Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Pseudo-Kaposi’s Sarcoma: A Rare Case and Review
Authors Li Y , Li W, Zhang M, Yang X, Yang C, Li D
Received 4 March 2023
Accepted for publication 17 May 2023
Published 23 May 2023 Volume 2023:16 Pages 1319—1323
DOI https://doi.org/10.2147/CCID.S411081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yuan Li,1,2,* Wanni Li,1,* Ming Zhang,1 Xianxu Yang,1 Changxiao Yang,1 Dan Li1
1Dermatology Department, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Changxiao Yang; Dan Li, Dermatology Department, The Fifth People’s Hospital of Hainan Province, No. 8 Longhua Road, Longhua District, Haikou, Hainan, 570100, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Our report concerns a 72-year-old female patient who presented with nodular ulcers on her right lower extremity and foot for a duration of 5 months. Based on the results of a dermatological examination, histopathological examination of the lesions, and immunohistochemical findings, we were able to diagnose the patient with Mari-type pseudocaposi sarcoma. Further research allowed us to clarify the distinction between this type of sarcoma and Kaposi’s sarcoma, which will be crucial in devising an effective treatment plan for the patient as we continue to monitor her progress during clinical supervision.
Keywords: pseudo-Kaposi’s sarcoma, Kaposi’s sarcoma, immunohistochemistry, CD34
Introduction
Pseudo-Kaposi’s sarcoma is a medical condition that can be either congenital or acquired, and it is characterized by vascular hyperplasia or reactive hyperplasia caused by abnormal changes in the microcirculation of small blood vessels and chronic venous valve insufficiency.1 This condition can be easily mistaken for Kaposi’s sarcoma, which is why we present a case of pseudocaposi’s sarcoma to aid clinicians in differentiating between the two diseases and making informed decisions about clinical treatment.
Case Presentation
Five months ago, the patient noticed the development of a few nodules on their right toe, which gradually increased in size and resulted in the formation of an ulcer causing pain. Over time, the nodules spread to the right calf, and scattered, irregular-shaped bruises appeared on the right foot. Despite seeking medical attention at a local hospital, the precise diagnosis and treatment remain unknown, and the condition did not improve. Consequently, the patient sought systematic treatment at our hospital and was diagnosed with “Kaposi’s sarcoma, varicose veins.” The patient had been previously healthy, with varicose veins in both lower extremities for almost a decade. They had no history of surgery, trauma, or blood transfusion. Additionally, no family members had similar illnesses or a history of hereditary diseases.
The systemic examination did not reveal any significant findings. However, dark purple nodules, ranging in size from that of a peanut to that of a rice grain, were observed on the right calf, right foot, and toes. The nodules had a hard texture and an uneven surface, and some of them had broken down to form ulcers that secreted a small amount of pus. Scattered irregularly shaped bruises were also visible on the right foot, while varicose veins were present in both lower extremities. Furthermore, there was sunken edema in both calves and feet, which was more apparent in the right calf (as shown in Figure 1A–C).
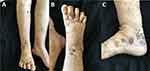 |
Figure 1 (A–C) Scattered dark purple peanut to thumb-sized nodules, ulcers, purulent discharge, bruising, and varicose veins in both lower extremities. |
The laboratory test results revealed a decreased hemoglobin (HGB) level of 101 g/L, as well as decreased levels of total protein (TP) at 56.0 g/L, albumin (ALB) at 36.8 g/L, and globulin (GLO) at 19.2 g/L. Low-density lipoprotein cholesterol (LDL-C) level was elevated at 3.84 mmol/L, and potassium (K) level was low at 2.73 mmol/L. Sedimentation (ESR) rate was elevated at 80 mm/h, and calcitonin (PCT) level was increased at 0.047 ng/mL. However, the remaining results of urine routine, stool routine + occult blood, renal function, blood glucose, cardiac enzymes, immuno-five, coagulation four, syphilis three, hepatitis B six, HIV antibody, and hepatitis C antibody were normal. A local bacterial smear (G+) showed the presence of Citrobacter graminearum through bacterial culture. Electrocardiogram showed sinus tachycardia. Vascular ultrasound of the right lower extremity showed scattered plaque formation in the right lower extremity artery in the indicated section, widened inner diameter sonogram of the right saphenous vein and right calf intermuscular vein, and thickened sonogram of the right calf soft tissue. Chest CT scan showed a few foci of fibrosis in the inferior lingual segment of the upper lobe of the left lung and aortic sclerosis.
