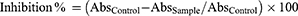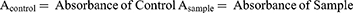Back to Journals » Drug Design, Development and Therapy » Volume 16
Protective Effects of p-CA Against Acute Liver Damage Induced by LPS/D-GalN in Wistar Albino Rats
Authors Mehdi S, Ahmad FUD , Lodhi AH, Khurshid U, Khalid AA, Sidiq SS, Hussain L, Baig MS
Received 20 July 2022
Accepted for publication 16 September 2022
Published 28 September 2022 Volume 2022:16 Pages 3327—3342
DOI https://doi.org/10.2147/DDDT.S380324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Seerat Mehdi,1 Fiaz-ud-Din Ahmad,1 Arslan Hussain Lodhi,1 Umair Khurshid,2 Ahmed Awais Khalid,1 Sheikh Safeena Sidiq,1 Liaqat Hussain,3 Mirza Shaharyar Baig1
1Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan; 2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan; 3Department of Pharmacology, Faculty of Pharmaceutical Sciences, Government College University Faisalabad, Faisalabad, Pakistan
Correspondence: Fiaz-ud-Din Ahmad, Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur, Khawaja Fareed Campus, Railway Road, Bahawalpur, 63100, Pakistan, Tel +92-320-8402376, Email [email protected]
Aim: Liver regulates metabolism of biomolecules and injury of liver causes distortion of metabolic functions. This injury may be oxidative or inflammatory induced by numerous factors including alcohol, pathogens and xenobiotics. This scientific study was planned to investigate the anti-inflammatory and anti-oxidant potential of p-coumaric acid (p-CA) on Lipopolysaccharide/D-Galactosamine (LPS/D-GalN) induced liver injury.
Methods: DPPH analysis, reducing power assay and HPLC analysis were performed during in-vitro studies of p-CA. Similarly, in-vivo experiments were performed using Wistar Albino rats. Normal control and intoxicated group received (5mL/kg normal saline p.o), standard treatment groups received ascorbic acid (100mg/kg p.o) and silymarin (25mg/kg p.o), while p-CA treatment groups received (100mg/kg p.o) for 28-days. After completion of 28-days, LPS/D-GalN injection (300 mg D-GalN/kg and 10 μg LPS/kg i.p.) was given at 6th, 12th and 24-hours to all groups except normal control group. Animals were sacrificed; serum and liver samples were harvested and subjected to biochemical and histological examinations, respectively.
Results: The results revealed that p-CA possess strong antioxidant activity. Increased levels of leukocyte infiltration (TLC), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), lipid panel (eg TG, TC, LDL-C, VLDL-C), whereas decreased HDL-C levels noticed in LPS/D-GalN groups as compared to normal control groups. Pro-Inflammatory markers (eg TNF-α, IL-6, IL-1β) and lipid peroxidation marker, eg malondialdehyde (MDA) increased while superoxide dismutase (SOD) and reduced glutathione (GSH) levels were decreased significantly in groups treated with LPS/D-GalN. ANOVA with Bonferroni post hoc analysis was used for statistical analysis of. H&E staining was done to assess architectural abnormalities among liver cells.
Conclusion: In conclusion, p-CA could ameliorate LPS/D-GalN induced hepatic injury via regulation of immune responses, liver function enzymes, lipid profile, oxidative stress and pro-inflammatory markers.
Keywords: p-coumaric acid, lipopolysaccharide, D-galactosamine, oxidative stress, inflammation
Introduction
The liver is the largest parenchymal organ in the body which performs essential metabolic regulation and biotransformation of most of the chemicals, drugs and toxins. It is a critical hub for macronutrient metabolism, immune system homeostasis, blood volume modulation, endocrine system control, cholesterol homeostasis, and the breakdown of xenobiotics, including many current drugs.1 However, liver is more susceptible to many drugs/toxins which lead towards precipitation of liver injury. Liver injury is frequently accompanied by endotoxins which causes severe damage to the hepatocytes. Its pathogenesis directly involves oxidative damage and immune mediated inflammatory reactions.2 A surfeit of high-fat diet,3 high-fructose diet,4 high-cholesterol diet,5 alcohol consumption,6 radiation,7 and some chemicals or drugs including Concanavalin A,8 Thioacetamide,9 CCl4,10 acetaminophen11 and Lipopolysaccharide/D-Galactosamine can cause liver damage.12 Liver transplantation is a well-known cost effective option available throughout the world, followed by a limitation due to the shortage of donors. Therefore, it is essential to find an optimized and effective drug to treat liver failure. Lipopolysaccharide (LPS) consist of hydrophobic domain known as lipid A (endotoxin), oligosaccharide (non-repeating core), and distal polysaccharide (O-antigen)13 It is major structural toxic component of the cell membranes of Gram-negative bacterium. It plays an principle role in endotoxic injury and destroy healthy tissues, eventually causing cancer or atherosclerosis.14 According to previous reports LPS/D-GalN induced liver injury result from the overproduction of cytokines.14 In resident macrophages of liver, LPS triggers the biosynthesis of diverse mediators of inflammation, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β),15,16 and activates the production of co-stimulatory molecules required for the adaptive immune response.17 In mononuclear and endothelial cells, LPS also stimulates tissue factor production.18,19 Tissue factors are needed for clearing local infections and inflammations, and they also act in synergy. When overproduced in systemic circulation leads to the onset of severe sepsis. However, various inflammatory mediators and clotting factors can destroy small blood vessels and precipitate gram-negative bacterial septic shock, accompanied by