Back to Journals » Drug Design, Development and Therapy » Volume 14
Protective Effects of Crocetin on Arsenic Trioxide-Induced Hepatic Injury: Involvement of Suppression in Oxidative Stress and Inflammation Through Activation of Nrf2 Signaling Pathway in Rats
Authors Liu Y, Liang Y, Zheng B , Chu L, Ma D , Wang H, Chu X, Zhang J
Received 1 February 2020
Accepted for publication 27 April 2020
Published 19 May 2020 Volume 2020:14 Pages 1921—1931
DOI https://doi.org/10.2147/DDDT.S247947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Yanshuang Liu,1,2,* Yingran Liang,3,* Bin Zheng,3 Li Chu,3 Donglai Ma,3 Hongfang Wang,3 Xi Chu,4 Jianping Zhang2,5
1Department of Diagnostics, School of Integrated Chinese and Western Medicine, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China; 2Hebei Key Laboratory of Integrative Medicine on Liver-Kidney Patterns, Shijiazhuang 050200, Hebei, People’s Republic of China; 3Department of Pharmaceutics, School of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China; 4Department of Pharmacy, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050011, People’s Republic of China; 5Department of Pharmacology, School of Basic Medicine, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Chu
The Fourth Hospital of Hebei Medical University, Shijiazhuang 050011, Hebei, People’s Republic of China
Tel/Fax +86 311 86095324
Email [email protected]
Jianping Zhang
School of Basic Medicine, Hebei University of Chinese Medicine, Shijiazhuang 050200, Hebei, People’s Republic of China
Tel/Fax +86 311 89926719
Email [email protected]
Purpose: Arsenic trioxide (ATO) has been shown to induce hepatic injury. Crocetin is a primary constituent of saffron, which has been verified to have antioxidant and anti-inflammatory effects. In the current experiment, we evaluated the efficacy of crocetin against ATO-induced hepatic injury and explored the potential molecular mechanisms in rats.
Methods: Rats were pretreated with 25 or 50 mg/kg crocetin 6 h prior to treating with 5 mg/kg ATO to induce hepatic injury daily for 7 days.
Results: Treatment with crocetin attenuated ATO-induced body weight loss, decreases in food and water consumption, and improved ATO-induced hepatic pathological damage. Crocetin significantly inhibited ATO-induced alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) increases. Crocetin prevented ATO-induced liver malondialdehyde (MDA) and reactive oxygen species (ROS) levels. Crocetin abrogated the ATO-induced decrease of catalase (CAT) and superoxide dismutase (SOD) activity. Crocetin was found to significantly restore the protein levels of interleukin 6 (IL-6), interleukin 1β (IL-1β), and tumor necrosis factor-alpha (TNF-α). Furthermore, crocetin promoted the expression of nuclear factor erythroid 2 related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and NADP(H): quinone oxidoreductase 1 (NQO1).
Conclusion: These findings suggest that crocetin ameliorates ATO-induced hepatic injury in rats. In addition, the effect of crocetin might be related to its role in antioxidant stress, as an anti-inflammatory agent, and in regulating the Nrf2 signaling pathway.
