact dermatitis, allergic exanthema, eczema, and skin inflammation.
 |
Table 1 Demographic and Clinical Characteristics of Enrolled Study Population |
We first performed SBT to determine the individual HLA-Cw genotypes among our study population (Supplementary Table S1) and found that the HLA-Cw*06:01 allele was significantly associated with psoriasis compared with 507 healthy controls with no history of severe skin disorders (P=0.0008, Supplementary Table S2). Further analysis of the correlations between HLA-Cw status and treatment response to Lindioil revealed that patients had poorer responses when carrying the HLA-Cw*01:02 allele (good responders vs poor responders carrying the HLA-Cw*01:02 allele, 29.4% vs 57.9%; odds ratio [OR]: 0.60, 95% confidence interval [CI], 0.39–0.92; P=0.019, Table 2), whereas those had better responses when carrying the HLA-Cw*06:02 allele (good responders vs poor responders carrying the HLA-Cw*06:02 allele, 29.4% vs 7.9%; OR: 1.31; 95% CI: 1.03–1.65; P=0.03, Table 2). In the multivariate analysis, HLA-Cw*01:02 was associated with the treatment response (OR: 0.35; 95% CI” 0.13–0.96; P=0.042, Supplementary Table S3). No significant differences of demographic, clinical features and adverse event were observed between the good and poor responder groups (Table 2). To improve the sensitivity to predict the response to Lindioil treatment, we combined these two associated HLA-Cw alleles and found that 73.5% (25 in 34 patients) patients who achieved PASI 75 (good responders) at week 8 were HLA-Cw*01:02-negative or HLA-Cw*06:02-positive (P=0.031, Table 2).
 |
Table 2 Demographic and Clinical Characteristics of Good and Poor Responders Treated with Lindioil Ointment |
We further analyzed and compared these two HLA genes after 20 weeks of Lindioil treatment. Among our cohort (Table 3 and Figure 1A–C), 32 patients (44.4%) were HLA-Cw*01:02-positive and 40 patients (55.6%) were HLA-Cw*01:02-negative. The HLA-Cw*01:02-negative group had a longer psoriasis duration than the HLA-Cw*01:02-positive group (mean 12.9 years vs 9.1 years, P=0.037). No other significant differences in clinical baseline parameters were identified between HLA-Cw*01:02 groups. The percentage of HLA-Cw*01:02-negative patients who achieved PASI 75 (good responders) was higher than that of HLA-Cw*01:02-positive patients (60.0% vs 31.3%, P=0.019) at week 8, and HLA-Cw*01:02-negative patients had better PASI improvements (score improvement 64.8% vs 46.3%, P=0.018) and BSA improvements (55.2% vs 28.6%, P=0.036) after 8 weeks of Lindioil treatment. HLA-Cw*01:02-negative patients also showed significantly larger improvements in mean PASI and BSA after 12 (PASI: 67.8% vs 50.0%, P=0.018; BSA: 62.0% vs 33.6%, P=0.026) and 20 weeks of treatment (PASI: 70.4% vs 51.6%, P=0.017; BSA: 65.1% vs 38.1%, P=0.041).
 |
Table 3 Demographic and Clinical Characteristics of HLA-Cw*01:02 Positive/Negative Patients Treated with Lindioil |
We then analyzed the HLA-Cw*06:02 allele (Table 4 and Figure 1D–F) and found that 13 patients (18.1%) were HLA-Cw*06:02-positive, and 59 patients (81.9%) were HLA-Cw*06:02-negative. No significant differences were identified in any clinical baseline parameters between these two groups. The percentage of HLA-Cw*06:02-positive patients who achieved PASI 75 (good responders) was higher than the percentage of HLA-Cw*06:02-negative patients (76.9% vs 40.7%, P=0.029), and HLA-Cw*06:02-positive patients presented with larger improvements in PASI (74.6% vs 52.6%, P=0.005) and BSA (73.3% vs 36.8%, P<0.001) after 8 weeks of Lindioil treatment. In addition, we observed that HLA-Cw*06:02-positive patients showed significantly larger improvements in mean PASI and BSA after 12 (PASI: 75.0% vs 56.5%, P=0.013; BSA: 79.3% vs 42.7%, P=0.001) and 20 weeks of treatment (PASI: 81.4% vs 57.8%, P=0.001; BSA: 84.9% vs 46.1%, P<0.001). Similarly, HLA-Cw*06:02-positive patients achieved PASI 75 more frequently at all time points, although no significant difference was observed between the two groups at week 20. We also analyzed the relationships between response outcomes, AEs, and HLA-Cw alleles and found no significant associations (Supplementary Table S4).
