Back to Journals » Drug Design, Development and Therapy » Volume 14
Protective Effects of Rocuronium Bromide on Ischemia-Reperfusion Injury in Skeletal Muscle Induced by Tourniquet in Patients Undergoing Elective Unilateral Total Knee Arthroplasty: A Prospective, Double Blind, Randomized, Controlled Study
Authors Chen H, Wei JQ, Wang YW , Zhou KP, He Y, Liu H , Zhang YY
Received 8 March 2020
Accepted for publication 26 July 2020
Published 18 August 2020 Volume 2020:14 Pages 3373—3384
DOI https://doi.org/10.2147/DDDT.S252546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Hui Chen,* Jing-Qiu Wei,* Yi-Wen Wang, Kun-Peng Zhou, Ying He, He Liu, Yue-Ying Zhang
Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yue-Ying Zhang Department of Anesthesiology
The Affiliated Hospital of Xuzhou Medical University, Huaihai Western Road, Quanshan District, Xuzhou, Jiangsu 221004, People’s Republic of China
Tel +86 138 1531 0789
Email [email protected]
Purpose: To investigate the effects of different doses of rocuronium on ischemia-reperfusion injury in skeletal muscle induced by tourniquet in patients undergoing elective unilateral total knee arthroplasty.
Patients and Methods: A total of 90 patients undergoing elective unilateral knee arthroplasty under general anesthesia combined with femoral nerve block were randomly divided into 3 groups: normal saline group (group S), rocuronium 0.6 mg/kg group (group L), and rocuronium 1.2 mg/kg group (group H). The primary outcome was the expression of dystrophin in skeletal muscle at 60 min after ischemia. Secondary outcomes included the concentration of malondialdehyde (MDA) and neuronal nitric oxide synthase (nNOS) in blood at 5 min and 30 min after reperfusion. In addition, thigh girth at 24 h and 48 h after operation, the leaving bed time, the incidence of tourniquet-related hypertension and short-term (3 days after operation) complications (nausea and vomiting, swelling, blister, wound infection) and long-term (3 months after operation) complications (joint instability, stiffness, nerve paralysis, pain) were recorded.
Main Results: The expression of dystrophin in the rocuronium group was higher than that in group S after ischemia (P < 0.05). The concentration of MDA in the rocuronium 1.2 mg/kg group was lower at 30 min after reperfusion (P < 0.05). There was no significant difference in nNOS among groups at each time point (P > 0.05). The change of thigh girth was the smallest in the rocuronium 1.2 mg/kg group after operation (P< 0.05). The leaving bed time was significantly earlier after operation in the rocuronium group than that in group S (P < 0.05).
Conclusion: Rocuronium can protect skeletal muscle from ischemia-reperfusion injury induced by tourniquet. The mechanism may be related to the fact that rocuronium can reduce the loss of dystrophin in skeletal muscle and have the effects of anti-oxidation and anti-stress.
Trial Registration: The study was registered at http://www.chictr.org.cn (ChiCTR1800019221, registered on 2018– 10-31).
Keywords: ischemia-reperfusion injury, rocuronium, dystrophin, TKA
Introduction
Total knee arthroplasty (TKA) is one of the most effective joint reconstructive surgeries in the past 30 years. It is mainly used for severe joint pain, joint instability, joint deformity, and daily activity disorder caused by various types of non-suppurative arthritis and traumatic arthritis in clinical practice, and ineffective after conservative treatment. Tourniquets can block blood circulation, reduce intraoperative blood loss, ensure a clear operative field, and provide a better bone–bone cement interface for TKA, but the potential risks caused by tourniquet use have gradually attracted attention.1 Inflation of the tourniquet can lead to early complications, such as nerve paralysis, vascular injury, postoperative pain exacerbation, postoperative limb swelling, stiffness, delayed recovery of muscle strength, delayed wound healing, and deep venous thrombosis of lower extremities induced by limb ischemia and hypoxia.2
After long-term ischemia, the tissue undergoes anaerobic metabolism, increasing the production of lactic acid and H+ and decreasing the level of adenosine triphosphate (ATP) and the intracellular pH value.3 A group of cytoskeleton proteins are distributed on the membrane, jointly maintaining the stability of the sarcolemma, including dystrophin, dystroglycan complex (DG), sarcoglycans, and syntrophin.4 Dystrophin is the main component of the cytoskeleton of the myofibrillar membrane, and it is the bridge between actin and a series of proteins on the sarcolemma. Acute ischemia can lead to the loss of dystrophin and the depletion of dystrophin-associated proteins.5 The loss of dystrophin was observed in the histological evidence of myocardial ischemia in varying degrees, indicating that it may be a marker of early ischemia.6