disseminated intra-vascular coagulation and finally multiple organ failure.20,21 D-Galactosamine (D-GalN) induces liver damage by interfering with the synthesis of RNA, protein and DNA. It is metabolized via the Leloir pathway, which leads to the production of uridine derivatives of d-galactosamine. The two enzymes in Leloir pathway, galactokinase and UDP-galactose uridyl transferase have low specificity for substrate so these enzymes convert galactosamine into galactosamine-1-phosphate and UDP-galactosamine, respectively. UDP-galactosamine blocks UDP-galactose-4′ epimerase enzyme which results in the accumulation of UDP-galactosamine in the liver cells. These reactions deplete uridine triphosphate (UTP), uridine diphosphate (UDP), uridine monophosphate (UMP), and sugar derivatives of uridine such as UDP-Galactose and UDP-glucose which are essential for the synthesis of RNA and proteins.22 d-Galactosamine is a potent toxin which sensitize animals to the lethal effects of LPS in the acute liver injury model and basically it is a fundamental way to amplify the endotoxin induced TNF-α and other pro-inflammatory interleukins.23 p-Coumaric acid (p-CA) is a polyphenolic compound which acts as secondary metabolite in plants and present in mushrooms, cereals (rice, corn, oats and wheat), fruits (apples, olives and grapes) and vegetables (carrots, beans, potatoes, tomatoes and onions).24 It has been proved to have anti-oxidative,25,26 antimicrobial, anti-inflammatory and immunomodulatory,27,28 antiviral,29 fungicidal,30 anti-mutagenic,31 neuroprotective,32 anticancer,33 antidiabetic and anti-hypercholesterolemic properties.34 In addition, recent studies have reported its protective effects against oxidative stress and lipid peroxidation.35 p-CA is a vital molecule showing great hepatoprotection against various toxic chemicals, eg acetaminophen,36 ethanol,37 and fipronil.38 For all that, p-CA has been suggested to have potential anti-inflammatory and antioxidant effects in LPS/D-GalN induced liver injury model. Thus, the current scientific research was come up with the main objectives to determine (1) in-vitro antioxidant activity of p-CA using DPPH chemical; (2) scrutinize the protective effects of p-CA against LPS/D-GalN induced inflammatory and oxidative stress-induced liver injury.
Materials and Methods
In vitro Assay
DPPH Free Radical Neutralizing Assay
Free radical scavenging activity of p-CA was measured by using a stable free radical DPPH (1,1-diphenyl-2-picrylhydrazyl). 50 mL of DPPH (0.2 mg/mL) was prepared in ethanol. 2 mL of DPPH solution was added into 1mL of p-CA (0.5 mg/mL) dissolved in methanol at different concentrations. The mixture was then shaken and allowed to stand for 30 minutes at room temperature. Absorbance was monitored at 517-nm using spectrophotometer. The standard compound was ascorbic acid and experiment was done in a triplicate. Low absorbance value of the reaction mixture indicated higher free radical scavenging activity of the sample. The percent DPPH scavenging effect of standard ascorbic acid and p-CA was calculated separately by using the following formula.39 All the above-mentioned protocol was performed in thrice and results were calculated by the following formula:
Reducing Power
Reducing power of p-CA was measured by direct reduction of Fe3+ to Fe2+ which imparts change in colour of the reaction mixture due to formation of Perl’s Prussian Blue complex. Absorbance of reaction mixture is directly proportional to the reduction potential of compounds. Ferric reducing antioxidant power (FRAP) method with slight modification40 was used to measure the reduction potential of p-CA. Different concentrations of p-CA (5–30 μg/mL) in 0.75 mL of distilled water were mixed with 1.25mL of 0.2 M, pH 6.6 PBS and 1.25 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The reaction mixture was incubated at 50 °C for 20 min. After 20-minutes, the reaction mixture was acidified with 1.25 mL of 10% trichloroacetic acid. Finally, 0.5 mL of FeCl3 (0.1%) was added to this solution. Absorbance was measured at wavelength of 700 nm in a spectrophotometer. Increased absorbance of reaction mixtures indicates greater reduction capability.41
High Performance Liquid Chromatography
Samples were kept at 4 °C before analysis. Stock solutions of standard and test compounds were prepared with ethanol in the range of 0.1–400μg/mL. 20 μL of sample was injected into the column. The HPLC analysis was performed using column Shim-pack CLC-ODS (C18) 25 cm × 4.6 mm, 5μm with 100 mm of column length (Shimadzu Scientific Instruments, Kyoto, Japan) and detector model (SPD-10AV, UV-Visible). The flow rate was 1 mL/min and detection was performed at 280 nm. 20 μL of sample was injected into shim-pack C18 column using the gradient elution. Two solvents used were 1% (v/v) acetic acid in water; pH = 2.27 (solvent A) and 100% ACN (solvent B). The gradient elution started with following conditions: 15% B for 0–15 minutes, 45% B for 15–30 min and 100% B for 30–45 min (end analysis). Identification and quantitative analysis were conducted in comparison with reference standard compounds.42
In vivo Assay
Animals and Ethical Decision
Wistar Albino rats (150 ± 10 g; n: 6 per group) were used in this experimentation. All animals were placed in room with the temperature (22 ± 1 °C), humidity (50% ± 5%), 12h day/night cycle, free access of diet and tap water ad libitum in their respective cages. The protocol of this experimental study was approved by the Pharmacy Animal Ethics Committee (PAEC/22/75), Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur. All the procedures of this study were done in accordance with Care and Use of Laboratory Animals Guide.