The histopathological examination revealed tumor masses growing both inward and outward in the mid-dermis section. The epidermis above the tumor was absent and was covered with pus and blood crust. The epidermis on both sides extended in a collar shape. The dermis showed the presence of dense narrow mature vascular lumen containing erythrocytes, spaced with loose collagen tissue. Additionally, the examination revealed plexiform vascular endothelial cell growth, which formed a lumen. There was no cellular anisotropy or erythrocyte spillage observed (as shown in Figure 2A and B).
Immunohistochemical analysis showed that vascular endothelial cells were CD34 positive, while mesenchymal cells were CD34 negative. Fli-1 was positive, HHV-8 was negative, Ki-67 was about 20%, and VEGF was positive (as shown in Figure 3A–E). Based on the clinical presentation and the results of the immunohistochemical analysis, the patient was diagnosed with pseudo-Kaposi’s sarcoma.
Upon admission, the patient’s ulcer was treated with boric acid wet compresses, semiconductor laser irradiation to enhance local anti-inflammation, vitamin C injections to improve vascular permeability, cumecoside tablets to promote wound healing, dipyridamole tablets to prevent platelet aggregation, furosemide tablets and spironolactone tablets to promote diuresis and reduce swelling, topical amikacin lotion, and other symptomatic treatments. The patient was also prescribed potassium chloride extended-release tablets for oral potassium supplementation and instructed to reduce walking and elevate the lower limbs. If the above treatments are ineffective, surgical treatment of chronic venous valve insufficiency may be necessary. The patient’s case is still being monitored.
Discussion
There exist two variations of pseudo-Kaposi’s sarcoma (PKS).2 The first type, known as Stewart-Bluefarb PKS with Klippel-Trenaunay-Weber syndrome, is identified by a combination of symptoms including erythema, varicose veins, and venous malformations. Additionally, soft tissue hypertrophy in the affected limb may be present, although not necessarily all symptoms will be present in every patient.3 This form of PKS typically emerges early in life and is limited to one side of the body. The condition arises primarily from congenital arteriovenous malformations and arteriovenous short circuits. The affected limb will display unilateral purple-blue patches and papules that are prone to ulceration, with the length or width of the limb increasing over time.4 The second variation is called Mali type, or vascular dermatitis of the extremities of the foot. This form of PKS results from chronic venous insufficiency and is characterized by lesions that resemble sarcoid plaques and nodular disease. The onset of Mali-type PKS usually occurs later in life and can initially present on one side of the body before spreading to both sides after several years. Lesions of this type appear as reddish-purple (or brown or dark) patches, papules, or plaques that are typically slow to develop and may become verrucous or form painful ulcers.5 These lesions typically affect the distal part of the lower leg and foot and are concentrated around the dorsal and dorsal toe triangles, particularly the 1st and 2nd toes. This form of PKS is more common among older men and is usually associated with severe venous insufficiency.6 It is considered a benign reactive vascular proliferative disease and is frequently misdiagnosed, thus making it less frequently reported in clinical settings.7 In the present case, the patient has Mali-type PKS.
Pseudo-Kaposi’s sarcoma (PKS) shares both clinical and histopathological similarities with Kaposi sarcoma (KS), but there are key differences that allow them to be distinguished from one another.8 PKS lesions exhibit capillary and fibroblast hyperplasia, erythrocyte extravasation, and iron-containing heme deposits in the dermis, which are identical to KS, but they lack vascular fissures, inflammatory cell infiltration, and the headland sign. Additionally, PKS generally has more edema than KS.9 On the other hand, KS has jagged vascular lacunae, atypical endothelial cells, spindle cell nuclei, lacunar and irregular lumen formation, headland signs, and common plasma cells. The dermal papillary layer is generally not involved in KS, and immunohistochemistry can help differentiate between the two diseases in some cases. It has been found that the early histopathology of KS is very similar to that of PKS, and both diseases have similar clinical manifestations. Immunopathology can also be used to distinguish between PKS and KS.10 CD34 expression is positive in both endothelial cells and surrounding mesenchyme in all stages of KS, whereas in PKS, CD34 expression is only positive in the vascular endothelium and negative in the vascular mesenchyme.10 Additionally, human herpesvirus 8 (HHV-8) can help identify these two diseases.11 KS is closely associated with HHV-8 infection, and all subtypes are associated with it, whereas the association between HHV-8 and PKS is less clear.11
Although the exact cause of pseudo-Kaposi’s sarcoma is not fully understood, numerous studies indicate that it may be linked to venous stasis, venous hypertension, calf muscle pump insufficiency, hypoxia resulting from venous stasis, endothelial cell damage, increased capillary permeability, and infiltration of blood components into the perivascular tissues leading to edema. This sequence of events can trigger neovascularization and inflammatory cell infiltration, which can result in the proliferation of the disease.12
The primary treatment for varicose veins involves using elastic bandages, while compression therapy is the primary treatment for venous insufficiency.13 Other treatment options for pseudo-Kaposi’s sarcoma include vein surgery, sclerotherapy, high ligation, valve grafting, and reconstruction. In cases of ulceration or infection, symptomatic treatment is available, and localized small nodules can be treated through freezing or surgery.14
Conclusions
The preceding analysis enables us to elaborate on the distinction between Kaposi’s sarcoma and Kaposi’s sarcoma, which holds great importance for the subsequent stages of the patient’s treatment regimen during clinical supervision.