The body is in a state of oxidative stress due to a large number of oxygen-free radicals being activated while blood flow and oxygen supply are restored after deflation.7 Neutrophil activation significantly increases oxygen consumption of tissues and stimulates phagocytes to produce a respiratory burst. L-arginine synthesizes arginine-nitric oxide under the action of NOS. A high concentration of NO reacts with O2- to form peroxynitrite anion (ONOO-), and its decomposition product hydroxyl radical (OH-) can cause cell and tissue damage. Radical oxygen species (ROS) form polyunsaturated fatty acids or lipoproteins through lipid peroxidation, which destroys the normal structure of the cytomembrane, and results in ischemia-reperfusion injury (IRI).8 Malondialdehyde (MDA) is one of the end-products of lipid peroxidation, which can indirectly reflect the production of ROS, and is an objective index to reflect the degree of lipid peroxidation and ischemia-reperfusion injury in the organism.9 It is difficult to directly measure enzyme-generated NO in vivo, and generally, the production of NO can be indirectly reflected by measuring NOS.10 There are three isoforms of NO synthase isozymes: neuronal nitric oxide synthase (NOSI/nNOS), endothelial nitric oxide synthase (NOSIII/eNOS) and inducible nitric oxide synthase (NOSII/iNOS). Both NOSI/nNOS and NOSIII/eNOS are related to the maintenance of adequate micro-vascular blood flow and the regulation of skeletal muscle function.11
There is evidence that the degree of muscle injury caused by IRI is closely related to the duration of ischemia,12 tourniquet inflation pressure,13 and mode.14 Therefore, surgeons often try to shorten the time of ischemia, reduce the pressure of the tourniquet, and change the method of using tourniquets in order to reduce the IRI of skeletal muscle of the operated limb.15 However, due to the limitations of surgical conditions and surgical techniques, it is often hard to succeed. Therefore, it has become a common concern of surgeons and anesthesiologists how to find additional strategies to reduce muscle ischemia-reperfusion injury, reduce short-term and long-term postoperative complications, and improve quality of life after discharge.
Previous studies prove that intraoperative use of dexmedetomidine,16 propofol,17 and sevoflurane18 has certain impacts on skeletal muscle IRI caused by tourniquet. The non-depolarizing neuromuscular block, rocuronium bromide, is an adjuvant for general anesthesia used for endotracheal intubation during routine induction anesthesia and for intraoperative muscle relaxation maintenance. Rocuronium induces skeletal muscle relaxation by blocking the N-cholinergic receptor. It has been shown that rocuronium has a protective effect on skeletal muscle ischemic injury in rats,19 but there is no clinical evidence to show the effects of rocuronium on muscle ischemia injury in humans. The main purpose of this study was to investigate whether different doses of rocuronium can alleviate the ischemia-reperfusion injury caused by tourniquet after TKA. It provides a new idea for clinical prevention of ischemia-reperfusion injury caused by perioperative orthopedic tourniquet.
Patients and Methods
This is a prospective, single-center, double-blind, randomized, controlled clinical trial. It has been approved by the Research Ethics Committee of Affiliated Hospital of Xuzhou Medical University (Xuzhou city, Jiangsu province, China) on 24 January (XYFY2019-KL014) and registered in the Chinese Clinical Trial Registry (ChiCTR1800019221). Written informed consent was obtained from all study participants before the operation.
Participants
From February 2019 to October 2019, 90 patients aged 50 to 80, ASA I–III, scheduled to undergo TKA at the Affiliated Hospital of Xuzhou Medical University, were selected for this study. Patients with metabolic diseases such as diabetes, rheumatoid arthritis, or long-term use of hormones, neuromuscular diseases, infectious diseases, cholinergic and adrenergic drug use, severe anemia or coagulation dysfunction, congestive heart failure or arrhythmia, severe damage to liver and kidney function, bilateral TKA, allergy to any of the drugs used in the present study and body mass index ≥30 kg/m2 were excluded. Elimination criteria: tourniquet inflation time >150 min or < 60 min; admission to ICU after surgery.
Randomization and Masking
All subjects were randomized into three groups using a computer-generated table of random numbers: Group S (normal saline), Group L (rocuronium 0.6 mg/kg), and Group H (rocuronium 1.2 mg/kg), and 30 patients in each group. The grouping was kept in a numbered opaque and sealed envelope by a nurse independent of the study. The grouping is blinded to patients and anesthesiologists. A doctor opened the sealed envelope and prepared the relevant drugs according to the grouping once the patient entered into the operating room. The intervention drugs were diluted to 10 mL and encapsulated in colorless syringes of the same size for anesthesia induction by the staff who did not participate in follow-up and data analysis. Another person who did not know about the grouping was responsible for perioperative data collection and statistics. Postoperative follow-up and postoperative data were recorded by another doctor whose grouping and intraoperative conditions were unknown.