Keywords: crocetin, arsenic trioxide, hepatotoxicity, oxidative stress, inflammation, Nrf2 signaling pathway
Introduction
Arsenic trioxide (ATO) is widely used as an effective component of traditional Chinese medicine that produces significant remission in individuals with refractory or relapsed acute promyelocytic leukemia (APL).1 The United States (US) Food and Drug Administration approved ATO for APL in September 2000.2 However, ATO is a known environmental toxicant that has become a widespread health concern because of its toxicity.3,4 Epidemiological studies have also confirmed an obvious association between the excess intake of inorganic arsenic and various hazardous effects in humans, including hepatotoxicity, nephrotoxicity, neurotoxicity, as well as dermatosis, which limit its clinical application.3,5,6
As the main organ for the metabolism of poisonous substances, the liver is the primary target for ATO.7 Arsenic is absorbed into the body and distributed to tissues and viscera, especially into the liver.5 In experimental research, arsenic induces liver injuries that result in changes in the biochemical indexes of liver functions, such as elevations of serum enzymes.7 Many investigators have also confirmed that ATO was able to cause serious histopathological changes in the liver.8
Hepatic injury induced by arsenicals is closely related to oxidative stress by triggering the production of intracellular reactive oxygen species (ROS), which can play a key role in the toxic effect of arsenic and its compounds.9,10 Oxidative stress is a state of an imbalance between oxidation and antioxidation involved in cellular damage.11,12 Excessive accumulation of ROS leads to cellular injuries, such as lipid peroxidation, DNA oxidative disruption, and enzyme inactivation.13,14 Synthetic or natural scavengers of ROS and antioxidants could attenuate ATO-induced toxicity, thereby enabling full exploitation of the therapeutic potential of ATO.15 The imbalance between the generation of ROS and the antioxidant systems is usually maintained by critical enzymes, for example, superoxide dismutase (SOD) and catalase (CAT).13
Exposure of arsenic to individuals involved in the production of pro-inflammatory cytokines.16,17 Interleukin 6 (IL-6), interleukin 1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) are important mediators of the inflammatory response and are involved with many early systemic inflammation events.18
Nuclear factor erythroid 2 related factor 2 (Nrf2) plays a dominant role as a central transcription factor in protecting against increased oxidative damage and toxic injury.19 Nrf2 is located in the cytoplasm of the resting cells.19,20 Upon stimulation, Nrf2 translocates to the nucleus and initiates transcription of its target genes, for instance, heme oxygenase-1 (HO-1) and NADP(H): quinone oxidoreductase 1 (NQO1).21,22 The activation of these proteins is now recognized as a strategy for cellular defense against the adverse effects of excessive ROS generation.22 A variation in the redox state means a variation in ROS production or metabolism.23 Furthermore, ATO produces excess ROS by combining with nearby mercaptans, causes lipid peroxidation, and promotes apoptosis.24 Nrf2 confers a protective effect against ROS and xenobiotics in healthy cells.25
Crocetin (CRO) (C20H24O4; molecular weight 328.4g/mol, Figure 1) is a primary constituent of saffron, which has been known to exert many kinds of pharmacological effects, including antioxidative stress,26 anti-inflammatory,27 anti-cancer, anti-apoptotic,28,29 heart disease preventive,30 and neuroprotective effects.31,32 These properties of CRO, especially its ability to reduce oxidative stress and inflammation, propose that it might be a significant candidate to attenuate liver injury.
 |
Figure 1 Chemical structure of crocetin. |
Accordingly, we supposed that CRO could effectively block hepatotoxicity induced by ATO. To test this prediction, we investigated the effects of CRO on ATO-induced hepatic injuries and explored the potential mechanisms of oxidative stress and inflammation through activation of the Nrf2 signaling pathway in rats. Thus, safe and effective drugs for decreasing ATO-induced hepatotoxicity will provide novel opportunities for the clinical use of ATO.
Materials and Methods
Materials
Crocetin with HPLC purity of ≥ 98% and arsenic trioxide (ATO, MW:197.84) were procured from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), malondialdehyde (MDA), glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) detection kits were procured from Jiancheng Bioengineering Institute of Nanjing (Nanjing, Jiangsu, China). The ROS detection kit was procured from Wuhan Servicebio Technology, Co., Ltd (Wuhan, China). Antibodies specific for Nrf2 and NQO1 were procured from Bioworld Technology, Co., Ltd. (Nanjing, Jiangsu, China). HO-1 antibodies were obtained from Wuhan Sanying Biotechnology Co., Ltd (Wuhan, China). β-actin was procured from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd (Beijing, China). The IL-6, IL-1β, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from Wuhan Servicebio Technology CO., Ltd (Wuhan, China).