Drugs and Experimental Models
p-CA and LPS (Cat. No. L2630) were obtained from Sigma-Aldrich, USA. D-Galactosamine was obtained from Acros organics. Animals were randomly divided into 15 groups comprises of 6 animals each; Group 1, 2, and 3 consist of control animals received NS vehicle (5mL/kg b.w.; i.p), for consecutive 28 days and sacrificed at 6h, 12h and 24h respectively. Group 4, 5 and 6 consist of intoxicated groups received normal saline (NS) vehicle (5mL/kg b.w.; i.p), group 7, 8 and 9 consist of standard treatment groups received ascorbic acid (100 mg/kg) in NS vehicle (5mL/kg b.w.; i.p), group 10, 11 and 12 consist of standard treatment groups received silymarin (25 mg/kg) in NS vehicle (5mL/kg b.w.; i.p.), group 13, 14 and 15 consist of treatment groups received p-CA (100mg/kg) in NS vehicle (5mL/kg b.w.; i.p) for consecutive 28 days32 followed by the subjected treatment with the total volume of combination of LPS (10μg/kg) and D-GalN (300mg/kg) dissolved in NS and sacrificed at 6 h, 12h and 24h respectively. All animals were kept fasted with water ad libitum for 12 hours prior to experimental procedures. Animals were given anesthesia with a mixture of ketamine and xylazine (10:1) i.p. surgical procedure was performed and blood samples were collected by procedure of cardiac puncture and sera was separated by centrifugation for 15 minutes at 3000-rpm. The liver tissues were harvested immediately, preserved in 10% formalin and subjected to homogenization43 for antioxidant enzymes tests and histopathological analysis.
Biochemical Investigation and Protein Determination
Freshly collected blood samples were diluted with Turk’s fluid in WBC pipette, in which red cells get lysed without affecting the leukocyte. Leukocyte count was done using a Neubauer’s chamber.44 Markers of hepatic damage; serum alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total bilirubin (TB) levels and lipid panel (TG, TC, HDL, LDL and VLDL) were measured in spectrophotometer by using commercial kits following the supplier’s protocol.45 Enzyme-linked immunosorbent assay (ELISA) kits (BT Lab, England) were used to quantify the levels of inflammatory markers; TNF-α, IL-6 and IL-1β, and oxidative markers; MDA, SOD and GSH in the supernatants. The absorbance was read at 450 nm wavelength by a microplate reader (Bio-Rad Laboratories). A standard curve was constructed using serial dilutions of standards provided within the kits and sample concentrations were calculated by interpolation method.46,47
Histopathology of Liver Tissues
After dissection, livers were excised and preserved in10% formalin solution. Microtome sections (5 μm thick) of the samples were taken and stained with hematoxylin and eosin. The images of the stained slides were viewed on an optical microscope at 10X resolution power.48
Statistical Evaluation
The results obtained were calculated atGraphPad Prism and MS excel and interpreted as mean ± SEM. All the values obtained from different groups were statistically analyzed by one-way ANOVA followed by Bonferroni’s post hoc test. p < 0.05 was considered significantly different.46
Results
In vitro Assay
DPPH Free Radical Neutralizing Assay
The DPPH free radical quenching capacity of ascorbic acid was 94.44% with an IC50 value of 80.33 μg/mL while that of p-CA was 74.31% with an IC50 value of 392.94μg/mL as shown in Figure 1.
 |
Figure 1 The graph representing free radical scavenging capacity of Ascorbic acid and p-CA in DPPH assay. The results were expressed as Mean ± SEM of triplicate and expressed as percent inhibition. |
Reducing Power
The reduction potential of a compound is a significant indicator of its antioxidant potential activity.40,41 p-CA displayed powerful reducing ability at different dilutions (5–40μg/mL) as compared to standard compounds. Reduction potential of p-CA and standard compounds presented the following sequence: α-tocopherol >BHT>p-CA as shown in Figure 2. The results illustrate that p-CA can neutralize free radicals by its reducing power. In this way, it is capable of terminating radical chain reactions that may be very damaging in the living system. The electron donating properties of p-CA for quenching free radicals by forming stable products.
High Performance Liquid Chromatography
The peak of p‑CA was detected at retention time of 16.500 minutes, while the retention time of gallic acid 4.533 min and of quercetin is 2.973 min as shown in Figure 3. The retention time (min) and peak area of standards and sample are tabulated in Table 1. The longer retention time may be due to higher affinity of the compounds towards the stationary phase and less affinity for the mobile phase. Xu and Howard49 supported that p‑CA is a less polar compound in comparison to other standard phenolic acids such as gallic acid, and quercetin thus leading to increased elution time for the compound.
 |
Table 1 Retention Time (Min), Height [mV]and Peak Area [mV.s]of Sample and Standards |
In vivo Assay
Evaluation of Immune Response
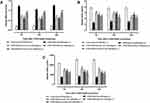
Leukocyte infiltration was the prominent phenomena during inflammatory reactions results from the effects of LPS and D-Galactosamine.50 The effects of standard drugs and p-CA treatment on serum TLC levels are shown in Figure 4. TLC levels in groups pretreated with p-CAdecreased significantly (p < 0.05) in serum at 6h, 12h and 24h after LPS/D-GalNcoinjection as compared to groups receiving only LPS/D-GalN treatment.