Anesthesia and Intervention
Patients did not receive premedication. ECG, pulse oxygen saturation, heart rate (HR), invasive arterial blood pressure (IBP), BIS, and nasopharyngeal temperature were routinely monitored after entering the room. Neuromyoelectric module (NMT) (GE Healthcare, China) was used during the operation. Train-of-four stimulation (TOF) and post-tetanic count stimulation (PTC) were used to monitor the muscle twitch of the adductor pollicis muscle to judge the degree of muscle relaxation and reflect the efficacy and elimination of rocuronium. Anesthesia was induced with midazolam 0.03 mg/kg, etomidate 0.3 mg/kg, sufentanil citrate 0.6 μg/kg and rocuronium (0.6–1.2 mg/kg), or saline. A laryngeal mask was used for mechanical ventilation for all patients, maintaining PETCO2 35–45 mmHg (1 mm Hg=0.133 kPa), and patients underwent nerve blocking after induction (5 mL of 2% lidocaine and 10 mL of 0.75% ropivacaine diluted to 20 mL with normal saline). During operation, 1% sevoflurane, propofol, and remifentanil were maintained. The dose of propofol and remifentanil was adjusted according to IBP, HR, and BIS (to maintain HR at 50–100 bpm, to keep systolic blood pressure fluctuation to no more than 20% of the baseline, and to maintain BIS 40–60 during operation). If the systolic blood pressure decreased more than 20% of the baseline or was lower than 90 mmHg, or HR was less than 50 bpm, ephedrine 5 mg or atropine 0.5 mg were given intravenously as appropriate. Automatic pneumatic tourniquet (Changzhou yanling co. LTD) was tied to the upper and middle thigh with inflating pressure as the SBP+150 mmHg.13 Tourniquet was inflated before skin incision and deflated after implant. Then, the incision was closed after safe hemostasis. Patient-Controlled Analgesic (PCA) (2 μg/kg sufentanil and dolasetron 25 mg diluted to 100 mL with normal saline, speed 2 mL/h, locking time 15 min, 0.5 mL each pressing) was linked 30 min before the end of the operation to relieve the postoperative pain. All operations were performed by the same team of surgeons. The perioperative use of antibiotics and postoperative rehabilitation treatment program were the same in the three groups. Patients with VAS > 4 were given flurbiprofen axetil intravenously by surgeons who did not know the grouping after the operation. The effective pressing times of PCA, and the dosage of sufentanil and flurbiprofen axetil were recorded.
Histological Outcome
The primary outcome measure was expression of dystrophin by immunohistochemistry (IHC) when the tourniquet was inflated for 60 min. Femoral medialis muscle 6 mm × 6 mm was taken from the incision edge of the surgical side and stored at −80°C immediately. Frozen tissues were cut into 4–5 μm sections and incubated with 1% acetone and 3% H2O2-methanol solution at room temperature (15–25°C); the slides were sealed with goat serum after being rinsed with phosphate-buffered saline (PBS); the sections were diluted with mouse anti-Dystrophin antibody (USA Proteintech Group 12715-1-ap) (diluted at 1:50), and incubated at 37°C for 2 hours. Subsequently, general mouse/rabbit polymer (MXB Biotechnologies, Fuzhou, China) was added and incubated at 37°C for 30 min. The immunoreactions were visualized using Elivision™ plus Polymer HRP (Mouse/Rabbit) (MXB Biotechnologies, Fuzhou, China, kit-9002), followed by color development with 3,3′-diaminobenzidine tetrahydrochloride (MXB Biotechnologies, Fuzhou, China, DAB-1031). Sections were stained with hematoxylin and examined in a high-resolution light microscope (Olympus BX43, JAPAN). The immunohistochemical positive reaction was located around the sarcolemma, dark brown staining was strongly positive, brown was positive, light yellow was weakly positive, and no staining was negative. The image was quantitatively analyzed by computer (HP Pro3005MT, USA), and the integral optical density (IOD) and positive area (AREA) were obtained.
Biochemical Outcomes
Blood samples were collected from the radial artery of each patient to examine the concentration of malondialdehyde (MDA) and neuronal nitric oxide synthase (nNOS) immediately after tourniquet inflation (T0), 60 min after inflation (Ti), 5 min after deflation (Td1) and 30 min after deflation (Td2). After centrifuging at 3000 r/min for 10 min, serum was stored at −80°C until determination. Serum MDA concentrations were assayed according to the thiobarbituric acid method (TBA) (Jiancheng Bioengineering, Nanjing, China). Serum nNOS concentrations were measured by Enzyme-Linked Immunosorbent Assay (ELISA) (Cloud.Clone corp., USA).
