Back to Journals » Clinical Interventions in Aging » Volume 18
Protein-Added Healthy Lunch-Boxes Combined with Exercise for Improving Physical Fitness and Vascular Function in Pre-Frail Older Women: A Community-Based Randomized Controlled Trial
Authors Park W, Lee J, Hong K, Park HY , Park S, Kim N, Park J
Received 11 October 2022
Accepted for publication 3 January 2023
Published 5 January 2023 Volume 2023:18 Pages 13—27
DOI https://doi.org/10.2147/CIA.S391700
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Wonil Park,1,2 Jaesung Lee,1 Kwangseok Hong,3 Hun-Young Park,4,5 Saejong Park,6 Nahyun Kim,1 Jonghoon Park1
1Exercise Nutrition and Biochemistry Laboratory, Department of Physical Education, Korea University, Seoul, South Korea; 2Physical Education Laboratory, Chung-Ang University, Seoul, South Korea; 3Department of Physical Education, College of Education, Chung-Ang University, Seoul, South Korea; 4Department of Sports Medicine and Science, Graduate School, Konkuk University, Seoul, South Korea; 5Physical Activity and Performance Institute (PAPI), Konkuk University, Seoul, South Korea; 6Department of Sports Science, Korea Institute of Sport Science, Seoul, South Korea
Correspondence: Jonghoon Park, Exercise Nutrition and Biochemistry Laboratory, Department of Physical Education, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul, 02841, South Korea, Tel/Fax +82 (2) 3290-2315, Email [email protected]
Purpose: Preventive or therapeutic interventions are key to maintaining independence in pre-frail and/or frail elderly. Therefore, we investigated whether multi-component interventions were effective in physical fitness levels and vascular functions in pre-frail older women.
Patients and Methods: Sixty participants aged ≥ 65 years (81.5 ± 4.3 yrs) were divided equally into control group, diet group, aerobic exercise and diet group, and aerobic exercise with electromyostimulation and diet group. For 8 weeks, the participants received a set of protein-added meals twice daily on weekdays. The aerobic exercise groups performed 45 mins of stepping exercise at 50– 70% of the maximal heart rate for 3 days/week, and the aerobic exercise with electromyostimulation was applied on each limb in 8 weeks. Blood pressure, physical fitness, cardiovascular biomarkers, pulse wave velocity, and flow-mediated dilation were measured before and after the 8-week.
Results: There were no group differences in age, height, weight, body mass index, free fat mass, and %body fat at baseline. The right grip strength significantly increased in the diet group, aerobic exercise and diet group, and aerobic exercise with electromyostimulation and diet group (p < 0.05). Short physical performance battery, 6-min walking distance, and flow-mediated dilation significantly increased in the aerobic exercise and diet group and aerobic exercise with electromyostimulation and diet group (p < 0.05). Blood pressure and pulse wave velocity did not differ between interventions. High-density lipoprotein-cholesterol levels significantly increased after 8 weeks in all intervention groups (p < 0.05). There were no significant differences in glucose, HbA1c, total cholesterol, low-density lipoprotein-cholesterol, triglyceride, insulin, Homeostatic Model Assessment for Insulin Resistance, nitric oxide, and C-reactive protein levels.
Conclusion: These results show that multi-component interventions appear to improve physical fitness and vascular function in pre-frail older women. Thus, possible strategies to prevent early frailty including proper nutrition and exercise may be needed.
Keywords: pre-frail, protein-added, aerobic exercise, electromyostimulation, physical fitness, vascular function
Introduction
A geriatric syndrome known as frailty is defined by age-related reductions in physiologic reserve and function across several organ systems, increasing vulnerability to unfavorable health consequences.1 A clinical syndrome called frailty was characterized as having three or more characteristics: unintended weight loss in the previous year, self-reported weariness, weakness, slow gait speed, and lower physical activity.2 The prevalence of frailty in community-dwelling older individuals ranged from 4.0 to 59.1%, and the prevalence of prefrailty ranged from 18.7% to 53.1%.3 Frailty prevalence was higher in females than males in the older population.4 This is because older adults did not meet the recommended level of physical activity5 and older women over 75 years showed the greater difficulty of functional limitation than older men in the Spanish population.6
Frailty is an independent risk factor linked to cardiovascular disease (CVD). Afilalo and colleagues observed the relationship between frailty and CVD that frailty increased the risk of CVD.7 In addition, a number of studies have reported that older women have greater aortic stiffness than older men, and this stiffness is fairly closely associated with blood pressure regulation, impaired ventricular coupling, and left ventricular remodeling in older women.8 Vascular dysfunction is to increase with the onset of frailty in the elderly,9 thereby inducing various cardiovascular diseases, such as atherosclerosis, stroke, and coronary artery disease. Atherosclerosis develops mainly due to impaired vascular functioning, including arterial stiffness and endothelial dysfunction.10 The brachial-ankle pulse wave velocity is a popular noninvasive method for determining arterial stiffness.9,11 A recent cross-sectional investigation of an elderly population in the community revealed a correlation between pre-frailty and increased vascular stiffness.12 Endothelial dysfunction is a prominent early indicator of the onset of atherosclerosis and manifests as clinical symptoms.13 In a recent cross-sectional study among hospitalized elderly, elderly who were pre-frail and frail had a lower flow-mediated dilation than elderly who were robust, indicating greater endothelial dysfunction.14
The onset and development of frailty are strongly influenced by low physical activity and inadequate nutritional intake. However, frailty can be managed with effective interventions, such as regular physical exercise and balanced nutrition.15 Habitual aerobic exercises and potential alternative strategies have shown a favorable effect on vascular function and related mechanisms in frail elderly.16 In a cross-sectional study involving sedentary older adults, moderate aerobic exercise training was negatively associated with arterial stiffness-reduced pulse wave velocity.17 Aerobic exercise has also improved brachial flow-mediated dilation, particularly in women with more impaired endothelial function.18 In summary, aerobic exercise mitigates the detrimental arterial stiffening and endothelial function in older adults overall.