Evaluation of Serum Liver Enzymes
Aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin (TB) levels among serum are important markers of liver cell damage.45 As shown in Tables 2 and 3, these factors increased significantly (p < 0.05) in rats administered with LPS/D-GalN. Pre-treatment with p-CA significantly reduced (p < 0.05) liver enzymes in serum in comparison totheLPS/D-GalN treated groups.
 |
Table 2 Effect of Treatment on Alanine Transaminase (ALT) and Aspartate Aminotransferase (AST) of LPS/D-GalN-Induced Liver Injury among Wistar Albino Rats |
 |
Table 3 Effect of Treatment on Alkaline Phosphatase (ALP) and Total Bilirubin (TB) of LPS/D-GalN-Induced Liver Injury among Wistar Albino Rats |
Evaluation of Serum Lipid Profile
The liver plays central role in the lipids and lipoproteins metabolism and homeostasis. Due to hepatocellular damage, cholesterol biosynthesis and elimination with bile are both disturbed and serum levels fluctuate from normal values. Serum levels of total cholesterol (TC), triglycerides (TG), low-density lipoproteins (LDL-C), very low-density lipoproteins (VLDL-C) were elevated significantly (p < 0.05) as well as high density lipoproteins (HDL-C) levels were reduced significantly (p < 0.05) observed in rats of the LPS/D-GalNgroupsasshown in Figure 5A–. Pre-treatment with standard drugs and p-CA significantly reduced TG, TC, LDL and VLDL levels as well as significantly raised HDL levels in serum when compared with LPS/D-GalNtreated groups (p < 0.05).
Evaluation of Inflammatory Markers
LPS trigger the release of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β which potentially deteriorate hepatic cells and lead towards cellular necrosis.46 The LPS/D-GalNadministration significantly increased levels of TNF-α, IL-6 and IL-1β in supernatants as compared to the control groups (p < 0.05). Pre-treatment with standard drugs and p-CA significantly decreased the levels of TNF-α, IL-6 and IL-1β in supernatants as compared to the LPS/D-GalN treated group (p < 0.05) as shown in Figure 6A–).
Evaluation of the Antioxidant Enzymes
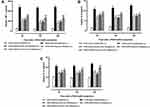
LPS/D-GalN challenge elevated oxidative stress due to un-controlled and un-balanced redox reactions and enervating antioxidant enzyme system in liver tissue (eg super-oxide dismutase and glutathione) and can lead to extensive liver damage. MDA is produced at the end of lipid peroxidation reactions. So, it is important biomarker of lipid oxidation.47 Therefore, we investigate the protective effects of p-CA on the levels of oxidative stress markers. Accordingly, rats that received LPS/D-GalN had significantly higher concentrations of MDA (P < 0.05), while significantly reduced levels of SOD and GSH (p < 0.05) in comparison to the normal control group as shown in Figure 7A–. p-CA pre-treatment significantly reduced the level of MDA (P < 0.05), while improved the levels of SOD and GSH (p < 0.05) in liver tissues when compared to the LPS/D-GalN group.
Histopathology
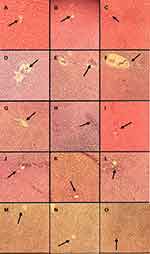
In this study, we evaluated the effects of p-CA on liver injury. The LPS/D-GalN administration led to liver tissue damage of the liver tissue. Treatment with p-CA greatly reduced liver injury which was accessed byHaematoxylin and Eosin staining. The normal control groups (a, b, c at 6h, 12h and 24h) showed the normal structure of liver cells. On the other hand, sections from the LPS/D-GalN treated groups (d, e, f at 6h, 12h and 24h) showed chronic inflammatory cells with immense neutrophils infiltration, derangement of hepatocyte architecture, blood congestion in central vein and hemorrhagic patches. (g-o) All the treatment groups showed significant protection against LPS/D-GalN induced liver damage as shown in Figure 8.
Discussion
There are numerous models for exploration of the role of inflammation and oxidative stress in liver injury caused by xenobiotics. LPS/D-GalNinduced liver injury is a well-known experimental model that has been widely used for the induction of liver injury to elucidate pathogenesis.51 It has been validated that LPS activates resident Kupffer cells, stimulates inflammatory cascade followed by production of pro-oxidative markers. Moreover, D-GalN, HPC selective inhibitor and hepatotoxic agent, sensitizes to LPS and augments its toxic effects. It can consume uridine monophosphate in the liver, inhibiting the nucleic acid, glycoprotein and cholesterol synthesis, damaging the hepatocytes and causing liver injury within a few hours. It is remarkable that D-GalN can intensify the toxic effects of LPS.52 In present study, LPS/D-GalNinjection was used to induce liver injury in male albino rats. LPS/D-GalN-induced liver injury is a well-known experimental model which presented substantial liver injury with profound changes in biochemical and histopathological and parameters.14,53 Following the challenge of LPS/D-GalN, levels of liver function enzymes: ALT, AST and ALP, which are indicators of hepatic damage, increased in the serum.45,54 The levels of bilirubin were also increased in response to LPS/D-GalN treatment.52,55 Bilirubin plays an important role as a physiologic antioxidant by scavenging peroxyl radicals and preventing oxidation of fatty acids and proteins.Its activity is augmented in oxidative stress as an adaptive mechanism.56,57 Our results in the present study revealed that p-CA significantly ameliorated the increased levels of liver function enzymes. LPS activates NF-κB by binding to the TLR4 complex in Kupffer cells.55 The activated NF-κB enters into the nucleus and causes the transcription of various inflammatory genes such as TNF-α, IL-1β, IL-6 etc.55 D-GalN, on the other hand, metabolized in the liver and selectively depletes uridine nucleotides, inhibits RNA synthesis, inhibits the transcription of hepatocytes and potentiates toxic effects of LPS.23,58 The combined effects of