Back to Journals » OncoTargets and Therapy » Volume 9
Proton beam therapy: clinical utility and current status in prostate cancer
Authors Yamoah K, Johnstone P
Received 23 May 2016
Accepted for publication 10 August 2016
Published 16 September 2016 Volume 2016:9 Pages 5721—5727
DOI https://doi.org/10.2147/OTT.S100518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Kosj Yamoah, Peter AS Johnstone
Radiation Oncology Department, Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Abstract: Proton beam therapy has recently become available to a broader population base. There remains much controversy about its routine use in prostate cancer. We provide an analysis of the existing literature regarding efficacy and toxicity of the technique. Currently, the use of proton beam therapy for prostate cancer is largely dependent on continued reimbursement for the practice. While there are potential benefits supporting the use of protons in prostate cancer, the low risk of toxicity using existing techniques and the high cost of protons contribute to lower the value of the technique.
Keywords: proton therapy, prostate cancer, radiotherapy
Introduction
The concept of treating patients with proton beam therapy (PrT) was first proposed by Robert Wilson in 1946.1 After 12 years, the first PrT patient series was published by researchers at the Lawrence-Berkeley National Laboratory.2 For several subsequent decades, many other proton treatment centers emerged including the Harvard cyclotron and Loma Linda University Medical Center (LLUMC) in California, the Midwest Proton Radiotherapy Institute (MPRI) in Bloomington, IN, the University of Florida Proton Therapy Institute (UFPTI) in Jacksonville, FL, and the MD Anderson Proton Therapy Center in Houston. At the time of writing, there are 23 active centers in the US with many more proposed or being built.3
PrT is an attractive – though expensive – modality of modern radiation delivery. Protons have unique physical properties that minimize dose to adjacent normal tissue and allow for potential escalation of delivered dose. We consider PrT to be critical in treating children,4 as well as rare clinical situations such as craniospinal radiotherapy (RT),5 recurrent chordomas, and midline central nervous system structures.6,7 The enthusiastic adoption of PrT for treating prostate cancer (CaP) was based on the theoretical promise for superiority in sparing organs at risk such as bladder, rectum, and femoral head when compared with photons. However, much of the investment in proton centers was made with the expectation of significant return on investment by using PrT for CaP.8 It was this practice that led to an unfortunate backlash against PrT at large that still exists. The purpose of this review is to discuss the role of PrT for CaP.
Physical and biological aspects of proton therapy
The predominant mechanism of radiation therapy in the US is delivered by X-ray photon energy. This involves a machine-generated beam as opposed to gamma rays, which originate in a radioactive source such as 60Cobalt. Both X-rays and gamma rays provide photons; these completely penetrate the target and may be captured on film after doing so. This penetration makes them very different from electrons, which enter the body at close to maximal energy and lose energy in a somewhat predictable fashion as distance increases.
Distinct from either of these is PrT. Protons are heavy charged particles with ~1,800 times the mass of electrons. This unique physical property offers superior dosimetric advantages over photons or electrons. Protons do not traverse the target; they stop at an energy-dependent depth within the target and have no exit dose. This obviously completely spares downstream normal tissue. A proton beam is first generated from a cyclotron or synchrotron and accelerated toward its intended target. The enormous mass and amount of acceleration applied gives each proton a specific momentum that is mostly dissipated after traveling a defined distance and then slowed down by interactions within the target. This causes a sharp increase in energy deposition (dose) at the end of the proton’s path is then followed by no further dose delivery, called the Bragg peak.9 As a result a proton has little tissue interaction (thus, little dose delivery) until it comes to rest. Figure 1 depicts the physical properties of protons, photons, and electrons.
Protons ionize tissue similarly to photons. Thus, we take advantage of our vast experience with X-rays to determine doses and to predict acute and late normal tissue effects. Simply: delivering 1 Gray equivalent (GyE) with protons is equivalent to delivering 1 Gy with photons. This has been validated in the proton clinic10,11 and translates to similar fractionation schedules: 1.8 GyE to 2 GyE per fraction, provided daily.