Clinical Outcomes
The clinical outcomes included recording the occurrence of tourniquet-related hypertension (tourniquet-related hypertension is defined as blood pressure increasing by more than 30% during 30–60 min after tourniquet use with sufficient depth of anesthesia), tourniquet inflation time, PTC count after induction, and time of TOF ratio (TOFr) recovering to 25% and 90%. Thigh girth (10 cm below the groin) was recorded at the end of the operation, 24 h after operation, and 48 h after operation. The leaving bed time after operation, the range of motion (ROM) (refers to the maximum range of motion that the joint can complete in a specific position) of the operated limb, postoperative VAS score, and sufentanil and flurbiprofen axetil dosage, and incidence of short-term (3 days) and long-term (3 months) complications were recorded.
Statistical Analysis
The sample size calculations were performed based on the expression of dystrophin. According to previous studies, the expression of dystrophin in rocuronium groups was 36% higher than that in normal saline groups after ischemia, which could significantly reduce the damage to muscle cells.19 Assuming that the expression of dystrophin in the rocuronium group increased by 36% or more than that in the normal saline group, the values 1-β = 0.9, α = 0.05 were selected. It was calculated that 25 patients were needed in each group. Given the 20% drop-out rate, 30 patients were enrolled in each group and the total sample size was 90.
Data were analyzed using SPSS Statistics software version 23.0 (IBM, USA). The Kolmogorov–Smirnov test was used to determine whether the continuous data conform to the normal distribution. The quantitative variables that obey normal distribution are presented as mean ± SD. Non-normal distribution data are represented by median (M) and interquartile range (IQR). Binomial variables are expressed as rate. The continuous data of normal distribution were analyzed by one-way analysis of variance (ANOVA) at the same time point among the three groups, by repeated ANOVA at different time points within each group, by multivariate-ANOVA at different time points among the three groups. Post-hoc analysis with an LSD test was performed. The continuous data of non-normal distribution among the three groups were analyzed by the Kruskal–Walis rank-sum test. Categorical data were analyzed using the chi-square test. P < 0.05 was considered to indicate statistical significance.
Results
Study Population
A total of 90 patients were screened between February 2019 and October 2019, 8 were excluded (1 patient used tourniquet for more than 150 min, 1 patient refused to use PCA, 3 patients were with incomplete data, 3 patients were lost to follow-up). Thus, 82 patients were enrolled in the study, and the records of the patients were available for the final analysis. The specific content is shown in the flow chart (Figure 1). Study methods for each group are shown in Figure 2. There were no significant differences in terms of age, sex, BMI, tourniquet pressure, or duration among the three groups (P>0.05) (Table 1).
 |
Table 1 Demographic and Perioperative Characteristics in the Three Groups |
 |
Figure 1 Flow chart of the study. |
 |
Figure 2 Study methods for each group. |
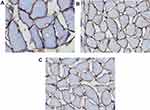
The H&E stained sections of skeletal muscle all showed that the myofibers were uniform in size and arranged in a grid with blue nuclei around. In group H, the membranous staining of dystrophin was dark brown and remained intact (Figure 3A); in group L, the membranous staining was brown with membrane integrity preserved (Figure 3B); in group S, the cell membrane staining was light yellow and some cells lysed (Figure 3C). The integrated optical density (IOD) (7.07±2.42 vs 5.46±2.19, P=0.019) and positive area (AREA) (11.63±3.64 vs 9.13±3.43, P=0.015) in group H were higher than those in group S; there were no significant differences of IOD and AREA between group H and group L at different time points (P>0.05) (Table 2).
 |
Table 2 IHC Results of Dystrophin |
There were no significant differences in the concentrations of MDA at different time points within three groups (P>0.05). Compared to group S, the concentrations of MDA decreased in group H at 30 min after reperfusion (1.226±0.495 nmol/mL and 2.285±1.082 nmol/mL, P=0.028). MDA concentrations at four time points in three groups are shown in Figure 4.
 |
Figure 4 Serum MDA concentrations at four time points in three groups. Data are expressed as mean ± standard deviation, *P=0.028, Group H vs Group S. |
The levels of nNOS in group H decreased at 30 min after reperfusion, whereas there was no significant difference in serum nNOS levels at every time point within three groups (P>0.05). There were no significant differences in nNOS levels after ischemia-reperfusion among three groups (P>0.05). Serum nNOS levels at four time points in three groups are shown in Table 3.