In contrast to the benefits of aerobic exercise, the effects of resistance exercises on the cardiovascular system are not well understood. Although various resistance exercises have crucial effects on the musculoskeletal system by maintaining functional capacity and preventing sarcopenia in the elderly,9 arterial stiffening can also increase.15 Many older adults seem to be either unable or unwilling to perform conventional resistance exercises because of fatigue and injury.19 Recently, electromyostimulation, a novel and innovative method that mimics resistance exercises, has been widely recognized as a time-saving and injury-preventing option for the elderly.20 Electromyostimulation, known as electrical muscle stimulation or neuromuscular electrical stimulation, involves the artificial activation of a muscle with a protocol intended to reduce the discomfort related to the stimulus. The electromyostimulation system may overcome the limitations of conventional resistance exercise programs for older populations, particularly frail ones. Therefore, combined aerobic exercise with electromyostimulation may be viable for frail elderly individuals at risk for CVD.
Muscle protein catabolism21 and decreased protein intake induce frailty,22 thus nutritional interventions, such as protein intake are also important for frailty management. Conversely, higher protein consumption has been linked to lower risk in frail older women.23 Thus, a higher protein dose in the diet may be a reasonable strategy for preventing frailty. In addition, increased protein intake may effectively prevent CVD in the elderly. Hu and colleagues reported that replacing carbohydrates with proteins may be reduced ischemic heart disease.24 In middle-aged or older adults, whey protein reduces arterial stiffness and improves endothelial function.25 Although dietary interventions may prevent CVD in older adults, data elucidating the effect of protein-added healthy dietary interventions on physical fitness and vascular function are limited.
To our knowledge, community-based studies on multi-component interventions for pre-frail elderly women are limited information on physical fitness and vascular function. Therefore, this is the first study to investigate the effects of 8-week community-based multi-component interventions including adding protein to healthy lunch-boxes, aerobic exercise, and EMS on physical fitness and vascular function in community-dwelling pre-frail elderly women. We hypothesized that a combination of nutritional supplementation and exercise with EMS may have favorable effects on physical fitness and vascular function in pre-frail older adults to prevent progression to frailty.
Materials and Methods
Participants
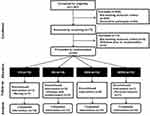
A four-arm, single-blind, randomized controlled trial of this investigation was carried out in senior community centers in S City, South Korea, from June 2019 to March 2020. Individuals aged ≥ 65 years were recruited for this study. During the screening period, we contacted 1063 senior citizens by phone or face-to-face, from which we identified 60 eligible participants. They described any measures to disguise the random allocation sequence until interventions were assigned, but they were unaware of it. Before the 8-week intervention, they were randomly and equally assigned by the blinded researcher into one of the following four groups: control, diet (DG), aerobic exercise and diet (ADG), aerobic exercise with EMS, and diet (AEDG) (Figure 1). Participants who were frail or robust, unable to walk independently, and had cognitive impairment and severe diseases, including CVD and cancer, were excluded from this study. Pre-frail older adults were identified based on five modified Fried criteria: 1) unintentional weight loss, 2) weakness, 3) exhaustion, 4) slow walking speed, and 5) low physical activity level. Scores of 0, 1–2, and 3–5 were classified as robust, pre-frail, and frail, respectively.2 With this, all recruited patients had scores of 1–2. All experimental procedures were explained to the individuals, and patients were informed of the potential risks of the study and given written informed consent to participate. The Institutional Review Board of Korea University approved the protocol and informed consent form (KUIRB-2019-0161-01) in South Korea, and all study procedures were performed in accordance with the Declaration of Helsinki. The data in the study are publicly available in the Clinical Research Information Service in Korea (KCT0006888, 05/01/2022) and WHO ICTRP (International Clinical Trials Registry Platform, http://www.who.int/ictrp).