As shown in Figure 1, a pristine monoenergetic proton beam delivers a Bragg peak far too small for functional dose delivery. To enable the beam to properly encompass a target across a range of depths, one must deliver various PrT energies to provide a spectrum of Bragg peaks. Usually, this is done by placing variable thicknesses of attenuating material in the beam. This modulated beam provides a summation of individual Bragg peaks, termed the Spread Out Bragg Peak. It must be remembered that while this process maintains the abrupt termination of dose beyond the target, it also increases the upstream dose somewhat.
The final area of new technology in PrT applies to the mechanism of delivering the proton beam to the target within the patient. In older centers, the beam is scanned across the target volume. This is a relatively uniform distribution, taking advantage of the unique physical properties of the beam but not allowing for heterogeneity, or “dose painting” within the volume. Newer, “pencil beam” nozzles allow for more flexibility in dose distribution within the target similar to the intensity modulation incorporated into photon RT over the past decade or more.12
Proton therapy for prostate cancer
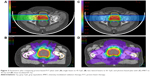
Modern photon therapy for prostate cancer incorporates both intensity modulation and image guidance. The former intensity-modulated radiation therapy (IMRT) incorporates both software and delivery hardware to conform multiple beams to a distinct target while simultaneously minimizing doses to adjacent normal tissue. The latter image-guided radiation therapy (IGRT) incorporates documentation of the location of prostate prior to each fraction; this allows for smaller margins on the target volume in treatment planning. While proton therapy uses IGRT, it uses only one or two lateral fields rather than the five to seven fields required for IMRT (Figure 2). The end result of this is that, while the volume getting a high dose is similar to IMRT,13 the amount of pelvic tissue receiving low-to-moderate dose is less.14 This dosimetric advantage has been the driving force for the increased utilization of PrT for CaP. However, the available data do not overwhelmingly support linking this sparing of normal tissue to a discernible difference in late effects, especially in the IMRT era. However, as a rule, radiation oncology best practice involves lowering dose to nontarget tissue to As Low As Reasonably Achievable (ALARA). Notably, the construction and operating costs for PrT results in higher costs than IMRT by factors of 1.7 to 2.4.15
Best practice until the development of conformal RT software limited the total radiation doses to the prostate because of a much higher risk of normal tissue toxicity. Not unexpectedly, doses of 66 Gy to 70 Gy to an intact prostate yield inferior disease control to doses of 81 Gy routinely available with image-guided IMRT. PrT, by virtue of its unique dose distribution, was a prime mechanism for study of such dose escalation in the early 1980s. The first clinical publication of PrT to a dose of 75.6 GyE for prostate cancer therapy was in 1983, from a team at the Harvard Cyclotron and Massachusetts General Hospital (MGH). In this case, photons were the primary mechanism of dose delivery and protons were used as a boost. Since the Harvard Cyclotron had a fixed beam, the boost was delivered using a perineal technique, which is not standard now. Still, no increased toxicity could be attributed to the additional dose.16 In a subsequent focused review of this boost technique by Gardner et al;17 they reported no grade 3 or more gastrointestinal (GI) toxicity and 8% grade 3 or more genitourinary (GU) toxicity. The relatively high GU toxicity is likely due to the perineal approach that puts more bladder at risk than current boost techniques using opposed lateral fields.
This team next undertook a randomized clinical trial,18 the first of several that would ultimately clarify and validate RT dose escalation for prostate cancer. After 50.4 Gy photons to the pelvis, 202 men were randomized between a standard photon prostate boost to 67.2 Gy and a PrT prostate boost to 75.6 GyE. Local control at 8 years was 77% (high dose) and 60% (standard dose), although this was not statistically significant (P=0.089). On subset analysis, a significant difference (84% vs 19%; P=0.0014) was noted for 57 men patients with high grade (Gleason sum 8–10) disease.18
The subsequent randomized trial investigating protons in dose escalation was a joint study of MGH and LLUMC.19 Between 1995 and 1999, 391 men were randomized between total doses of 70.2 Gy or 79.2 Gy, achieved by proton boost. At a median follow-up of 8.9 years, the high-dose arm had statistically superior local control (P<0.0001). The 10-year biochemical failure (nadir PSA + 2) was 17.4% for the high-dose cadre and 32% for the standard dose arm (P<0.001).