 |
Table 4 Demographic and Clinical Characteristics of HLA-Cw*06:02 Positive/Negative Patients Treated with Lindioil |
The cytokine analysis showed that patients who achieved PASI 75 after 8 weeks of treatment presented with lower baseline plasma IL-17 expression levels than those who did not achieve PASI 75 after 8 weeks (11.28 pg/mL vs 15.82 pg/mL, P=0.05, Figure 2), whereas no significant differences were identified in other baseline cytokine expression levels, including IL-6, IL-22, IL-23 and TNF-α (Supplementary Table S5). No significant differences in baseline cytokine levels were observed between patients who did and did not achieve PASI 50 after 8 weeks of treatment.
Discussion
The application of predictive genetic markers to treatment selection could reveal whether a given treatment is likely to result in a good response for the patient. Psoriasis is a chronic, long-term disease with a long average duration, and the identification of more effective treatments is a high priority for patients. HLA-Cw*06:02 has previously been identified as a major genetic susceptibility allele for psoriasis,24 and the findings of the present study are consistent with previous genetic findings. Both methotrexate and ustekinumab have been shown to be more effective in HLA-Cw*06:02-positive patients,7,8,25 whereas no relationships have been identified between HLA-Cw6 status and treatment response to secukinumab,26 etanercept, or infliximab.27 Another biologic agent, adalimumab, was found to result in a poorer response than ustekinumab in HLA-Cw*06:02-positive patients.28 Moreover, fewer methotrexate treatment-related AEs were reported among HLA-Cw*06:02-positive patients.7 HLA-Cw*01 frequently appears among patients with psoriasis in several countries, particularly Asian countries, with a frequency ranging from 2.4% to 27%.29–33 Cai and others highlighted that HLA-Cw*01:02 is a psoriasis-associated allele in Southern Han Chinese. Moreover, HLA-Cw*01:02-positive patients tended to show no response to conventional therapies.30,34 In our study, 32 of 72 (44.4%) participants were HLA-Cw*01:02-positive, and all patients had received conventional psoriasis treatment prior to our study. The relatively high HLA-Cw*01:02 frequency of our study population may indicate a tendency for this population to experience an overall poor response to conventional therapies, highlighting the need to develop alternative treatment strategies. The identification of gene status among patients with psoriasis can improve personalized medicine approaches, preventing the waste of time and money on treatments that are unlikely to be effective. However, currently, no evidence exists to support a relationship between genetic factors and the treatment response to Lindioil, a fast-acting topical therapy for psoriasis patients. In this study, we used the DNA database from our previous clinical trial11 to analyze the correlation between HLA-Cw allele status and the treatment response to Lindioil.