these two agents produce a severe hepatocyte necrosis and inflammatory reactions.48 TNF-α is a key pathogenic factor in liver injury which produce primarily in liver macrophages. It induces apoptosis and necrosis in hepatocytes by inducing other inflammatory mediators eg IL-6 and IL-1β that orchestrate inflammatory reactions.59 p-CA inhibits activation and transfer of NF-kB to nucleus and inhibits the production of inflammatory cytokines.36,55 Following the progression of liver damage, TNF-α increases extravasation of leukocytes from sinusoids into liver parenchyma by different mechanisms, which exacerbates the liver injury.60 Increased accumulation and infiltration of inflammatory cells may induce apoptosis.61 p-CA pre-treatment during this study clearly elaborated the protective effects against LPS/D-GalN induced inflammatory response while improving hepatic necrosis and increasing the level of anti-oxidants.62 p-CA had alleviated the overproduction of inflammatory mediators eg TNF-α, IL-6 and IL-1β in liver tissue, which suggested its anti-inflammation activity in liver injury.36 Neutrophils infiltration is the important factor which is responsible for the liver damage during systemic inflammation. These cells also produce inflammatory mediators and ROS and exacerbate liver injury.63 Our study elaborated that using p-CA led to reduce total leukocyte count in liver tissue. Thus, it seems that p-CA attenuates liver damage by the alleviation of neutrophils infiltration. The liver is an important organ which plays a significant role in metabolism of drugs, de-toxification of toxic chemicals and biomolecule’s synthesis. Alteration in metabolic functions of liver cells leads to imbalance in cellular antioxidant defense which results in ROS production and tissue injury.47 Injection of LPS/D-GalN in rats augmented lipid peroxidation reactions due to metabolic disturbances. D-GalN is an amino sugar that when metabolized by hepatocytes produces ROS.52 Oxidative stress is actually redox imbalance in which levels of ROS exceed the magnitude of antioxidants as shown by increased hepatic malondialdehyde (MDA) levels. Lipid peroxidation64 alters the cell membranes structure and fluidity resulting in cytolysis and cellular destruction.65 LPS/D-GalN treatment significantly elevated total cholesterol levels which may be due to inability of necrotic liver to remove or synthesize cholesterol.66 Pretreatment of p-CA showed protection against lipid changes caused by LPS/D-GalN induced oxidative insult. These findings could be correlated with the previous studies.67 Hepatocellular damage due to alcohol, virus and drug-induced hepatitis causes modest hypertriglyceridemia68 which is due to the biochemical changes interfering with the transport of triglycerides out of liver. Our study showed an increased accumulation of triglycerides, low density lipoproteins and very low-density lipoproteins in LPS/D-GalN induced rats as reported in previous research.67,69 Reduced glutathione (GSH) is ubiquitous antioxidant, constantly synthesized intracellularly from cysteine, glutamate, and glycine. GSH plays a major role in regulation of intracellular redox balance by detoxifying ROS. Lower levels of GSH and reduced GSH to oxidized GSH ratio (GSH: GSSG), is the measure of redox balance.70 Glutathione plays a major role in the immune regulation, DNA synthesis, and cellular detoxification71 by combating with oxidative stress through scavenging free radicals reactive oxygen/nitrogen species.72 In present study, the GSH levels were reduced significantly in LPS/D-GalN challenged group, indicating that LPS/D-GalN induced oxidative reactions converted glutathione to its oxidized form.12,72 Pretreatment with p-CA significantly elevated glutathione levels in hepatocytes (p < 0.05). In addition to GSH, superoxide dismutase (SOD) counteracts oxidation of biomolecules (proteins, lipids and DNA) by quenching ROS. SOD reduces superoxide ion to less toxic product, namely hydrogen peroxide, which further reduced to water by the action of catalase and glutathione peroxidase.73 In this experimental work, hepatic levels of SOD decreased significantly in LPS/D-GalN treated groups while p-CA tried to overcome this depletion in comparison to LPS/D-GalN treated groups. LPS/D-GalN treatment significantly increase total cholesterol levels which may be due to the inability of the injured liver to remove cholesterol from the circulation.66 Pretreatment of p-CAalso showed protection against serum and liver lipid changes caused by LPS/D-GalN evidencing a broad spectrum of hepatoprotective property. This finding could be correlated with the results of the previous studies.67 Hepatocellular damage due to virus, alcohol and drugs causes hypertriglyceridemia which may be due to interference of oxidative stress with transport of triglycerides.66,68 Our study showed that LPS/D-GalN treatment increased serum levels of triglycerides, low density lipoproteins and very low-density lipoproteins in rats as reported in previous research.67,69 H&E staining was used in current study to detect the histological features of p-CA treated liver tissues in comparison to LPS/D-GalN-induced liver damage. Liver tissues of the control group showed normal structure of livers.LPS/D-GalN injected animals showed the histopathological changes, including extensive blood congestion in central vein and loss of hepatic architecture and massive inflammatory cells infiltration.14,64 These liver histopathological changes were restored by the treatment with p-CA. By summarizing the outcomes, p-CA inhibited LPS/D-GalN-induced liver injury by suppressing pro-inflammatory cytokines, maintaining oxidative balance and homeostasis in the liver and regulating hepatic enzymes and lipid profile in serum. Thus, p-CA could be used as an antioxidant and anti-inflammatory agent in the management of liver injury.