Experimental Animals
Forty healthy male Sprague Dawley rats (weighing 180–220g, 7–8 weeks old) were provided by the Center for Experimental Animals at Hebei Medical University (Shijiazhuang, China). The rats were maintained in cages under standard laboratory conditions (indoor temperature: 25±2°C; humidity: 50±10%, 12/12h dark/light cycle). Rats were offered food pellets and tap water freely. All rats were allowed to adapt to the environment for 7 days before the experiment. All experimental and animal handling procedures were approved by the Ethics Committee for Animal Experiments of Hebei University of Chinese Medicine (DWLL2019011) and conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Design
Forty rats were randomly and evenly divided into five treatment groups, with eight rats in each group, and were processed as follows:
Group I (Con): control rats were intraperitoneally injected with 0.9% normal saline (5 mg/kg) once per day for 7 days.
Group II (Cro): rats received crocetin (50 mg/kg) orally by gastric intubation once per day for 7 days.
Group III (ATO): rats were intraperitoneally injected with ATO (5 mg/kg) once per day for 7 days.
Group IV (L-Cro): rats pretreated with crocetin (25 mg/kg) orally by gastric intubation 6 h prior to treating with ATO (as in group III) for 7 days.
Group V (H-Cro): rats pretreated with crocetin (50 mg/kg) orally by gastric intubation 6 h prior to treating with ATO (as in group III) for 7 days.
The applied doses for ATO and CRO were performed according to the method of previous experiments,33,34 as well as our preliminary studies.
Sample Preparations
During the period of feeding, body weights were recorded at the beginning and end of the experiment, food and water consumption were recorded daily in the morning before supplemental diet.
On the 8th day, rats were starved overnight and sacrificed under 10% urethane solution anesthesia; the blood samples were collected through arteria femoralis into heparinized vials and centrifuged (1000×g, 4°C) for 10 min to obtain the serum. The liver was carefully removed immediately in ice-cold media. Partial liver specimens were fixed for histochemistry, and the remaining tissues were frozen in −196°C liquid nitrogen until further analysis.
Preparation of Liver Homogenates
The isolated liver tissue was homogenized in ice cold phosphate buffered saline at pH 7.4 and centrifuged at 3000 g for 10 min at 4°C. The supernatant was harvested for further experiments.11
Histopathological Examinations
To assess the changes in the liver, a portion of the liver tissues was placed in a 4% paraformaldehyde solution overnight, dehydrated in an ethanol series, embedded in paraffin, cut into 5-μm thick sections, and stained with hematoxylin-eosin. Finally, sections were observed under a light microscope.
Assessment of Biochemical Indices
Serum ALT, AST, and ALP activity were assayed spectrophotometrically according to the instructions using commercially available diagnostic kits to assess hepatotoxicity.
Detection of Oxidative and Antioxidant Indicators
The levels of MDA, GSH, CAT, and SOD in hepatic tissue supernatants obtained from liver tissue homogenization were detected by commercial kits according to each manufacturer’s instruction.
ROS Detection by Fluorescence Microscopy
Rat liver tissue was embedded in OCT (optimal cutting temperature compound) buffer, frozen in liquid nitrogen, and cut into 10-μm thick sections.35 Frozen sections of rat liver were incubated in 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA) solution at room temperature for 20min under dark conditions. DCFH-DA is rapidly turned into fluorescent compound 2,7-dichlorofluorescein (DCF) by ROS.36 The fluorescence intensity was measured at 488 nm of excitation wavelength and 525 nm of emission wavelength after washing with phosphate buffer solution. Sections were observed under a fluorescence microscope, and images were collected.
Inflammatory Cytokine Measurements by ELISA
IL-6, IL-1β, and TNF-α expression in hepatic tissue were measured to justify the inflammatory status in rats of the different groups using ELISA kits in accordance with the manufacturer’s instructions. Briefly, 80 μL of test buffer and 20 μL of the sample were added to the sample hole and then 50 μL diluted detection antibody (1:100 diluted) was added to each well. Reagents were washed gently six times with washing buffer after incubation for 2 h at room temperature. Termination fluid (100 μL) was added to each well after the 100 μL chromogen reagent was added. The optical density was detected at 450 and 570 nm. Finally, the concentrations of IL-6, IL-1β, and TNF-α were expressed as pg/mL.