We identified that HLA-Cw*06:02-positive and HLA-Cw*01:02-negative patients were more likely to be good responders to Lindioil treatment than patients with other allele presentations. Our results further revealed that HLA-Cw*06:02-positive and HLA-Cw*01:02-negative patients showed better responses to Lindioil therapy than patients with other allele presentations across several response evaluations, including mean PASI improvement, the proportion of patients achieving PASI 75, and mean BSA improvement. When examining clinical baseline parameters, HLA-Cw*01:02-negative patients were found to have longer psoriasis history; however, no association was reported between disease duration and treatment response by Edson-Heredia and others.35 Among those with good responses to Lindioil treatment, 73.5% were HLA-Cw*01:02-negative or HLA-Cw*06:02-positive, suggesting that both the HLA-Cw*01:02 and HLA-Cw*06:02 alleles participate in the pharmacological mechanism underlying the effects of Lindioil. The finding that HLA-Cw*01:02-positive patients showed a worse response to Lindioil than HLA-Cw*01:02-negative patients is consistent with one alefacept response study.30 Further study remains necessary to determine the pathogenic mechanisms associated with HLA-Cw*01:02 in psoriasis and the biogenetic mechanism that determines the response of patients with HLA-Cw*01:02 or HLA-Cw*06:02 alleles to Lindioil treatment.
The reduced incidence of treatment-related AEs is among the advantages of topical Lindioil use. Among the 6 patients who experienced Lindioil-related AEs, 2 were HLA-Cw*01:02-negative, and only 1 was HLA-Cw*06:02-positive. None of these 6 patients were both HLA-Cw*01:02-negative and HLA-Cw*06:02-positive. Although an insufficient number of cases experienced AEs for statistical analyses, our findings suggested that HLA-Cw*06:02-positive and HLA-Cw*01:02-negative patients were less likely to suffer from treatment-related AEs than patients with other allelic combinations. We also identified that the IL-17 signaling pathway is involved in the treatment response to Lindioil. Significantly decreased plasma levels of IL-6, IL-17, IL-22, IL-23, and TNF-α were observed in good responders compared with poor responders. The primary active compounds in indigo naturalis have been reported to attenuate IL-17A expression, and our results indicate that topical treatment with Lindioil ointment in patients with psoriasis may also decrease the plasma expression levels of psoriasis-related cytokine expression. These findings indicate that psoriasis can be regarded as a systemic disease, and Lindioil may significantly decrease the Th17 cell population or associated pathways. In addition, patients who achieved PASI 75 after 8 weeks of Lindioil treatment presented with lower baseline plasma IL-17 expression; however, IL-17 was the only psoriasis-related cytokine found to be significantly reduced in patients with better treatment response, making this unlikely to reflect an overall reduction in inflammation status among the good-responder population. However, patients with a poor response to Lindioil may require combination treatments using other Medicines (eg, traditional immunosuppressants or anti-IL-23/IL-12 biologic agents) or therapeutic approaches (phototherapy) to improve treatment response and suppress psoriasis-related cytokine expression.
One of the limitations of this study was the number of cases recruited at two hospital centers. Not all patients with psoriasis who were treated during the trial study period agreed to the extraction of sufficient blood samples for the performance of genetic analyses; however, all patients who received a genetic analysis were included in our cohort study. We were unable to demonstrate any significant correlation between HLA-Cw allele status and cytokine expression patterns among patients with psoriasis (data not shown), and only five types of psoriasis-related cytokines were investigated. Cytokine expression analysis performed using skin specimens obtained from psoriasis lesion might be more strongly correlated with disease severity and treatment response than the cytokine expression pattern in plasma. Large-scale studies remain necessary to fully evaluate the influence of HLA-Cw allele status and cytokine expression patterns on the response to Lindioil treatment.
Conclusion
In conclusion, HLA-Cw*01:02-negative and HLA-Cw*06:02-positive status and lower plasma IL-17 levels were correlated with a good Lindioil treatment response among psoriasis patients. Our study provides predictive markers for treatment response which may lead to improvements in the topical Lindioil treatment in patients with psoriasis.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from first author, Yin-Ku Lin, on reasonable request.