Study Design
A single-blind, randomized controlled experimental trial was used. The order of interventions was determined by a random sequence generator and administered by the primary investigator. In addition to screening, all participants fasted for ≥ 8 hours before venous blood collection between 8:00–9:00 a.m., both before and after the 8-week intervention. After that, body composition, blood pressure, and vascular function were measured. Physical function was then measured after the lunch sessions. Following baseline measurement, the intervention groups (DG, ADG, and AEDG) were assigned to 8-week supervised diet and exercise programs, which were performed at a set of 2 or 3-day intervals before and after testing to testing to minimize the impacts of training and/or testing (Figure 2). After interventions, all of the data were analyzed by the same researcher, blinded to the identification of participants and interventions.
 |
Figure 2 Study design. Abbreviations: CG, control group; DG, diet group; ADG, aerobic exercise and diet group; AEDG, aerobic exercise with electromyostimulation and diet group. |
Exercise Intervention
All pre-frail older women in the exercise intervention groups (eg, ADG and AEDG) performed stepping aerobic exercises. We conducted 45-min exercises three times a week for 8 weeks, with constant temperature (22–24°C) and humidity (60%). The maximal heart rate (HRmax) of the participants was calculated using the formula [208-(0.7 x age)] introduced by Tanaka equation.26 They performed about 45 mins of exercise, corresponding to 50%-60% of HRmax. The training sessions included a 5-min warm-up, six repetitions of 5-min stepping sessions, followed by a 1-min rest in the standing position (for a total of about 35 min), and an approximately 5-min cool-down before returning to the resting state. The participants in the exercise interventions received instructions to monitor their target heart rate during the sessions using a heart rate monitor (M400, Polar, Helsinki, Finland). Participants received specialized instructions and coaching from a certified trainer in the senior community center for the whole intervention period. In contrast, the control group was asked to keep participants engaged with the same level of activities during the 8-week intervention period. The 8-week supervised exercise intervention participants received expert training and coaching from a knowledgeable trainer. Participants did not experience any apparent exercise-related events; nevertheless, musculoskeletal issues such as delayed onset muscle soreness and joint discomfort were the most common phenomenon reported during the first phase of the exercise intervention.
Electromyostimulation Intervention
ADEG performed a stepping exercise with electromyostimulation. A specially designed Velcro-tape muscle stimulator (Miracle, Gyeonggi-do, South Korea) powered by a 9-V battery was used for electromyostimulation in this investigation. The waveform of the stimulator current was created to induce regular contractions (2-s stimulation/2-s recess) in the upper (eg, biceps and triceps) and lower extremity (eg, thigh) muscle groups generating at a 4 Hz. During the stepping workouts, impulses were sent through silicone-rubber electrodes on each arm and leg.20 EMS was applied at an exercise intensity of 5–6, corresponding to moderate intensity, based on the Borg’s revised rating of perceived exertion (RPE) scale from 0 (rest) to 10 (extreme intensity).
Diet Intervention
The nutritional intervention was to maintain the 2015 Korean Food Guide’s guidelines for senior women.27 The evaluation of caloric needs to be considered both sex and weight. A set of personalized lunch boxes was given to the participants, including carbohydrates, proteins, fats, vitamins, and minerals twice a day (eg, lunch and dinner) during weekdays for 8 weeks in the senior community center. The healthy diet intervention’s primary goal was to increase protein intake. The recommended amount of protein for Korean senior citizens aged ≥ 65 years was 0.8g/kg/day. The lunch boxes were divided into three types of color-customized meal pads indicating the personalized protein amounts (pink, red, and black for 20 g, 30 g, and 40 g of protein per serving, respectively), which also made it easier for participants to obtain their assigned meals. The number of leftovers was assessed, and we discussed reasons and possible solutions for non-adherence.
Measurements
Anthropometry and Body Composition
The participants were minimally clothed and barefoot when their height and weight were measured using a physicians’ balance scale (Seca, Germany). Body mass (kg)/height squared was used to determine body mass index (kg/m2). Using a bioelectrical impedance analysis instrument, lean mass and body fat percentage were measured (Inbody 270, Biospace, South Korea).
Dietary Analyses
Participants were given detailed instructions by the nutritionist on how to keep dietary records for 3 days (eg, 2- weekdays and 1- weekend day). Moreover, all intervention group participants were instructed to track how well they ate and the amount of food offered. Dietary records were collected before and after intervention and analyzed with Can-pro 5.0 (The Korean Nutrition Society, South Korea) by a well-trained nutritionist.
Physical Fitness
A grip strength dynamometer was used to measure the maximal grip strength of both arms (T.K.K 5401, Takei Instruments, Japan). Three objectives lower body function tests were used to evaluate the short physical performance battery (SPPB).: 1) the standing balancing test, in which individuals were timed until they moved or after 10seconds had passed; this test included tandem, semi-tandem, and side-by-side standing. 2) the faster of the two walking speed trials was also used to compute the walking speed. 3) repeated chair sit-to-stand test, in which participants performed a pre-test of standing up from the sitting position with arms folded anteriorly and, if successful, performed five chair stands as quickly as possible, to which they were timed. The pre-test involved the participants standing up from the sitting position with arms folded anteriorly. Each subtest’s performance was graded from 0 to 4, yielding total scores that ranged from 0 to 12. Higher scores indicated better function.28 For a 6-minute walk test on a flat surface, participants were encouraged to walk as far as they could in that time. They were not given any comments or encouragement during the test, although they were free to take breaks as needed.