Protein Expression Analysis of Nrf2, HO-1, and NQO1 by Western Blotting
Liver samples were homogenized in precooled protein extraction buffer and centrifuged at 12,000×g at 4°C for 30 min to obtain protein. The total protein content was quantified using a Coomassie brilliant blue reagent kit (Jiancheng Bioengineering Institute of Nanjing, China). Total protein was separated by electrophoresis and transferred onto polyvinylidene fluoride membrane (Millipore, American) and blocked in TBS-T buffer with 5% skimmed dry milk for 1 h at room temperature. Subsequently, the membranes were incubated overnight in specific diluted antibodies of anti-Nrf2 (1:1000), anti-HO-1 (1:1000), and anti-NQO1 (1:500) at 4°C. Anti-β-actin (1:5000) was used as the internal standard. The next day, the blots were washed with PBS-T and incubated in respective secondary diluted antibodies (1:10,000) for 1 h. The membrane was visualized using chemiluminescence detection reagent and exposed onto an X-ray film. The density of the protein signals was analyzed using the Image J system.
Statistical Analysis
The data analyses were performed with the Statistical Package for Social Sciences (SPSS) 20.0 software. All values were presented as the mean ± standard deviation (SD). Differences among groups were determined by one-way analysis of variation (ANOVA). P-values of less than 0.05 were considered statistically significant.
Results
Body Weight, Food and Water Consumption
As shown in Table 1, after 7 days feeding, final body weight, food and water consumption in ATO group were significantly lower than that of control, these parameters were elevated with crocetin treatment. On the other hand, no rat deaths occurred in any of the treatment groups.
 |
Table 1 General Observations in Rats |
Effects of CRO on Liver Histopathology
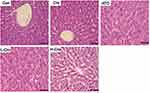
To assess the protection of crocetin against liver damage induced by ATO, pathologic changes in the liver were detected in this experiment. As shown in Figure 2, livers from the control group and crocetin alone group displayed regular cell distribution and lobular architecture. The liver tissues from ATO-treated rats showed obvious pathological changes, including hepatocyte steatosis, apoptosis, disorganization of parenchyma, and those in the H-Cro group indicated that pre-treatment with crocetin markedly ameliorated apoptosis and steatosis of hepatocytes.
Effects of CRO on Biochemical Markers of Liver Function
Treatment with crocetin alone did not produce any marked changes in the activities of ALT, AST, and ALP versus the control group. ATO treatment brought about a marked increase in ALT, AST, and ALP levels versus the control group. Whereas pretreatment with crocetin obviously suppressed the ATO-induced increase of ALT, AST, and ALP activities (Figure 3).
Effects of CRO on Levels of MDA, GSH, CAT, and SOD
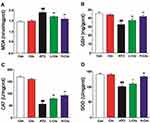
To assess the anti-oxidative effects of crocetin, the levels of MDA, GSH, CAT, and SOD were measured. Figure 4A shows the MDA levels were elevated in ATO groups versus the control group, and this effect was decreased with crocetin pretreatment. As shown in Figure 4B, treatment with ATO resulted in a significant depletion of GSH level versus the control group; treatment with crocetin increased the level of GSH versus ATO group. Figure 4C and D show the effect of pretreatment with crocetin on the activities of CAT and SOD. CAT and SOD activities obviously decreased after ATO exposure. Pretreatment with crocetin inhibited the decrease of CAT and SOD activity.
Effects of CRO on the ROS Generation
As shown in Figure 5, no obvious dichlorofluorescein fluorescence was detected in the control group. Strong fluorescence was detected in the ATO treated group. Crocetin reduces the level of ROS induced by ATO.
Effects of CRO on the Pro-Inflammatory Markers of IL-6, IL-1β, and TNF-α
Results indicate that IL-6, IL-1β, and TNF-α were dramatically elevated in the ATO treated group compared with the control group. CRO, meanwhile, was found to markedly restore the protein levels of IL-6, IL-1β, and TNF-α to normal levels, as shown in Figure 6A–C.