Acknowledgment
We thank Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan, for supporting the HLA control database in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-182A-037-MY3, 107-2320-B-182A-002-MY3, 108-2622-B-182A-003-CC2, 108-2314-B-182A-104-MY3, 108-2320-B-182A-024-MY2, 108-2314-B-182A-006-MY3, and 109-2326-B-182A-001-, 110-2320-B-182A-014 -MY3) and Chang Gung Memorial Hospital (CLRPG3J0011-3, CMRPG3I0381-2, CORPG1J0011~3, CORPG3J0321-3, and CMRPG3K0561-2).
Disclosure
The authors declare no conflicts of interest for this work and that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi:10.1146/annurev-pathol-011811-132448
2. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi:10.1016/s0140-6736(07)61128-3
3. Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59(5):772–780. doi:10.1016/j.jaad.2008.06.043
4. Tadros A, Vergou T, Stratigos AJ, et al. Psoriasis: is it the tip of the iceberg for the quality of life of patients and their families? J Eur Acad Dermatol Venereol. 2011;25(11):1282–1287. doi:10.1111/j.1468-3083.2010.03965.x
5. Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4(3):e1000041. doi:10.1371/journal.pgen.1000041
6. Borroni RG, Costanzo A. HLA-C*06 and psoriasis: susceptibility, phenotype, course and response to treatment. Br J Dermatol. 2018;178(4):825. doi:10.1111/bjd.16412
7. West J, Ogston S, Berg J, et al. HLA-Cw6-positive patients with psoriasis show improved response to methotrexate treatment. Clin Exp Dermatol. 2017;42(6):651–655. doi:10.1111/ced.13100
8. Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. Br J Dermatol. 2014;171(5):1181–1188. doi:10.1111/bjd.13056
9. Lin YK, Yen HR, Wong WR, Yang SH, Pang JH. Successful treatment of pediatric psoriasis with Indigo naturalis composite ointment. Pediatr Dermatol. 2006;23(5):507–510. doi:10.1111/j.1525-1470.2006.00295.x
10. Lin YK, See LC, Huang YH, et al. Comparison of refined and crude indigo naturalis ointment in treating psoriasis: randomized, observer-blind, controlled, intrapatient trial. Arch Dermatol. 2012;148(3):397–400. doi:10.1001/archdermatol.2011.1091
11. Lin YK, See LC, Huang YH, Chi CC, Hui RC. Comparison of indirubin concentrations in indigo naturalis ointment for psoriasis treatment: a randomized, double-blind, dosage-controlled trial. Br J Dermatol. 2018;178(1):124–131. doi:10.1111/bjd.15894
12. Cheng HM, Kuo YZ, Chang CY, et al. The anti-TH17 polarization effect of Indigo naturalis and tryptanthrin by differentially inhibiting cytokine expression. J Ethnopharmacol. 2020;255:112760. doi:10.1016/j.jep.2020.112760
13. Lin YK, Wong WR, Chang YC, et al. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque-type psoriasis. Dermatology. 2007;214(2):155–161. doi:10.1159/000098576
14. Moon MJ, Lee SK, Lee JW, et al. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg Med Chem. 2006;14(1):237–246. doi:10.1016/j.bmc.2005.08.008
15. Marko D, Schätzle S, Friedel A, et al. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer. 2001;84(2):283–289. doi:10.1054/bjoc.2000.1546
16. Lin YK, Leu YL, Yang SH, Chen HW, Wang CT, Pang JH. Anti-psoriatic effects of indigo naturalis on the proliferation and differentiation of keratinocytes with indirubin as the active component. J Dermatol Sci. 2009;54(3):168–174. doi:10.1016/j.jdermsci.2009.02.007
17. Hsieh WL, Lin YK, Tsai CN, Wang TM, Chen TY, Pang JH. Indirubin, an acting component of indigo naturalis, inhibits EGFR activation and EGF-induced CDC25B gene expression in epidermal keratinocytes. J Dermatol Sci. 2012;67(2):140–146. doi:10.1016/j.jdermsci.2012.05.008