Blood Pressure and Arterial Stiffness
The participant’s blood pressure (BP) was monitored twice with an automatic monitor (HEM-7200-AP3; Omron Healthcare, Kyoto, Japan) while they were seated and their arms were at heart level, with a 5-minute gap between each reading. Following a 15-min rest, arterial stiffness was assessed by brachial-ankle pulse wave velocity (PWV) utilizing an automatic oscillometric instrument (VP-1000plus, Omron Healthcare, Japan). The participants were supine in a quiet, semi-lit, temperature-controlled room.29 Using oscillometric pressure sensors wrapped around the arms, ankles, and extremity cuffs coupled to plethysmographic sensors, bilateral brachial and post-tibial arterial pressure waveforms were recorded for 10s. The PWV was estimated as the transit time divided by the distance between the two artery recording sites. The time difference between the proximal and distal “foot” waveforms was used to calculate the transit time. The sharp systolic upstroke began at the foot in the waveform, which was automatically recognized by a band-pass filter (5–30 Hz). Based on the participants’ height, the system automatically calculated the length of the artery path.
Brachial Flow-Mediated Dilation
Flow-mediated dilation (FMD) is a non-invasive method of measuring endothelial vasodilation using ultrasonography recordings of the brachial artery’s reaction to ischemia circumstances. The FMD was measured using a noninvasive Doppler ultrasonography system with an online computer-assisted semi-automatic analysis software before and after the 8-week intervention (UNEX EF-38G, UNEX Co. Ltd, Nagoya, Japan). A 10 MHz electric linear-array transducer was included in the system. A BP cuff was placed on the forearm of the participant while they were lying down and relaxed, with the proximal edge of the cuff above the participant’s antecubital fossa. A second blood pressure cuff was placed on the participant’s opposite arm to take a standard blood pressure reading. The automated probe, self-adjusted to offer a crisp longitudinal image of the artery and record baseline data, was used to capture cross-sectional images of the artery. After taking the baseline reading, the arm below the antecubital fossa was successfully occluded for five minutes by inflating the cuff to 50 mmHg above the resting systolic blood pressure. Following cuff deflation, blood flow velocities and brachial artery diameters were measured using ultrasound during a 2-min period.30 ((peak diameter - baseline diameter)/baseline diameter) x 100 was used to determine FMD.
Blood Chemistry
The Green Cross Medical Foundation examined venous blood factors (Certified Organization in the Korea Society for Laboratory Medicine). The levels of glucose, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), insulin, C-reactive protein (CRP), and nitric oxide were measured in the blood (NO). A tube for separating serum was filled with a 5-mL sample of venous blood (SST). The SST sample was centrifuged at 3000 rpm for 20 min to guarantee clot formation. Samples of fasted blood were frozen at −80°C and thawed for the test. A TG kit (Roche, Basel, Switzerland), CHOL kit (Roche, Basel, Switzerland), HDL-C plus 3rd generation kit (Roche, Basel, Switzerland), and LDL-C plus 2nd generation kit were used in the enzymatic colorimetric assay (Roche, Germany). The following formula was used to determine Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) values: Insulin (U/mL) divided by glucose (mg/dL) equals HOMA-IR. Total NO was measured using the colorimetry method with total NO and a nitrate/nitrite assay (R&D Systems, Minneapolis, MN). CRP was measured using the turbidimetric immunoassay method with cardiac C-reactive protein (Latex) High Sensitive (Roche, Basel, Switzerland). All blood tests were carried out twice.
Statistical Analysis
With the aid of the G*power analysis tool, the sample size was determined. Based on data from a similar study in frail older adults,16 a power analysis using an ɑ-level and power of 0.05 and 0.80, respectively, showed a sufficient sample size of 15 participants. The means and standard deviations (SDs) were computed for each main dependent variable. Data normality was demonstrated via the Kolmogorov–Smirnov test. The means (baseline vs post-intervention) were compared with a paired t-test or Wilcoxon test (based on data normality). To assess the impact of intrasubject factors (time: pre and post), intersubject factors (group: intervention protocol), and the interaction between group and time, a two-way analysis of variance (group time) with repeated measurements was performed. The Greenhouse-Geisser test was used to determine the differences in the univariate analysis of this model, and Tukey’s test was utilized for post-hoc analysis. We used of Cohen’s d, which expresses the importance of a statistic derived from a sample of data and standardized mean differences. Cohen’s d was calculated using a 95% confidence interval and a significance level of p 0.05. We evaluated the significant effects using Cohen’s d (effect sizes: small d = 0.2, medium d = 0.5, and large d = 0.8). The Statistical Package for Social Science was used for all analyses (SPSS, ver 25.0, IBM Corp., Armonk, NY, USA). The cutoff for statistical significance was p < 0.05.