 |
Table 3 Serum nNOS Levels at Four Time Points in Three Groups |
All patients experienced deep muscle relaxation after induction in group H and group L. The time of TOF ratio (TOFr) back to 25% and 90% was significantly extended in group H compared with group L (61.6 ±6.3 min vs 33.8 ±6.4 min, P = 0.001; 85.2±5.7 min vs 56.7 ±6.2 min, P = 0.001). The incidence of tourniquet-related hypertension showed no significant difference among the three groups (P=0.127). There was no significant difference in MAP fluctuation immediately after deflation among the three groups (P=0.236). Recovery times of muscle relaxation and blood pressure fluctuations are demonstrated in Table 4.
 |
Table 4 Recovery Time of Muscle Relaxation and Blood Pressure Fluctuations |
Increases of thigh girth 24 h after operation from that before surgery were 0.33±1.32 cm in group H, 1.22±1.83 cm in group L, and 1.62±1.01 cm in group S (P=0.191,P=0.002, and P=0.001, respectively); increases of thigh girth 48 h after operation were 1.12±1.51 cm in group H, 1.83±2.04 cm in group L, and 2.07±1.45 cm in group S (P=0.001, P=0.001, and P=0.000, respectively). The change of thigh girth after operation in group H is smaller than that in group S (P=0.001 24 h after operation, P=0.039 48 h after operation), showing a significant difference between group H and group S. The time leaving bed after operation in group H was the earliest, group L was the second, and that in group S was significantly later than that in group H (P =0.026). Compared to group S, the hospital stay decreased in the rocuronium group, but the difference was not statistically significant (P=0.728). Evaluation of the postoperative recovery index after operation is demonstrated in Table 5.
 |
Table 5 Evaluation of Postoperative Recovery Index After Operation |
Compared to group S, VAS scores of group H at 24 h and 48 h after surgery were reduced and the difference was statistically significant (P=0.006 and P=0.015). There were no statistically significant differences in the number of effective presses of postoperative analgesia pump, the doses of sufentanil and flurbiprofen axetil among the three groups (P>0.05). Postoperative VAS score and dosage of analgesics are demonstrated in Table 6.
 |
Table 6 Postoperative VAS Score and Dosage of Analgesics |
No residual neuromuscular blockade was observed in all groups. There was no significant difference in the incidence of short-term (3 days after operation) complications (nausea and vomiting, swelling, blister, wound infection) and long-term (3 months after operation) complications (joint instability, stiffness, nerve paralysis, pain after a long walk) (P>0.05). The incidence of short- and long-term postoperative complications is demonstrated in Table 7.
 |
Table 7 The Incidence of Short- and Long-Term Postoperative Complications |
Discussion
The study reveals that non-depolarizing muscle relaxant rocuronium can prevent the loss of dystrophin on the cytomembrane and maintain the integrity of the sarcolemma before ischemia. Large doses of rocuronium can prevent the up-regulation of MDA after reperfusion, so it can be speculated that rocuronium can alleviate the oxidative stress induced by excessive release of ROS after deflation. However, these effects were not obvious in rocuronium at 0.6 mg/kg.
Rocuronium, as an adjuvant for general anesthesia, is used for endotracheal intubation during routine induction anesthesia and for maintaining intraoperative muscle relaxation. The ED95 (95% effective doses) of rocuronium was 0.3 mg/kg, the conventional intubation dose (2 times ED95) was 0.6 mg/kg, the clinical action time (the time from the end of injection to 25% recovery of muscle twitch) was 30 to 40 min, and the total action time (90% recovery time of muscle twitch) was 50 min. The dose of rapid intubation (4 times ED95) was 1.2 mg/kg and the total duration was about 70 min.20 The PTC was 0 either with 0.6 mg/kg or 1.2 mg/kg after induction, indicating that both doses could obtain a state of deep muscle relaxation. Combined with the TOF recovery time of different doses of rocuronium, the patients in the high-dose group were still in a state of complete muscle relaxation when deflated, the 0.6 mg/kg group was already recovering, while the saline group was in the state of no muscle relaxation. Therefore, perfect muscle relaxation can significantly reduce the oxygen consumption of the muscle and reduce the injury caused by ischemia to the muscle in the period of muscle ischemia.21 In addition, no residual neuromuscular blockade was caused due to the action time of 1.2 mg/kg rocuronium matching the operation time of TKA.