In 2004, investigators at LLUMC published outcomes of over 1,250 patients treated between 1991 and 1997.20 Approximately 40% of the patients received PrT alone to 74 GyE; the remainder had initial photon pelvic fields with a proton boost. Since high- and intermediate-risk patients were combined, results are difficult to reconcile with more stratified reports. Nevertheless, 8-year biochemical disease-free survival was 73%. Grade 3 toxicity was 1% for both GI and GU symptoms; grade 4 levels were only noted in GI (0.2%).
Since the results of dose-escalated conventional photon RT for prostate cancer have been so good, many payers feel there is little incentive to pay the extra cost for protons for prostate cancer. In this case, some centers have tried to validate the therapy by virtue of fewer side effects. Table 1 is a collation of reports of PrT toxicity to date. The University of Florida Proton Center has managed several prospective trials of proton therapy. With 2-year minimum follow-up, 211 patient records from the first three of these were reported.21 Patients received 78–82 GyE. Grade 3 GI toxicity was noted in <0.5% and grade 3 GU toxicity in 2%. These data have been updated several times; most recently on 1,285 patients treated between 2006 and 2010.22 No grade 4 or 5 GI events were noted, and rectal bleeding (grade 3) was noted in 0.9% of patients. Five-year GU data were reported separately and excluded high-risk patients.23 In 171 patients, 2.9% grade 3 side effects were noted. Finally, one multicenter Japanese series reported 151 patients treated to 74 GyE with >24-month follow-up. In this cohort, grade 2 or more GI or GU toxicity was 2% and 4.1%, respectively.24
More recently, a population-based study using Surveillance, Epidemiology, and End Results (SEER)–Medicare-linked data evaluated the morbidity and disease outcomes associated with the use of IMRT and PrT for localized prostate cancer.25 Using a propensity score matching approach, it was determined that IMRT patients had a lower rate of GI morbidity, with an absolute risk of 12.2 vs 17.8 per 100 person-years, and a relative risk of 0.66 in favor of IMRT.
Practical considerations
Most prostate cancer patients will be candidates for either photon or proton therapy. However, there are some specific instances for which protons are not feasible. These are mentioned briefly as follows:
- Many radiation oncologists feel that the pelvic lymph nodes should be treated in the case of higher risk prostate cancer. This is more challenging with scanned protons than with standard photons, and in some cases is frankly impossible.
- Prostate cancer patients not infrequently present after hip replacements. Such patients are easily treated with conventional IMRT. However, protons should not traverse the prosthesis. Some proton centers have expertise to treat men with a single prosthesis, but men with two would be ineligible for care at most centers.
- The depth of a proton beam is dictated by the energy of the beam. Very large men may not be eligible for treatment in a center with a relatively low energy beam.
Thus, the calculus for PrT for prostate cancer is dependent on cost and availability, not by outcomes. The simple act of dosimetric avoidance of normal tissue is always a nice thing to show, however, in the absence of increased survival or decreased toxicity it is hard to reconcile the much greater cost of protons. Prostate cancer patients were key to many proton centers’ business cases in the recent past,8 but the US has overdeveloped proton sites in some geographical areas while leaving major areas uncovered.26 Costs remain quite high.27 More recently, the implementation of hypofractionated PrT techniques that requires less total number of fractions per treatment course might help to decrease overall costs for the use of PrT in prostate cancer.28,29 This will result in a lower overall cost per treatment course to a level that is more comparable to the cost for a conventional prostate IMRT.
Innovative technology of PrT by itself alone is not beneficial always. The value of this technology must be based on its ability to improve survival, decrease morbidity, or decrease cost and not in its unique physical properties. It goes without saying that if the cost of PrT was comparable to that of electron therapy, there will be widespread use of this technology based on its unique physical properties without the need for Level I evidence. Perhaps, the cost of PrT needs to be reset in order to justify its use as an alternative treatment modality, based on the current available evidence that offers no major advantage over conventional IMRT/IGRT techniques for prostate cancer.