Limitations of the Study
Lipid metabolizing enzymes, eg lipoprotein lipase (LPL), lecithin-cholesterol acyl transferase (LCAT) and hepatic triglyceride lipase (HTGL) are limitations of this study.
Conclusion
The present study showed LPS/D-GalN-induced liver injury followed by hepatic dysfunction and morphologic pathology among experimental rats. In conclusion, our study elaborated that the use of p-CA in the treatment of LPS/D-GalN-induced liver injury can alleviate the damage to liver by ameliorating inflammation, oxidative stress and leukocytes infiltration.
Provision of Data
All the data is provided within the manuscript submitted.
Acknowledgments
Authors would like to thank the Department of Pharmacology, Faculty of Pharmacy, the Islamia University of Bahawalpur for laboratory access and uninterrupted research facilities.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declared that they had no competing interests.
References
1. Wei X, Yang D, Xing Z, et al. Quercetin loaded liposomes modified with galactosylated chitosan prevent LPS/D-GalN induced acute liver injury. Mater Sci Eng C. 2021;131:112527. doi:10.1016/j.msec.2021.112527
2. Huang S, Mo C, Zeng T, et al. Lupeol ameliorates LPS/D-GalN induced acute hepatic damage by suppressing inflammation and oxidative stress through TGFβ1-Nrf2 signal pathway. Aging. 2021;13(5):6592. doi:10.18632/aging.202409
3. Zhang J-K, Zhou X-L, Wang X-Q, et al. Que Zui tea ameliorates hepatic lipid accumulation and oxidative stress in high fat diet induced nonalcoholic fatty liver disease. Food Res Int. 2022;156:111196. doi:10.1016/j.foodres.2022.111196
4. Alwahsh SM, Dwyer BJ, Forbes S, Van Thiel DH, Starkey Lewis PJ, Ramadori G. Insulin production and resistance in different models of diet-induced obesity and metabolic syndrome. Int J Mol Sci. 2017;18(2):285. doi:10.3390/ijms18020285
5. Zhao C-Z, Jiang W, Zhu -Y-Y, et al. Highland barley Monascus purpureus went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J Ethnopharmacol. 2022;286:114922. doi:10.1016/j.jep.2021.114922
6. Alwahsh SM, Xu M, Schultze FC, et al. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS One. 2014;9(8):e104220. doi:10.1371/journal.pone.0104220
7. Li Y-H, Wu J-X, He Q, et al. Amelioration of radiation-induced liver damage by p-coumaric acid in mice. Food Sci Biotechnol. 2022;31(10):1315–1323. doi:10.1007/s10068-022-01118-8
8. Zhang P, Yin Y, Wang T, et al. Maresin 1 mitigates concanavalin A-induced acute liver injury in mice by inhibiting ROS-mediated activation of NF-κB signaling. Free Radic Biol Med. 2020;147:23–36. doi:10.1016/j.freeradbiomed.2019.11.033
9. Harn H-J, Lin S-Z, Hung S-H, et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21(12):2753–2764. doi:10.3727/096368912X652959
10. Wu Z, Sun L, Chen R, et al. Chinese tea alleviates CCl4-induced liver injury through the NF-κBorNrf2Signaling pathway in C57BL-6J mice. Nutrients. 2022;14(5):972. doi:10.3390/nu14050972
11. Xu L, Yang Y, Jiang J, et al. Eosinophils protect against Acetaminophen‐induced liver injury through cyclooxygenase‐mediated IL‐4/IL‐13 production. Hepatol. 2022;2022:1–10.
12. Chai F-N, Zhang J, Xiang H-M, et al. Protective effect of Coptisine from RhizomaCoptidis on LPS/D-GalN-induced acute liver failure in mice through up-regulating expression of miR-122. Biomed Pharmacother. 2018;98:180–190. doi:10.1016/j.biopha.2017.11.133
13. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635. doi:10.1146/annurev.biochem.71.110601.135414
14. Gong X, Yang Y, Huang L, et al. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure in mice. Int Immunopharmacol. 2017;46:124–132. doi:10.1016/j.intimp.2017.03.003
15. Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57(1):505–518. doi:10.1146/annurev.bi.57.070188.002445
16. Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77(8):1627–1652. doi:10.1182/blood.V77.8.1627.1627
17. Medzhitov R, Janeway JC. Innate immunity. N Engl J Med. 2000;343(5):338–344. doi:10.1056/NEJM200008033430506
18. Drake T, Cheng J, Chang A, Taylor F. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. AmJPathol. 1993;142(5):1458.
19. Li A, Chang A, Peer GT, Hinshaw LB, Taylor FB. Comparison of the capacity of rhTNF-alpha and Escherichia coli to induce procoagulant activity by baboon mononuclear cells in vivo and in vitro. Shock. 1996;5(4):274–279. doi:10.1097/00024382-199604000-00007