Effects of CRO on the Expression of Nrf2, HO-1, and NQO1
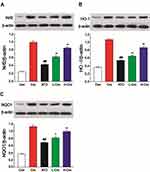
To explore the mechanism of action, the effect of CRO on Nrf2 expression was analyzed by Western blotting in liver samples. Experimental results suggested that Nrf2 protein was markedly higher in CRO-treated rats than the control and ATO alone treated rats (Figure 7A). To further explore the effects of CRO on the Nrf2 signal pathway, the HO-1 and NQO1 proteins (two representative Nrf2 downstream regulatory proteins) were also analyzed in the hepatic tissues. Figure 7B and C also show a clear induction of HO-1 and NQO1 proteins in the corresponding ATO-treated groups. The experiments also indicate that the expression levels of Nrf2, HO-1, and NQO1 were elevated by ATO treatment. Meanwhile, CRO up-regulated the protein expression of Nrf2, HO-1, and NQO1 induced by ATO. Furthermore, the treatment of CRO alone could increase the expression of Nrf2, HO-1, and NQO1 even without the ATO induction.
Discussion
CRO is an aglycone of crocin found in the fruit of gardenia and the stigma of saffron,37 which in its free-acid form is insoluble in water and most organic solvents.38 CRO has been reported to have antioxidant and anti-inflammatory actions.39 Unfortunately, the precise mechanisms involved remain unclear. In our current research, we investigated the effects of CRO on hepatic injury in ATO-induced rats according to its antioxidant and anti-inflammatory effects through activating the Nrf2 signaling pathway.
General observations in rats showed that ATO induced the body weight loss, food and water consumption decreases compared with control group, these parameters were improved in the L-Cro and H-Cro groups.
We found that significant pathological changes discovered in the liver verified the side effects of ATO. The histological analysis showed that crocetin alleviated liver pathologic changes, such as hepatocyte vacuolation and cellular apoptosis. Meanwhile, these results demonstrated that ATO caused liver injury as measured by the elevated activities of ALT, AST, and ALP compared with control group treatment. Crocetin markedly inhibited ATO-induced ALT, AST, and ALP production, which suggests crocetin could attenuate liver injury.
The underlying mechanism of ATO-induced liver damage requires further investigation. It has been reported that arsenic exposure leads to the production of ROS.25 ROS are formed in aerobic cells as byproducts of mitochondrial respiration or oxidases.40 The alteration of the redox environment of the tissue implies the production or metabolism of ROS.41 Peroxidases, such as CAT and GSH peroxidase, are further metabolized to produce O2 and H2O.40 The generation of excessive ROS might result in free radical-mediated injuries, loss of cellular function, and eventual apoptosis or necrosis.42 MDA is the product of endogenous lipid peroxidation and reflects the status of oxidative stress.43,44 Arsenic-induced oxidative damages the liver, which not only increases ROS and MDA but also reduces GSH contents and inhibits many antioxidant enzyme activities, including CAT and SOD.5 Thus, antioxidant therapy might play an important role in reducing liver tissue damage caused by oxidative stress.9,45 After a 7-day treatment with ATO, liver homogenate from rats treated with ATO indicated markedly elevated MDA content, decreased GSH content, and reduced CAT and SOD levels compared to the control group. Nevertheless, crocetin suppressed (dose-dependently) ROS and MDA production induced by ATO. Furthermore, crocetin was found to up-regulate the generation of SOD, CAT, and GSH (which are suppressed by ATO). Our results confirm that ATO-induced liver oxidative stress damages and destroys the balance between oxidative agents and antioxidants. These results also suggested that crocetin protects against ATO induced liver damage by suppressing oxidative stress.
Enhanced oxidative stress causes the disruption of the biological membrane and results in inflammation and the generation of pro-inflammatory cytokines.16,46 Our study also demonstrates that CRO has anti-inflammatory actions. Cytokine (IL-6, IL-1β, and TNF-α) levels were also higher in the ATO group than the control group. After treatment with CRO for 7 days, these parameters were decreased in the L-Cro and H-Cro groups.