Results
A total of 60 participants aged ≥ 65 (mean age, 81.5 ± 4.3 yrs) were registered in this study (Table 1). The participant’s compliance with the study protocol was 97% and 92% in the exercise and dietary interventions, respectively, based on the exercise log and dietary survey. Four participants dropped out either because of relocation (n = 1) because they were unsatisfied with the randomized result (n = 2) or for other personal reasons (n = 1). Therefore, 56 participants (93.3%) completed the 8-week intervention program (Figure 1). The 8-week adherence rate was 95%, 97%, and 91% for the DG, ADG, and AEDG, respectively. There were no safety issues related to exercise and diet during the 8-week intervention period. Selected participant characteristics are listed in Table 1. The groups did not differ in age, height, weight, BMI, free fat mass, and body fat percentage.
 |
Table 1 Selected Participant Characteristics |
Frailty Status
As shown in Table 2, frailty status significantly decreased in all intervention groups after 8 weeks (p < 0.05) (Interaction: p = 0.007, η2 = 0.497; Group: p = 0.33, η2 = 0.074; Time: p = 0.001, η2 = 0.24).
 |
Table 2 Changes in Frailty Status Before and After the 8-Week Intervention Period |
Dietary Composition
Pre-frail individuals have a lower energy intake, with an optimal intake of 1500 kcal/day. Therefore, after the intervention, as measured by the dietary log, total energy was optimized at approximately 1500 kcal/day in the DG, ADG, and AEDG. Total energy (Interaction: p = 0.066, η2 = 0.15; Group: p = 0.001, η2 = 0.35; Time: p = 0.001, η2 = 0.263), carbohydrate (Interaction: p = 0.082, η2 = 0.14; Group: p = 0.003, η2 = 0.265; Time: p = 0.007, η2 = 0.156), protein (Interaction: p = 0.117, η2 = 0.124; Group: p = 0.001, η2 = 0.372; Time: p = 0.007, η2 = 0.156), and fat (Interaction: p = 0.164, η2 = 0.108; Group: p = 0.001, η2 = 0.396; Time: p = 0.001, η2 = 0.316) significantly increased in all intervention groups (p < 0.05) (Table 3).
 |
Table 3 Changes in Macro-Nutrition Components Before and After the 8-Week Intervention Period |
Physical Fitness
As shown in Table 4, the right-hand grip strength significantly increased in all intervention groups after 8 weeks (p < 0.05), while left grip strength did not change. SPPB and walking speed significantly increased after 8 weeks in the ADG and AEDG (p < 0.05), but those in the CG and DG did not increase (Interaction: p = 0.037, η2 = 0.174; Group: p = 0.251, η2 = 0.088; Time: p = 0.001, η2 = 0.481). A submaximal aerobic exercise test, as measured by the 6-min walk test, was significantly increased in the ADG and AEDG after 8 weeks (Interaction: p = 0.001, η2 = 0.419; Group: p = 0.048, η2 = 0.163; Time: p = 0.001, η2 = 0.475).
 |
Table 4 Changes in Physical Fitness Before and After the 8-Week Intervention Period |
BP and Biomarkers
BP and biomarkers for cardiovascular risk factors are shown in Tables 5 and 6. There were no significant changes in baseline measurements for systolic and diastolic BP, mean arterial pressure, pulse pressure, and resting HR after 8 weeks of intervention. Differences in glucose, HbA1c, TC, LDL-C, TG, insulin, HOMA-IR, NO, and CRP levels were not observed after the interventions. However, all intervention groups significantly increased HDL-C levels after 8 weeks (Tables 5 and 6).
 |
Table 5 Changes in Blood Pressure Before and After the 8-Week Intervention Period |
 |
Table 6 Changes in Biomarkers Before and After the 8-Week Intervention Period |
Vascular Function
PWV and FMD results are shown in Figure 3. PWV was not statistically different among the intervention groups (Figure 3A). FMD was significantly increased in the ADG and AEDG after 8 weeks (Figure 3B) (Interaction: p = 0.003, η2 = 0.275; Group: p = 0.729, η2 = 0.029; Time: p = 0.002, η2 = 0.199). FMD was significantly increased in ADG and AEDG (ADG: Δ25.8%, 3.1±0.9% to 3.9±0.8%, AEDG: Δ25%, 3.1±0.9% to 4±1.2%, respectively).
Discussion
This study aimed to determine how an 8-week multifactorial intervention affected the physical health and vascular function of pre-frail older women. Most studies focusing on improvement in body composition, muscle, and function on physiological mechanisms such as blood vessel function and CVD have been insufficient. To our knowledge, our study is the first to demonstrate the positive effects of regular exercise and healthy diet intake on physical fitness and vascular function, despite a relatively short intervention period of 8 weeks.