Dystrophin, an indispensable cytoskeleton protein, can protect the integrity of free sarcolemma from the destruction caused by longitudinal forces of contraction.22 The location and abundance of dystrophin changed and most of it translocated to myofibril or was lost after ischemia. A series of cellular stress events such as the production of reactive oxygen species (ROS) and calcium overload weaken the sarcolemma through unknown mechanisms, and lead to myocyte necrosis when muscle contractile force is reintroduced after reperfusion.5 Dystrophin-deficient muscles are more sensitive to oxidative stress-induced muscle damage and cell death, with membrane repair and regeneration ability decreased, resulting in impaired muscle function and decreased myodynamia.23 Ledowski et al confirmed for the first time at the cellular level that rocuronium can prevent the down-regulation of dystrophin and reduce the decrease of cellular metabolic activity induced by ischemia, and thus prevent the release of intracellular free calcium.19 In this study, immunohistochemistry was used to determine the expression of dystrophin on the skeletal muscle cell membrane. The results showed that rocuronium 1.2 mg/kg prevented the up-regulation of dystrophin after ischemia and had a certain protective effect on ischemic injury caused by inflation of tourniquet. The results are consistent with the previous experiment.
The mechanism of ischemia-reperfusion injury in skeletal muscle has not been fully elucidated, and may be related to the damage of oxygen free radicals, calcium overload, and NO to skeletal muscle cells after ischemia-reperfusion. Jeong et al showed that rocuronium has the function of anti-oxidation and scavenging oxygen free radicals to a certain extent, and dose-dependently protects vascular endothelial injury induced by ROS.24 This study showed that rocuronium reduced serum MDA 30 min after reperfusion. It can be seen that rocuronium also has a protective effect on reperfusion injury induced by oxidative stress.
NO can aggravate skeletal muscle ischemia-reperfusion injury, but its nature is unstable, so this study measured nitric oxide synthase (NOS). Tsui et al indicated that up-regulation of NOS may be associated with muscle dysfunction following acute ischemia and reperfusion, which elevates both the NOS I/nNOS mRNA and protein during reperfusion occurs in skeletal muscle.11 However, nNOS did not change significantly during either ischemia or reperfusion in this study. One possible reason is that nNOS does not play a role in ischemia-reperfusion injury. A study confirmed this conjecture that there was no difference in infarct size between wild-type and nNOS knockout mice after myocardial ischemia, suggesting that nNOS does not play a role in myocardial reperfusion injury.25 These results seem to be contradictory and need to be further studied. Another possible reason is that we detected nNOS in the blood of the radial artery rather than in the femoral vessels of the operated limb, which reduced the sensitivity of the results. Garcia et al showed that the level of oxidative stress markers in blood from reperfused limbs was higher than that from peripheral blood.9 This is a limitation of this study, which may affect the accuracy of the results.
The clinical indicators observed in this study also showed that rocuronium can alleviate ischemia-reperfusion injury in skeletal muscle. The results showed that the thigh girth of patients 24 hours and 48 hours after TKA was greater than that before operation, the increase of thigh girth was more obvious in the saline group, and the change was the smallest in the 1.2 mg/kg group, consistent with the research of Dennis which demonstrated that the thigh girth increased after tourniquet use in TKA, which may be related to myocyte swelling and tissue edema after compression.2 Janda et al detected heart rate variability (HRV) in children who received endotracheal intubation, demonstrating that children without muscle relaxant had higher HRV parameters such as low frequency/high frequency ratio after intubation, and they had stronger stress response to endotracheal intubation than those with muscle relaxant.26 Relaxation of skeletal muscle is conducive to reducing the confrontation between the skeletal muscle of the affected limb and the great pressure of the tourniquet, thus alleviating the postoperative swelling, pain, and discomfort of the affected limb’s tourniquet compression area, relieving the stress response caused by the tourniquet and surgical operation, and reducing the IRI to skeletal muscle induced by the tourniquet.19
It is clear that muscle weakness translates into declines in functional mobility, as do severe pain, large wound, compression bandaging, and lower limb swelling.27 A previous study proved that ischemia can lead to microcirculatory disorder, and rocuronium can inhibit the inflammatory response and relieve pain after ischemia.28 Range of motion (ROM) was used to reflect the flexibility of knee joint after operation in an experiment which showed that rocuronium can improve knee mobility and flexibility, significantly in larger doses.29 It can be seen that the application of different doses of rocuronium during TKA can reduce the tourniquet-related reaction of the affected limb, relieve the postoperative pain, improve the flexibility of the affected limb, shorten bed time after operation, and speed recovery. It is preliminarily inferred that the protective effect of rocuronium on skeletal muscle is dose-dependent, and the beneficial effect of high-dose rocuronium on skeletal muscle ischemic injury is more obvious. All the patients included in the study were successfully extubated, and there were no adverse reactions such as delayed recovery of muscle relaxation, dyspnea, or residual neuromuscular blockade. The dosage of general anesthesia during the operation was adjusted according to the entropy index, so the dosage was reduced to a minimum under the condition of meeting the requirements of the operation. Combined with the use of a laryngeal mask instead of an endotracheal catheter, the postoperative respiratory complications of the patient could be effectively reduced.