The Nrf2 signal pathway is generally considered to enhance the cellular defenses against increased oxidative injuries.24 Furthermore, Nrf2 has been shown to play essential roles in stimulating antioxidant enzymes against oxidative injury.21 Nrf2 manages a series of downstream antioxidative genes encoding antioxidant enzymes, including HO-1 and NQO1, to defend against oxidative damage.8 We discovered that ATO exposure somewhat increases hepatic Nrf2 expression under the tested conditions, which has also been reported by other authors.8,9
The results showed that the expressions of Nrf2, HO-1, and NQO1 were enhanced in the ATO group, these enhancements of Nrf2, HO-1, and NQO1 were up-regulated by CRO. Nevertheless, the protective effect of the Nrf2 signal path might be covered by tissue damage at high doses of arsenic compounds, and the Nrf2-dependent defense reaction is counteracted by the adverse effects induced by ATO, ultimately leading to oxidative stress and toxic damage.25
HO-1 has an anti-oxidant activity that can resist oxidative damage.21 In this research, the up-regulation of HO-1 and NQO1 protein expression are considered to be beneficial and to play key roles in opposing redox imbalances induced by ATO.
Conclusion
In summary, the outcomes of the current study show that crocetin exhibits a protective effect against ATO-induced hepatic oxidant stress, inflammatory injuries and the ability of crocetin to active Nrf2 might help to protect against the arsenic hepatotoxicity. Therefore, crocetin provides a safe and natural option for preventing ATO-induced hepatotoxicity in acute promyelocytic leukemia patients.
Acknowledgments
This work was supported by the Research Foundation of Administration of Traditional Chinese Medicine of Hebei Province, China (No.2020188) and the open projects of Hebei Key Laboratory of Integrative Medicine on Liver-kidney Patterns (B 201907).
Disclosure
Yanshuang Liu and Yingran Liang are co-first authors. The authors declare no conflict of interest.
References
1. Raghu KG, Yadav GK, Singh R, et al. Evaluation of adverse cardiac effects induced by arsenic trioxide, a potent anti-APL drug. J Environ Pathol Toxicol Oncol. 2009;28(3):241–252. doi:10.1615/JEnvironPatholToxicolOncol.v28.i3.60
2. Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl 2):1–2. doi:10.1634/theoncologist.6-suppl_2-1
3. Paul MK, Kumar R, Mukhopadhyay AK. Dithiothreitol abrogates the effect of arsenic trioxide on normal rat liver mitochondria and human hepatocellular carcinoma cells. Toxicol Appl Pharmacol. 2008;226(2):140–152. doi:10.1016/j.taap.2007.09.020
4. Lau A, Whitman SA, Jaramillo MC, et al. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol. 2013;27(2):99–105. doi:10.1002/jbt.21463
5. Gao S, Duan X, Wang X, et al. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol. 2013;59:739–747. doi:10.1016/j.fct.2013.07.032
6. Ling S, Shan Q, Liu P, et al. Metformin ameliorates arsenic trioxide hepatotoxicity via inhibiting mitochondrial complex I. Cell Death Dis. 2017;8(11):e3159. doi:10.1038/cddis.2017.482
7. Zhang Z, Gao L, Cheng Y, et al. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. Biomed Res Int. 2014;2014:617202. doi:10.1155/2014/617202
8. Zhang Y, Wei Z, Liu W, et al. Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget. 2017;8(3):3773–3780. doi:10.18632/oncotarget.13931
9. Xie G, Meng X, Wang F, et al. Eriodictyol attenuates arsenic trioxide-induced liver injury by activation of Nrf2. Oncotarget. 2017;8(40):68668–68674. doi:10.18632/oncotarget.19822
10. Chayapong J, Madhyastha H, Madhyastha R, et al. Arsenic trioxide induces ROS activity and DNA damage, leading to G0/G1 extension in skin fibroblasts through the ATM-ATR-associated Chk pathway. Environ Sci Pollut Res Int. 2017;24(6):5316–5325. doi:10.1007/s11356-016-8215-7
11. Nili-Ahmadabadi A, Alibolandi P, Ranjbar A, et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Res Pharm Sci. 2018;13(6):500–508. doi:10.4103/1735-5362.245962
12. Rahimifard M, Navaei-Nigjeh M, Mahroui N, et al. Improvement in the function of isolated rat pancreatic islets through reduction of oxidative stress using traditional Iranian medicine. Cell J. 2014;16(2):147–163.