Ethics Statement
The publications of images were included in the patient’s consent for publication of the case. The Hospital Ethics Committees of the Fifth People’s Hospital of Hainan Province approved to publish the case details.
Consent Statement
Informed consent was provided by the patient for publication of the case.
Funding
This project supported by Hainan Province Clinical Medical Center.
Disclosure
Yuan Li and Wanni Li are co-first authors for this study. Changxiao Yang and Dan Li are co-correspondence authors for this study. The authors have no conflicts of interest to declare for this work.
References
1. Gaurav V, Grover C. ”Pseudotumors” in dermatology. Indian Dermatol Online J. 2022;13(2):294–301. doi:10.4103/idoj.idoj_226_21
2. Parsi K, O’Connor AA, Bester L. Stewart-Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30(8):505–514. doi:10.1177/0268355514548090
3. Sun L, Duarte S, Soares-de-almeida L. Acroangiodermatitis of Mali-An unusual cause of painful ulcer. Actas Dermosifiliogr. 2022:S0001-7310(22)00630–5. Spanish. doi:10.1016/j.ad.2022.07.013
4. Lauck K, Nguyen QB, Klimas N, Rogge M. Acroangiodermatitis presenting as unilateral hypertrophic verrucous plaques. Dermatol Online J. 2022;28(2). doi:10.5070/D328257400
5. Santosa A, Chandran NS. Novel manifestations of acroangiodermatitis: a report of two cases. Indian J Dermatol. 2020;65(3):246–247. doi:10.4103/ijd.IJD_669_18
6. Mine T, Koike Y, Ehara D, Murota H. A case of bilateral plantar pseudo-Kaposi sarcoma successfully treated with propranolol. JAAD Case Rep. 2021;18:74–78. doi:10.1016/j.jdcr.2021.09.032
7. Chhabra G, Verma P, Khullar G, Shruti S. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62(1):e156–e157. doi:10.1111/ajd.13386
8. Horiguchi Y, Takahashi K, Tanizaki H, Miyachi Y. Case of bilateral acroangiodermatitis due to symmetrical arteriovenous fistulas of the soles. J Dermatol. 2015;42(10):989–991. doi:10.1111/1346-8138.12934
9. Dabas G, De D, Handa S, Chatterjee D. Dermoscopic features in two cases of acroangiodermatitis. Australas J Dermatol. 2018;59(4):e290–e291. doi:10.1111/ajd.12820
10. Goorney BP, Newsham J, Fitzgerald D, Motta L. Acroangiodermatitis mimicking Kaposi’s sarcoma in an HIV-positive man. Int J STD AIDS. 2018;29(7):729–731. doi:10.1177/0956462417750709
11. Hronek AL, Clark SN, Young G, Kinikini D, Wells J. AcroangiodermatitisA presentation of two cases of nonhealing ulcerations in the lower extremity. J Am Podiatr Med Assoc. 2016;106(5):364–369. doi:10.7547/14-162
12. Singh SK, Manchanda K. Acroangiodermatitis (Pseudo-Kaposi sarcoma). Indian Dermatol Online J. 2014;5(3):323–325. doi:10.4103/2229-5178.137791
13. Huguen J, Bonsang B, Lemasson G, Misery L, Brenaut E. Image Gallery: acroangiodermatitis or pseudo-Kaposi sarcoma: two cases in patients with paralyzed legs. Br J Dermatol. 2016;174(6):e84. doi:10.1111/bjd.14743
14. Trennheuser L, Fink C, Haenssle HA, Enk AH, Toberer F. Diagnostic workup of acroangiodermatitis of Mali (pseudo-Kaposi sarcoma) demasking metastasized epithelioid angiosarcoma. J Dtsch Dermatol Ges. 2020;18(12):1475–1477. doi:10.1111/ddg.14290
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