The limitation of this study is that due to the limited surgical conditions, there is a lack of tissue of the operated limb before inflation as a control, which can be used to reflect the degree of ischemia-reperfusion injury after tourniquet application. Although there was no significant difference in the length of stay and the incidence of complications in this study, the length of stay and the incidence of complications in the non-muscle relaxant group were higher than those in the rocuronium group. The probable cause is that the sample size of this trial is calculated according to the changes in patients’ dystrophin after ischemia led to the relatively small sample size. Therefore, future research needs to expand the samples’ quantity and extend the follow-up time.
Conclusion
In summary, rocuronium bromide administered intravenously under general anesthesia can alleviate the ischemia-reperfusion injury of skeletal muscles induced by tourniquet after total knee arthroplasty. The mechanism may be related to the fact that rocuronium can reduce the loss of dystrophin in skeletal muscle and have the effects of anti-oxidation and anti-stress.
Abbreviations
ASA, American Society of Anesthesiologists; ATP, adenosine triphosphate; BMI, Body Mass Index; ED95, 95% effective dose; ELISA, Enzyme-Linked Immunosorbent Assay; HR, heart rate; HRV, heart rate variability; IBP, invasive blood pressure; IHC, immunohistochemistry; IOD, integrating optical density; IRI, ischemia reperfusion injury; MDA, malondialdehyde; NMT, neuromuscular transmission; NO, nitric oxide; NOSI/nNOS I/nNOS, neuronal nitric oxide synthase; NOSII/iNOS, inducible nitric oxide synthase; NOS ш/eNOS, endothelial nitric oxide synthase; PETCO2, end tidal carbon dioxide partial; PTC, post-titanic count stimulation; ROM, range of motion; ROS, reactive oxygen species; SBP, systolic blood pressure; SpO2, pulse oxygen saturation; TKA, total knee arthroplasty; TOF, train-of-four stimulation; VAS, Visual Analogue Scale.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study has been approved by the Research Ethics Committee of Affiliated hospital of Xuzhou Medical University (Xuzhou, Jiangsu, People’s Republic of China) on 24 January (XYFY2019-KL014). In conformation with the Declaration of Helsinki, every patient or patient’s legally authorized representative provided written informed consent before entering the trial.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. Wang K, Ni S, Li Z, et al. The effects of tourniquet use in total knee arthroplasty: a randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2849–2857. doi:10.1007/s00167-015-3964-2
2. Dennis DA, Kittelson AJ, Yang CC, Miner TM, Kim RH, Stevens-Lapsley JE. Does tourniquet use in TKA affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res. 2016;474(1):69–77. doi:10.1007/s11999-015-4393-8
3. Paradis S, Charles AL, Meyer A, et al. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am J Physiol Cell Physiol. 2016;310(11):C968–982. doi:10.1152/ajpcell.00356.2015
4. Ohlendieck K, Ervasti JM, Snook JB, Campbell KP. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991;112(1):135–148. doi:10.1083/jcb.112.1.135
5. Kido M, Otani H, Kyoi S, et al. Ischemic preconditioning-mediated restoration of membrane dystrophin during reperfusion correlates with protection against contraction-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2004;287(1):H81–90. doi:10.1152/ajpheart.01140.2003
6. Campos EC, Romano MM, Prado CM, Rossi MA. Isoproterenol induces primary loss of dystrophin in rat hearts: correlation with myocardial injury. Int J Exp Pathol. 2008;89(5):367–381. doi:10.1111/j.1365-2613.2008.00604.x
7. Jiang L, Li L, Shen J, Qi Z, Guo L. Effect of dexmedetomidine on lung ischemiareperfusion injury. Mol Med Rep. 2014;9(2):419–426. doi:10.3892/mmr.2013.1867
8. Leurcharusmee P, Sawaddiruk P, Punjasawadwong Y, Chattipakorn N, Chattipakorn SC. The possible pathophysiological outcomes and mechanisms of tourniquet-induced ischemia-reperfusion injury during total knee arthroplasty. Oxid Med Cell Longev. 2018;2018:8087598. doi:10.1155/2018/8087598
9. Garcia-de-la-Asuncion J, Perez-Solaz A, Carrau M, Belda FJ, Perez-Griera J, Garriges B. Different oxidative stress marker levels in blood from the operated knee or the antecubital vein in patients undergoing knee surgery: a tourniquet-induced ischemia-reperfusion model. Redox Rep. 2012;17(5):194–199. doi:10.1179/1351000212Y.0000000022