13. Dugo EB, Yedjou CG, Stevens JJ, et al. Therapeutic potential of Arsenic Trioxide (ATO) in treatment of hepatocellular carcinoma: role of oxidative stress in ATO-induced apoptosis. Ann Clin Pathol. 2017;5(1).
14. Choudhury S, Ghosh S, Mukherjee S, et al. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J Nutr Biochem. 2016;38:25–40. doi:10.1016/j.jnutbio.2016.09.001
15. Binu P, Gifty K, Vineetha RC, et al. Eugenol, a plant-derived phenolic nutraceutical, protects thiol (SH) group in myocardium from ROS-mediated oxidation under chemotherapeutic stress induced by arsenic trioxide – a in vivo model study. Drug Chem Toxicol. 2018;41(3):352–357. doi:10.1080/01480545.2018.1424179
16. Singh MK, Yadav SS, Yadav RS, et al. Protective effect of Emblica-officinalis in arsenic induced biochemical alteration and inflammation in mice. Springerplus. 2015;4(1):438. doi:10.1186/s40064-015-1227-9
17. Islam LN, Nabi AH, Rahman MM, et al. Association of respiratory complications and elevated serum immunoglobulins with drinking water arsenic toxicity in human. J Environ Sci Health a Tox Hazard Subst Environ Eng. 2007;42(12):1807–1814. doi:10.1080/10934520701566777
18. Tajiki H, Salomao R. Association of plasma levels of tumor necrosis factor alpha with severity of disease and mortality among patients with leptospirosis. Clin Infect Dis. 1996;23(5):1177–1178. doi:10.1093/clinids/23.5.1177
19. Hirotsu Y, Katsuoka F, Funayama R, et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40(20):10228–10239. doi:10.1093/nar/gks827
20. Li S, Ding Y, Niu Q, et al. Lutein has a protective effect on hepatotoxicity induced by arsenic via Nrf2 signaling. Biomed Res Int. 2015;2015:315205.
21. Li L, Liu Q, Fan L, et al. Protective effects of oxymatrine against arsenic trioxide-induced liver injury. Oncotarget. 2017;8(8):12792–12799. doi:10.18632/oncotarget.12478
22. Liu D, Duan X, Dong D, et al. Activation of the Nrf2 pathway by inorganic arsenic in human hepatocytes and the role of transcriptional repressor Bach1. Oxid Med Cell Longev. 2013;2013:984546. doi:10.1155/2013/984546
23. Han YH, Moon HJ, You BR, et al. The effect of MAPK inhibitors on arsenic trioxide-treated Calu-6 lung cells in relation to cell death, ROS and GSH levels. Anticancer Res. 2009;29(10):3837–3844.