20. Esmon CT. Regulation of blood coagulation. BiochimBiophysActa. 2000;1477(1–2):349–360.
21. Bernard GR, Vincent J-L, Laterre P-F, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi:10.1056/NEJM200103083441001
22. Keppler D, Decker K. Studies on the mechanism of galactosamine hepatitis: accumulation of galactosamine‐1‐phosphate and its inhibition of UDP‐glucose pyrophosphorylase. Eur J Biochem. 1969;10(2):219–225. doi:10.1111/j.1432-1033.1969.tb00677.x
23. Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res. 2004;10(3):147–162. doi:10.1179/096805104225004879
24. Ferreira PS, Victorelli FD, Fonseca-Santos B, Chorilli M. A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit Rev Anal Chem. 2019;49(1):21–31. doi:10.1080/10408347.2018.1459173
25. Ramorobi LM, Matowane GR, Mashele SS, et al. Bioactive synergism between zinc mineral and p‐coumaric acid: a multi‐mode glycemic control and antioxidative study. J Food Biochem;2022. e14360. doi:10.1111/jfbc.14360
26. Pei K, Ou J, Huang J, Ou S. p‐Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96(9):2952–2962. doi:10.1002/jsfa.7578
27. Pragasam SJ, Venkatesan V, Rasool M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36(1):169–176. doi:10.1007/s10753-012-9532-8
28. Kilani-Jaziri S, Mokdad-Bzeouich I, Krifa M, Nasr N, Ghedira K, Chekir-Ghedira L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: a structure–activity relationship study. Drug Chem Toxicol. 2017;40(4):416–424. doi:10.1080/01480545.2016.1252919
29. Chiang L, Chiang W, Chang M, Ng L, Lin C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 2002;55(1):53–62. doi:10.1016/S0166-3542(02)00007-4
30. Ferreira P, Victorelli F, Rodero C, et al. p-Coumaric acid loaded into liquid crystalline systems as a novel strategy to the treatment of vulvovaginal candidiasis. Int J Pharm. 2021;603:120658. doi:10.1016/j.ijpharm.2021.120658
31. Ferguson LR, Lim IF, Pearson AE, Ralph J, Harris PJ. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat Res Genet Toxicol Environ Mutagen. 2003;542(1–2):49–58. doi:10.1016/j.mrgentox.2003.08.005
32. Daroi PA, Dhage SN, Juvekar AR. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. J Pharm Pharmacol. 2022;74(4):556–564.
33. Wang L, You X, Dai C, Fang Y, Wu J. Development of poly (p-coumaric acid) as a self-anticancer nanocarrier for efficient and biosafe cancer therapy. Biomater Sci. 2022;10(9):2263–2274. doi:10.1039/D2BM00027J
34. Amalan V, Vijayakumar N, Indumathi D, Ramakrishnan A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: in vivo approach. BiomedPharmacother. 2016;84:230–236.
35. Shen Y, Song X, Li L, et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. BiomedPharmacother. 2019;111:579–587.
36. Cha H, Lee S, Lee JH, Park J-W. Protective effects of p-coumaric acid against Acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 2018;121:131–139. doi:10.1016/j.fct.2018.08.060
37. Sabitha R, Nishi K, Gunasekaran VP, et al. p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models. Chem Biol Interact. 2020;321:109044. doi:10.1016/j.cbi.2020.109044
38. Bal SS, Leishangthem GD, Sethi RS, Singh A. P-coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress and inflammation. Pestic Biochem Physiol. 2022;180:104997. doi:10.1016/j.pestbp.2021.104997
39. Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Food Sci Tech. 2003;36(2):263–271.
40. Işıl Berker K, Güçlü K, Tor İ, Demirata B, Apak R. Total antioxidant capacity assay using optimized ferricyanide/Prussian blue method. Food Analy Method. 2010;3(3):154–168. doi:10.1007/s12161-009-9117-9
41. Yesiloglu Y, Aydin H, Kilic I. In vitro antioxidant activity of various extracts of ginger (Zingiber officinale L.) seed. Asian J Chem. 2013;25(7):3573. doi:10.14233/ajchem.2013.13657
42. Imtiaz SM, Aleem A, Saqib F, Ormenisan AN, Elena Neculau A, Anastasiu CV. The potential involvement of an ATP-dependent potassium channel-opening mechanism in the smooth muscle relaxant properties of Tamarix dioica Roxb. Biomolecules. 2019;9(11):722. doi:10.3390/biom9110722
43. Li J, Zhang X, Huang H. Protective effect of linalool against lipopolysaccharide/D-galactosamine-induced liver injury in mice. Int Immunopharmacol. 2014;23(2):523–529. doi:10.1016/j.intimp.2014.10.001
44. Mukherjee D, Khatua TN, Venkatesh P, Saha B, Mukherjee PK. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. JEthnopharmacol. 2010;128(2):490–494. doi:10.1016/j.jep.2010.01.015
45. El‐Beshbishy HA. Aqueous garlic extract attenuates hepatitis and oxidative stress induced by galactosamine/lipoploysaccharide in rats. Phytother Res. 2008;22(10):1372–1379. doi:10.1002/ptr.2505
46. Tian Q, Wang G, Zhang Y, et al. Engeletin inhibits Lipopolysaccharide/d-galactosamine-induced liver injury in mice through activating PPAR-γ. J Pharmacol Sci. 2019;140(3):218–222. doi:10.1016/j.jphs.2019.06.011
47. Lv H, Qi Z, Wang S, Feng H, Deng X, Ci X. Asiatic acid exhibits anti-inflammatory and antioxidant activities against lipopolysaccharide and d-galactosamine-induced fulminant hepatic failure. Front Immunol. 2017;8:785. doi:10.3389/fimmu.2017.00785
48. Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed Pharmacother. 2016;83:302–313. doi:10.1016/j.biopha.2016.06.036
49. Xu Z, Howard LR. Analysis of Antioxidant-Rich Phytochemicals. John Wiley & Sons; 2012.
50. Ma L, Gong X, Kuang G, Jiang R, Chen R, Wan J. Sesamin ameliorates lipopolysaccharide/d-galactosamine-induced fulminant hepatic failure by suppression of Toll-like receptor 4 signaling in mice. Biochem Biophys Res Commun. 2015;461(2):230–236. doi:10.1016/j.bbrc.2015.03.154
51. Chen W, Lin Y-J, Zhou X-Y, Chen H, Jin Y. Rosiglitazone protects rat liver against acute liver injury associated with the NF-κB signaling pathway. Can JPhysiolPharmacol. 2016;94(1):28–34.