As mentioned, grip strength is a quick, easy, and validated measure of frailty. Weak handgrip strength in frailty is linked to a higher risk of cardiovascular and all-cause death and predicts decreasing muscle strength. In the English Longitudinal Study of Ageing (ELSA), reduced grip strength was significantly associated with sedentary behavior in older adults.31 Our results show a prominent improvement in right-hand grip strength in all intervention groups. Hsieh and colleagues observed similar results during a 12-week intervention period and a 12-week self-maintenance duration.16 Although the additional effects of exercise with nutrition were not shown, muscle strength can be increased by nutritional treatment alone in our study.
SPPB could be utilized as a screening tool to identify frailty in elderly people living in their communities.32 According to the SPPB score, functional degradation and disability of the elderly can be predicted, with the score associated with the risk of disability in those likely to be admitted to long-term care facilities due to reduced function.33 In this study, it was found that the SPPB score improved more significantly in the exercise groups. In a cross-sectional study, Lee reported a similar significant difference in SPPB between the elderly with and without regular exercise.34 Therefore, continuous aerobic exercises of the pre-frail female elderly could reduce lower limb weakening, delay functional degradation, and maintain physical function. Most of the participants in our study ameliorated by at least 1-point in the SPPB at week 8. Our results imply that aerobic exercise and nutrition intervention should have a considerable effect on lower limb function in pre-frail elderly women, although no additional effect was seen by EMS.
We found that the 6-min walk improved significantly in the exercise groups. Various physiological changes caused by aging have a particularly negative impact on the cardiopulmonary endurance of the elderly. In particular, it has been reported that reduced muscle mass is accompanied by degradation of heart function, decreased contractility, maximum arteriovenous oxygen difference, and maximum cardiac output.35 EMS training significantly increased aerobic capacity in healthy sedentary adults.36 In this study, aerobic exercise and EMS were simultaneously conducted. However, EMS did not show any additional impact on the improvement of aerobic capacity in the pre-frail elderly. This may be attributed to methodological issues, such as intensity, duration, attached position (eg, whole-body vs localized extremities), or individual training characteristics.
It should be noted that increased arterial stiffness is related to frailty.9 Furthermore, the level of frailty is associated with arterial stiffening in community-dwelling older adults in cross-sectional studies.12 The effect of protein added to a healthy lunch-box, and stepping exercise on arterial stiffness has not been observed in our pre-frail participants, probably because the pre-frail and frail elderly already had very high levels of arterial stiffness. The elevated arterial stiffness might have been difficult to alter because the elevated arterial stiffness status had already induced structural changes in the arterial wall. Chronic low-grade inflammation and excessive oxidative stress are characteristics of fragility, which are thought to contribute significantly to cardiovascular dysfunction.15
The risk of frailty has been linked to worse endothelial function.37 Frailty can be improved with effective interventions such as regular physical exercise and balanced nutrition.15 However, in this study, greater protein in well-nourished lunch boxes without exercise did not show a favorable effect on endothelial function after 8 weeks. We cautiously thought that the period of nutrition intervention was shorter in order to alter endothelial function. Another possible explanation is that increased fat intake and protein intake with the lunch boxes might have reduced beneficial effects on endothelial function. Macronutrients are thought to predominantly induce oxidative stress caused by high-fat meals and triglyceride-rich lipoprotein, resulting in endothelial dysfunction.25
In a study in which postmenopausal women with impaired endothelial function (FMD <4.5%) who did not have exercise habits were selected and performed aerobic exercise (walking/jogging, 4 times a week, moderate (RPE, 10–12) to high intensity (RPE, 15–17), and 20~40 minutes/day, progressively) for 12 weeks, a significant improvement in FMD was founded following aerobic training.38 These results are consistent with our results that FMD was significantly increased in ADG and AEDG (ADG: Δ25.8%, 3.1±0.9% to 3.9±0.8%, AEDG: Δ25%, 3.1±0.9% to 4±1.2%, respectively). Most studies have demonstrated that aerobic training improves endothelial function via NO.39,40 Aerobic exercise directly affected the vasculature by sporadic increases in the shear stress. With evidence of aerobic training, there may be an upregulation of eNOS or a decrease in the free radical destruction of NO as a result. As most of the studies were derived from cross-sectional studies, the multifactorial interventions with nutrition and exercise for pre-frail elderly women in this study are considered to be valuable data. In this study, improvement in cardiovascular function as assessed by FMD was found by aerobic exercise intervention, despite a short period of intervention. Although muscular stimulation provides greater vascular remodeling and increased peripheral vascular adaptation by exercise training,20 EMS was not an effective strategy for vascular function in this study.
In this study, the combination of exercise and nutrition interventions did not affect blood chemistry parameters, suggesting that the intervention was limited to better adaptations to systemic metabolism. One possible explanation may be the short duration of the intervention. Dose-response to training and history of exposure to exercise could be other possible reasons. In contrast, all intervention groups in this study demonstrated significantly increased HDL-C levels after 8 weeks. High doses of protein are associated with higher HDL-C production.41 Even though the status of body weight regulates this response, increased protein in the habitual diet was independently associated with higher HDL-C regardless of the total amount of nutritional energy, carbohydrates, and fat.42 A cross-sectional trial suggested that consuming a higher protein diet is associated with greater HDL-C and has a lower risk of developing cardiometabolic disease in US adults.42 Most studies have demonstrated that aerobic exercise increased HDL-C levels.43 Even though substantial increases in carbohydrate, protein, and fat were made in our study, protein-added healthy lunchboxes and aerobic exercise had a positive effect on HDL-C levels.