10. Csonka C, Pali T, Bencsik P, Gorbe A, Ferdinandy P, Csont T. Measurement of NO in biological samples. Br J Pharmacol. 2015;172(6):1620–1632. doi:10.1111/bph.12832
11. Tsui JC, Baker DM, Shaw SG, Dashwood MR. Alterations in nitric oxide synthase isoforms in acute lower limb ischemia and reperfusion. Angiology. 2007;58(5):586–592. doi:10.1177/0003319707305466
12. Jawhar A, Ponelies N, Schild L. Effect of limited ischemia time on the amount and function of mitochondria within human skeletal muscle cells. Eur J Trauma Emerg Surg. 2016;42(6):767–773. doi:10.1007/s00068-015-0600-2
13. Kim TK, Bamne AB, Sim JA, Park JH, Na YG. Is lower tourniquet pressure during total knee arthroplasty effective? A prospective randomized controlled trial. BMC Musculoskelet Disord. 2019;20(1):275. doi:10.1186/s12891-019-2636-7
14. Harsten A, Bandholm T, Kehlet H, Toksvig-Larsen S. Tourniquet versus no tourniquet on knee-extension strength early after fast-track total knee arthroplasty; a randomized controlled trial. Knee. 2015;22(2):126–130. doi:10.1016/j.knee.2014.12.010
15. Halladin NL, Zahle FV, Rosenberg J, Gogenur I. Interventions to reduce tourniquet-related ischaemic damage in orthopaedic surgery: a qualitative systematic review of randomised trials. Anaesthesia. 2014;69(9):1033–1050. doi:10.1111/anae.12664
16. Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186(1):240–245. doi:10.1016/j.jss.2013.07.052
17. Ozkan D, Akkaya T, Yalcindag A, et al. Propofol sedation in total knee replacement: effects on oxidative stress and ischemia-reperfusion damage. Anaesthesist. 2013;62(7):537–542. doi:10.1007/s00101-013-2192-8
18. Kosucu M, Coskun I, Eroglu A, et al. The effects of spinal, inhalation, and total intravenous anesthetic techniques on ischemia-reperfusion injury in arthroscopic knee surgery. Biomed Res Int. 2014;2014:846570. doi:10.1155/2014/846570
19. Ledowski T, Nissler S, Wenk M, Pogatzki-Zahn EM, Segelcke D. Effects of muscle relaxants on ischaemia damage in skeletal muscle. Sci Rep. 2018;8(1):5794. doi:10.1038/s41598-018-24127-2
20. Schepens T, Cammu G. Neuromuscular blockade: what was, is and will be. Acta Anaesthesiol Belg. 2014;65(4):151–159.
21. Vernon DD, Witte MK. Effect of neuromuscular blockade on oxygen consumption and energy expenditure in sedated, mechanically ventilated children. Crit Care Med. 2000;28(5):1569–1571. doi:10.1097/00003246-200005000-00051
22. Matre PR, Mu X, Wu J, et al. CRISPR/Cas9-based dystrophin restoration reveals a novel role for dystrophin in bioenergetics and stress resistance of muscle progenitors. Stem Cells. 2019;37(12):1615–1628. doi:10.1002/stem.3094
23. Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2016;96(1):253–305. doi:10.1152/physrev.00007.2015
24. Jeong JS, Suh JK, Cho ES, Kim DW, Jeong MA. Antioxidant effect of muscle relaxants (vecuronium, rocuronium) on the rabbit abdominal aortic endothelial damage induced by reactive oxygen species. Korean J Anesthesiol. 2013;65(6):552–558. doi:10.4097/kjae.2013.65.6.552
25. Jones SP, Girod WG, Huang PL, Lefer DJ. Myocardial reperfusion injury in neuronal nitric oxide synthase deficient mice. Coron Artery Dis. 2000;11(8):593–597. doi:10.1097/00019501-200012000-00004
26. Janda M, Bajorat J, Kudlik C, et al. Comparison of heart rate variability response in children undergoing elective endotracheal intubation with and without neuromuscular blockade: a randomized controlled trial. Paediatr Anaesth. 2013;23(12):1153–1159. doi:10.1111/pan.12236
27. Judd DL, Eckhoff DG, Stevens-Lapsley JE. Muscle strength loss in the lower limb after total knee arthroplasty. Am J Phys Med Rehabil. 2012;91(3):220–226. doi:10.1097/PHM.0b013e3182411e49
28. Baek SB, Shin MS, Han JH, et al. Rocuronium bromide inhibits inflammation and pain by suppressing nitric oxide production and enhancing prostaglandin E2 synthesis in endothelial cells. Int Neurourol J. 2016;20(4):296–303. doi:10.5213/inj.1632796.398
29. Zhang Y, Li D, Liu P, Wang X, Li M. Effects of different methods of using pneumatic tourniquet in patients undergoing total knee arthroplasty: a randomized control trial. Ir J Med Sci. 2017;186(4):953–959. doi:10.1007/s11845-017-1585-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.