24. Yang D, Lv Z, Zhang H, et al. Activation of the Nrf2 signaling pathway involving KLF9 plays a critical role in allicin resisting against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace Elem Res. 2017;176(1):192–200. doi:10.1007/s12011-016-0821-1
25. Li J, Duan X, Dong D, et al. Hepatic and nephric NRF2 pathway up-regulation, an early antioxidant response, in acute arsenic-exposed mice. Int J Environ Res Public Health. 2015;12(10):12628–12642. doi:10.3390/ijerph121012628
26. Wang X, Zhang G, Qiao Y, et al. Crocetin attenuates spared nerve injury-induced neuropathic pain in mice. J Pharmacol Sci. 2017;135(4):141–147. doi:10.1016/j.jphs.2017.08.007
27. Nam KN, Park YM, Jung HJ, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648(1–3):110–116. doi:10.1016/j.ejphar.2010.09.003
28. Xiang M, Yang M, Zhou C, et al. Crocetin prevents AGEs-induced vascular endothelial cell apoptosis. Pharmacol Res. 2006;54(4):268–274. doi:10.1016/j.phrs.2006.06.010
29. Moradzadeh M, Sadeghnia HR, Tabarraei A, et al. Anti-tumor effects of crocetin and related molecular targets. J Cell Physiol. 2018;233(3):2170–2182. doi:10.1002/jcp.25953
30. Zhuang X, Dong A, Wang R, et al. Crocetin treatment inhibits proliferation of colon cancer cells through down-regulation of genes involved in the inflammation. Saudi J Biol Sci. 2018;25(8):1767–1771. doi:10.1016/j.sjbs.2017.04.005
31. Tashakori-Sabzevar F, Hosseinzadeh H, Motamedshariaty VS, et al. Crocetin attenuates spatial learning dysfunction and hippocampal injury in a model of vascular dementia. Curr Neurovasc Res. 2013;10(4):325–334. doi:10.2174/15672026113109990032
32. Zhang H, Shang Q, An J, et al. Crocetin inhibits PDGF-BB-induced proliferation and migration of retinal pigment epithelial cells. Eur J Pharmacol. 2019;842:329–337. doi:10.1016/j.ejphar.2018.11.001
33. Hemmati AA, Olapour S, Varzi HN, et al. Ellagic acid protects against arsenic trioxide-induced cardiotoxicity in rat. Hum Exp Toxicol. 2018;37(4):412–419. doi:10.1177/0960327117701986
34. Zhang W, Li Y, Ge Z. Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed Pharmacother. 2017;93:376–382. doi:10.1016/j.biopha.2017.06.032
35. Zhu W, Chen S, Li Z, et al. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr Metab (Lond). 2014;11(1):35. doi:10.1186/1743-7075-11-35
36. de Castro AL, Tavares AV, Fernandes RO, et al. T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol Cell Biochem. 2015;408(1–2):235–243. doi:10.1007/s11010-015-2501-4
37. Umigai N, Takeda R, Mori A. Effect of crocetin on quality of sleep: a randomized, double-blind, placebo-controlled, crossover study. Complement Ther Med. 2018;41:47–51. doi:10.1016/j.ctim.2018.09.003
38. Hashemi M, Hosseinzadeh H. A comprehensive review on biological activities and toxicology of crocetin. Food Chem Toxicol. 2019;130:44–60. doi:10.1016/j.fct.2019.05.017
39. Zhang J, Wang Y, Dong X, et al. Crocetin attenuates inflammation and amyloid-beta accumulation in APPsw transgenic mice. Immun Ageing. 2018;15(1):24. doi:10.1186/s12979-018-0132-9
40. Han YH, Kim SH, Kim SZ, et al. Apoptosis in arsenic trioxide-treated Calu-6 lung cells is correlated with the depletion of GSH levels rather than the changes of ROS levels. J Cell Biochem. 2008;104(3):862–878. doi:10.1002/jcb.21673
41. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757(5–6):509–517. doi:10.1016/j.bbabio.2006.04.029
42. Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11(1):300–312. doi:10.2741/1798
43. Zhong G, Wan F, Yan H, et al. Methionine sulfoxide reductases are related to arsenic trioxide-induced oxidative stress in mouse liver. Biol Trace Elem Res. 2019.
44. Varghese MV, Manju A, Abhilash M, et al. Oxidative stress induced by the chemotherapeutic agent arsenic trioxide. 3 Biotech. 2014;4(4):425–430. doi:10.1007/s13205-013-0170-0
45. Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 2009;47(1):145–149. doi:10.1016/j.fct.2008.10.020
46. Chang SI, Jin B, Youn P, et al. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol. 2007;218(2):196–203. doi:10.1016/j.taap.2006.11.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.