This study has several limitations. First, it is not possible to generalize the results for pre-frail elderly women because of the small sample size. Second, the intervention period of 8 weeks was relatively short compared to the corresponding period of intervention. However, we compared these periods, trying intervention at six weeks for most EMS studies. Third, dietary intake was conducted twice daily for 5 weekdays for the 8 weeks. It was recommended to take a balanced meal when lunch-boxes were not provided during weekends, but most participants might have failed to do so. Due to financial and management constraints, we could not provide dietary intake during weekends. Fourth, we could not control chronic diseases, including diabetes, obesity, and high blood pressure. Therefore, follow-up studies with more specific controls are needed to support this study. Fourth, we did not present the exercise group alone, so the dose-response relationship with exercise was not compared. Finally, a double-blind design was only partially applied to this study. It was difficult to intervene as participants spent most of their time together at the senior community center during the day. While this study has provided the important first data conducted in real-life conditions to make multifactorial interventions, further studies are warranted in the future to elucidate the effects over the longer term in a similar population.
Conclusion
Combined protein-added to healthy lunch-boxes and stepping exercises had a positive effect on physical fitness and endothelial function for a short duration of 8 weeks in pre-frail older women. However, the additional effect of EMS did not show. This study provides clinical aspects with cardiovascular benefits that could improve the management of frailty in community-dwelling older women.
Data Sharing Statement
The authors can offer complete individual participant data without identifying information if available. Please send your request for original data to the e-mail address of Jonghoon Park, Ph.D. at [email protected].
Institutional Review Board Statement
The Institutional Review Board of Korea University approved the protocol and informed consent form (KUIRB-2019-0161-01) in South Korea, and all study procedures were performed in accordance with the Declaration of Helsinki. The data in the study are publicly available in the Clinical Research Information Service in Korea (KCT0006888, 05/01/2022) and WHO ICTRP (International Clinical Trials Registry Platform, http://www.who.int/ictrp).
Acknowledgments
This research was supported by NRF (National Research Foundation of Korea) Grant funded by the Korean Government (NRF-2017-Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program). This research was supported by the Chung-Ang University Research Grants in 2022. We thank the company Massng Ltd, for providing lunchbox and financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–441. doi:10.2147/CIA.S45300
2. Fried P, Tangen M, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56:146–156. doi:10.1093/gerona/56.3.M146
3. Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi:10.1111/j.1532-5415.2012.04054.x
4. Zhang Q, Guo H, Gu H, et al. Gender-associated factors for frailty and their impact on hospitalization and mortality among community-dwelling older adults: a cross-sectional population-based study. Peer J. 2018;6:e4326. doi:10.7717/peerj.4326
5. Boente-Antela B, Leiros-Rodriguez R, Garcia-Soidan JL. World Health Organization on the practice of physical activity in people over 65 years in Spain. J Hum Sport Exerc. 2022;17(1):29–38.
6. Leiros-Rodriguez R, Romo-Perez V, Garcia-Soidan JL, et al. Prevalence and factors associated with functional limitations during aging in a representative sample of Spanish population. Phys Occup Ther Geriatr. 2018;36(2–3):156–167. doi:10.1080/02703181.2018.1449163
7. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. doi:10.1016/j.jacc.2013.09.070
8. Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30(7):756–764. doi:10.1016/j.cjca.2014.03.020
9. Angulo J, Assar M, Alvarez-Bustos A, et al. Physical activity and exercise: strategies to manage frailty. Redox Biol;2020. 101513. doi:10.1016/j.redox.2020.101513
10. Maruhashi T, Kajikawa M, Kishimoto S, et al. Vascular function is further impaired in subjects aged 80 years or older. Hypertens Res. 2020;43(9):914–921. doi:10.1038/s41440-020-0435-z
11. Jennings A, MacGregor A, Welch A, et al. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. 2015;145(9):2130–2138. doi:10.3945/jn.115.214700
12. Orkaby R, Lunetta L, Sun J, et al. Cross-Sectional Association of Frailty and Arterial Stiffness in Community-Dwelling Older Adults: the Framingham Heart Study. J Gerontol a Biol Sci Med Sci. 2019;74(3):373–379. doi:10.1093/gerona/gly134
13. Ardestani S, Eftedal I, Pedersen M, et al. Endothelial dysfunction in small arteries and early signs of atherosclerosis in ApoE knockout rats. Sci Rep. 2020;10(1):15296. doi:10.1038/s41598-020-72338-3
14. Santillo E, Migale M, Balestrini F. Frailty and flow-mediated dilation: a pilot study in hospitalized elderly. J Curr Res Sci Med. 2016;2:92–97. doi:10.4103/2455-3069.198368
15. Soysal P, Isik T, Carvalho F, et al. Oxidative stress and frailty: a systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. doi:10.1016/j.maturitas.2017.01.006
16. Hsieh J, Su C, Chen W, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):119. doi:10.1186/s12966-019-0855-9
17. Xue Q, Qin Z, Jia J, et al. Association between frailty and the cardio-ankle vascular index. Clin Interv Aging. 2019;26(14):735–742. doi:10.2147/CIA.S195109