18. Hoessel R, Leclerc S, Endicott JA, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1(1):60–67. doi:10.1038/9035
19. Lin YK, Chen HW, Leu YL, et al. Indigo naturalis upregulates claudin-1 expression in human keratinocytes and psoriatic lesions. J Ethnopharmacol. 2013;145(2):614–620. doi:10.1016/j.jep.2012.11.044
20. Cheng HM, Wu YC, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of Indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17(1):439. doi:10.1186/s12906-017-1947-1
21. Bungsu I, Kifli N, Ahmad SR, Ghani H, Cunningham AC. Herbal Plants: the Role of AhR in Mediating Immunomodulation. Mini Rev. 2021;12(2491). doi:10.3389/fimmu.2021.697663
22. Peter Guengerich F, Martin MV, McCormick WA, Nguyen LP, Glover E, Bradfield CA. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch Biochem Biophys. 2004;423(2):309–316. doi:10.1016/j.abb.2004.01.002
23. Chang HN, Pang JH, Yang SH, et al. Inhibitory effect of indigo naturalis on tumor necrosis factor-α-induced vascular cell adhesion molecule-1 expression in human umbilical vein endothelial cells. Molecules. 2010;15(9):6423–6435. doi:10.3390/molecules15096423
24. Chen L. Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178(4):854–862. doi:10.1111/bjd.16083
25. Indhumathi S, Rajappa M, Chandrashekar L, Ananthanarayanan PH, Thappa DM, Negi VS. Pharmacogenetic markers to predict the clinical response to methotrexate in south Indian Tamil patients with psoriasis. Eur J Clin Pharmacol. 2017;73(8):965–971. doi:10.1007/s00228-017-2255-x
26. Papini M, Cusano F, Romanelli M, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: results from extension phase of the SUPREME study. Br J Dermatol. 2019;181(2):413–414. doi:10.1111/bjd.18013
27. Gallo E, Cabaleiro T, Román M, et al. The relationship between tumour necrosis factor (TNF)-α promoter and IL12B/IL-23R genes polymorphisms and the efficacy of anti-TNF-α therapy in psoriasis: a case-control study. Br J Dermatol. 2013;169(4):819–829. doi:10.1111/bjd.12425
28. Dand N, Duckworth M, Baudry D, et al. HLA-C*06:02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol. 2019;143(6):2120–2130. doi:10.1016/j.jaci.2018.11.038
29. Cai M, Huang H, Ran D, et al. HLA-C*01:02 and HLA-A*02:07 confer risk specific for psoriatic patients in Southern China. J Invest Dermatol. 2019;139(9):2045–2048.e4. doi:10.1016/j.jid.2019.02.027
30. Chiu HY, Huang PY, Jee SH, et al. HLA polymorphism among Chinese patients with chronic plaque psoriasis: subgroup analysis. Br J Dermatol. 2012;166(2):288–297. doi:10.1111/j.1365-2133.2011.10688.x
31. Nakagawa H, Asahina A, Akazaki S, et al. Association of Cw11 in Japanese patients with psoriasis vulgaris. Tissue Antigens. 1990;36(5):241–242. doi:10.1111/j.1399-0039.1990.tb01835.x
32. Szczerkowska Dobosz A, Rebała K, Szczerkowska Z, Nedoszytko B. HLA-C locus alleles distribution in patients from northern Poland with psoriatic arthritis–preliminary report. Int J Immunogenet. 2005;32(6):389–391. doi:10.1111/j.1744-313X.2005.00543.x
33. Zhang XJ, Zhang AP, Yang S, et al. Association of HLA class I alleles with psoriasis vulgaris in southeastern Chinese Hans. J Dermatol Sci. 2003;33(1):1–6. doi:10.1016/s0923-1811(0300157-9)
34. Huang YW. Tsai TF. HLA-Cw1 and Psoriasis. Am J Clin Dermatol. 2021;22(3):339–347. doi:10.1007/s40257-020-00585-1
35. Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134(1):18–23. doi:10.1038/jid.2013.326
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.