Conclusion
This review restricts its focus to PrT for CaP. Given the unique physical properties and superior dosimetric parameters, protons do offer a theoretical advantage to photons and electrons in delivering higher radiation doses to the prostate while sparing surrounding normal tissue. However, the currently available and rather limited body of evidence suggest that the use of PrT in treating CaP offers no proven superiority over conventional IMRT. Further, well-conducted research studies with enough follow-up data are required to rigorously evaluate the clinical advantage of PrT in treating CaP to improve disease control and/or reduce acute and long-term radiation toxicity. Protons are an essential aspect of a sophisticated radiotherapy portfolio. Their cost is significant, but their benefit in treating children and advanced spine and skull base lesions is becoming a matter of record. Avoidance of dose to uninvolved tissue is a valid goal in clinical radiotherapy. It may simply matter more in children and in the skull base or spine than in the older adult male pelvis.
Disclosure
The authors report no conflicts of interest in this work.
References
Wilson RR. Radiological use of fast protons. Radiology. 1946;47(5):487–491. | ||
Lawrence JH, Tobias CA, Born JL, et al. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res. 1958;18(2):121–134. | ||
The National Association for Proton Therapy. Proton therapy centers. Available from: http://www.proton-therapy.org/map.htm. Accessed August 31, 2016. | ||
Johnstone PAS, McMullen KP, Buchsbaum JC, Douglas JG, Helft PR. Pediatric CSI: are protons the only ethical approach? Int J Radiat Oncol Biol Phys. 2013;87(2):228–230. | ||
Ray G, Buchsbaum JC, McMullen KP, et al. Definitive treatment of leptomeningeal spinal metastases in children. Pediatr Blood Cancer. 2013;60(11):1839–1841. | ||
McDonald MW, Linton OR, Shah MV. Proton therapy for reirradiation of progressive or recurrent chordoma. Int J Radiat Oncol Biol Phys. 2013;87(5):1107–1114. | ||
Estabrook N, McDonald MW, Hoene T, et al. Proton radiotherapy for midline CNS lesions: a class solution. Oncology. 2015;89(2):111–117. | ||
Johnstone PAS, Kerstiens J, Helsper R. Proton facility economics: the importance of ‘simple’ treatments. J Am Coll Radiol. 2012;9(8): 560–563. | ||
Bragg W, Kleeman R. On the alpha particles of radium, and their loss of range in passing through various atoms and molecules. Philos Mag. 1905;S6:318–340. | ||
Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53(2):407–421. | ||
Lomax AJ. Charged particle therapy: the physics of interaction. Cancer J. 2009;15(4):285–291. | ||
Kooy HM, Grassberger C. Intensity modulated proton therapy. Br J Radiol. 2015;88(1051):20150195. | ||
Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):994–1002. | ||
Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69(2): 444–453. | ||
Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol). 2003;15(1):S37–S50. | ||
Duttenhaver JR, Shipley WU, Perrone T, et al. Protons or megavoltage X-rays as boost therapy for patients irradiated for localized prostatic carcinoma. An early phase I/II comparison. Cancer. 1983;51(9):1599–1604. | ||
Gardner BG, Zietman AL, Shipley WU, Skowronski UE, McManus P. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol. 2002;167(1):123–126. | ||
Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32(1):3–12. | ||
Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–1111. | ||
Slater JD, Rossi CJ Jr, Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59(2):348–352. | ||
Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):213–221. | ||
Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the University of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91(1):172–181. | ||
Henderson RH, Hoppe BS, Marcus RB Jr, et al. Urinary functional outcomes and toxicity five years after proton therapy for low- and intermediate-risk prostate cancer: results of two prospective trials. Acta Oncol. 2013;52(3):463–469. | ||
Nihei K, Ogino T, Onozawa M, et al. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys. 2011;81(2):390–396. | ||
Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307(15):1611–1620. | ||
Kerstiens J, Johnstone PAS. Proton therapy expansion under current US reimbursement models. Int J Radiat Oncol Biol Phys. 2014;89(2):235–240. | ||
Johnstone PA, Kerstiens J. Reconciling reimbursement for proton therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):9–10. | ||
Wang Y, Efstathiou JA, Lu HM, Sharp GC, Trofimov A. Hypofractionated proton therapy for prostate cancer: dose delivery uncertainty due to interfractional motion. Med Phys. 2013;40(7):071714. | ||
Vargas CE, Hartsell WF, Dunn M, et al. Hypofractionated versus standard fractionated proton-beam therapy for low-risk prostate cancer: interim results of a randomized trial PCG GU 002. Am J Clin Oncol. Epub 2015 Oct 29. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.