18. Seals D, Nagy E, Moreau K. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol. 2019;2:1–14.
19. Kemmler W, von Stengel S. New strategies to fight against sarcopenia at old age. J Aging Res. 2012;109013. doi:10.1155/2012/109013
20. Kemmler W, Teschler M, Weissenfels A, et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteo Int. 2016;27:3261–3270. doi:10.1007/s00198-016-3662-z
21. Beasley J, LaCroix A, Neuhouser M, et al. Protein intake and incident frailty in the Women’s Health Initiative observational study. J Am Geriatr Soc. 2010;58:1063e1071. doi:10.1111/j.1532-5415.2010.02866.x
22. Coelho-Junior H, Rodrigues B, Uchida M, et al. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10:1334. doi:10.3390/nu10091334
23. Miller M, Bannerman E, Daniels L, et al. Lower limb fracture, cognitive impairment and risk of subsequent malnutrition: a prospective evaluation of dietary energy and protein intake on an orthopaedic ward. Eur J Clin Nutr. 2006;60:853e861. doi:10.1038/sj.ejcn.1602390
24. Hu F, Stampfer M, Manson J, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr. 1999;70(2):221–227. doi:10.1093/ajcn.70.2.221
25. LaRocca T, Martens C, Seals D. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev. 2017;39:106–119. doi:10.1016/j.arr.2016.09.002
26. Tanaka H, Monahan K, Seals D. Age-predicted maximal heart rate revisited. J. Am Coll Cardiol. 2001;37(1):153–156. doi:10.1016/S0735-1097(00)01054-8
27. Korea National Health and Nutrition Examination Survey. 2015.
28. Pritchard J, Kennedy C, Karampatos S, et al. Measuring frailty in clinical practice: a comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr. 2017;17(1):264. doi:10.1186/s12877-017-0623-0
29. Park W, Miyach M, Tanaka H. Does aerobic exercise mitigate the effects of cigarette smoking on arterial stiffness? J Clin Hypertens. 2014;16(9):640–644. doi:10.1111/jch.12385
30. Park W, Lee J, Park H-Y, et al. Vascular function and frailty in community-dwelling older individuals. Artery Res. 2022;28:31–39. doi:10.1007/s44200-022-00012-2
31. Hamer M, Stamatakis E. Screen-based sedentary behavior, physical activity, and muscle strength in the English longitudinal study of ageing. PLoS One. 2013;8(6):1–5. doi:10.1371/journal.pone.0066222
32. Fishe S, Ottenbacher K, Goodwin J, et al. Short physical performance battery in hospitalized older adults. Aging Clin Exp Res. 2009;21:445–452. doi:10.1007/BF03327444
33. Perracini M, Mello M. Maximo de O, et al. Diagnostic accuracy of the short physical performance battery for detecting frailty in older people. Phys Ther. 2020;100(1):90–98. doi:10.1093/ptj/pzz154
34. Lee K. Effects of balance control on physical performance of elderly women. Neurotherapy. 2012;16(1):37–43.
35. Figueroa A, Park S, Seo D, et al. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18(9):980–984. doi:10.1097/gme.0b013e3182135442
36. Banerjee P, Caulfield B, Crowe L, et al. Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J Appl Physiol. 2005;99(6):2307–2311. doi:10.1152/japplphysiol.00891.2004
37. Alonso-Bouzon C, Carcaillon L, Garcia-Garcia F. Association between endothelial dysfunction and frailty: the Toledo study for healthy aging. Age. 2014;36(1):495–505. doi:10.1007/s11357-013-9576-1
38. Swift DL, Weltman JY, Patrie JT, et al. Predictors of improvement in endothelial function after exercise training in a diverse sample of postmenopausal women. J Womens Health. 2014;23(3):260
39. Linke A, Schoene N, Gielen S, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi:10.1016/S0735-1097(00)01108-6
40. Walsh J, Best M, Maiorana A, et al. Exercise improves conduit vessel endothelial function in CAD patients. J Appl Physiol. 2003;285:20–25. doi:10.1152/japplphysiol.00012.2003
41. Jennings A, MacGregor A, Welch A, et al. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. 2015;145(9):2130–2138.
42. Pasiakos S, Lieberman H. Fulgoni 3rd V. Higher-protein Diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US Adults. J Nutr. 2015;145(3):605–614. doi:10.3945/jn.114.205203
43. Sunami Y, Motoyama M, Kinoshita F. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism. 1999;48:984–988. doi:10.1016/S0026-0495(99)90194-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.