52. Peng Z, Gong X, Yang Y, et al. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-κB and MAPK signal pathways. Int Immunopharmacol. 2017;52:281–289. doi:10.1016/j.intimp.2017.09.022
53. Kemelo M, Wojnarova L, Canová NK, Farghali H. D-galactosamine/lipopolysaccharide-induced hepatotoxicity downregulates sirtuin 1 in rat liver: role of sirtuin 1 modulation in hepatoprotection. Physiol Res. 2014;63(5):615–623. doi:10.33549/physiolres.932761
54. Wen J, Lin H, Zhao M, et al. Piceatannol attenuates D-GalN/LPS-induced hepatoxicity in mice: involvement of ER stress, inflammation and oxidative stress. Int Immunopharmacol. 2018;64:131–139. doi:10.1016/j.intimp.2018.08.037
55. Wang H, Wei X, Wei X, et al. 4-hydroxybenzo [d] oxazol-2 (3H)-one ameliorates LPS/D-GalN-induced acute liver injury by inhibiting TLR4/NF-κB and MAPK signaling pathways in mice. Int Immunopharmacol. 2020;83:106445. doi:10.1016/j.intimp.2020.106445
56. Zeashan H, Amresh G, Singh S, Rao CV. Protective effect of Amaranthus spinosus against D-galactosamine/lipopolysaccharide-induced hepatic failure. PharmBiol. 2010;48(10):1157–1163.
57. Vera‐Ramirez L, Pérez‐Lopez P, Varela‐Lopez A, Ramirez‐Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. Biofactors. 2013;39(1):88–100. doi:10.1002/biof.1057
58. Bradham CA, Manns MP, Brenner DA, Trautwein CI, Trautwein C. TNF-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 1998;275(3):G387–G392. doi:10.1152/ajpgi.1998.275.3.G387
59. Sethi G, Sung B, Aggarwal B. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13(13):5094–5107.
60. Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF‐α‐induced apoptosis in liver injury. J Cell Mol Med. 2004;8(4):445–454.
61. Wang L, Wang X, Kong L, et al. Isoliquiritigenin alleviates LPS/D-GalN-induced acute liver failure by activating the PGC-1α/Nrf2 pathway to reduce oxidative stress and inflammatory response. Int Immunopharmacol. 2021;100:108159. doi:10.1016/j.intimp.2021.108159
62. Godarzi SM, Gorji AV, Gholizadeh B, Mard SA, Mansouri E. Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrología. 2020;40(3):311–319. doi:10.1016/j.nefro.2019.10.003
63. Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26(8):912–919. doi:10.1111/j.1478-3231.2006.01327.x
64. Xia X, Su C, Fu J, et al. Role of α-lipoic acid in LPS/d-GalN induced fulminant hepatic failure in mice: studies on oxidative stress, inflammation and apoptosis. Int Immunopharmacol. 2014;22(2):293–302. doi:10.1016/j.intimp.2014.07.008
65. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46(12):2027–2049. doi:10.1093/clinchem/46.12.2027
66. Sheriff SA, Devaki T. Lycopene stabilizes lipoprotein levels during D-galactosamine/lipopolysaccharide induced hepatitis in experimental rats. Asian Pac J Trop Biomed. 2012;2(12):975–980. doi:10.1016/S2221-1691(13)60009-X
67. Black DD, Tso P, Weidman S, Sabesin SM. Intestinal lipoproteins in the rat with D-(+)-galactosamine hepatitis. J Lipid Res. 1983;24(8):977–992. doi:10.1016/S0022-2275(20)37912-8
68. Prabhakaran V, Kumar BSA, Shekar DS, Nandeesh R, Subramanyam P, Ranganayakulu D. Evaluation of the hepatoprotective activity of Portulaca oleracea L. On D-galactosmaine-induced hepatic injury in rats. BoletínLatinoamericano y del Caribe de PlantasMedicinales y Aromáticas. 2010;9(3):199–205.
69. Cartwright CK, Ragland JB, Weidman SW, Sabesin SM. Alterations in lipoprotein composition associated with galactosamine-induced rat liver injury. J Lipid Res. 1982;23(5):667–679. doi:10.1016/S0022-2275(20)38099-8
70. Nagasaki T, Schuyler AJ, Zhao J, et al. 15LO1 dictates glutathione redox changes in asthmatic airway epithelium to worsen type 2 inflammation. J Clin Invest. 2022;132(1). doi:10.1172/JCI151685
71. Gate L, Paul J, Ba GN, Tew K, Tapiero H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacother. 1999;53(4):169–180. doi:10.1016/S0753-3322(99)80086-9
72. Pizzorno J. Glutathione! Integr Med. 2014;13(1):8.
73. Valdivia A, Perez-Alvarez S, Aroca-Aguilar J, Ikuta I, Jordan J. Superoxide dismutases: a physiopharmacological update. J Physiol Biochem. 2009;65(2):195–208.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.