Back to Journals » Drug Design, Development and Therapy » Volume 14
Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway
Authors Liu D, Wang H, Zhang Y, Zhang Z
Received 26 August 2019
Accepted for publication 27 November 2019
Published 7 January 2020 Volume 2020:14 Pages 51—60
DOI https://doi.org/10.2147/DDDT.S228751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Dequan Liu,1 Huilin Wang,1 Yangang Zhang,2 Zhan Zhang2
1Department of Neurology, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang, Henan 471000, People’s Republic of China; 2Department of Ultrasound, The Affiliated Children’s Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710003, People’s Republic of China
Correspondence: Zhan Zhang
The Affiliated Children’s Hospital of Xi’an Jiaotong University, No. 69, Xijuyuan Lane, Lianhu District, Xi’an City, Shaanxi Province 710003, People’s Republic of China
Tel +86-29-13991941239
Email [email protected]
Introduction: Cerebral ischemia-reperfusion (CI/R) injury is caused by blood flow recovery after ischemic stroke. Chlorogenic acid (CGA, 5-O-caffeoylquinic acid) is a major polyphenol component of Coffea canephora, Coffea arabica L. and Mate (Ilex paraguariensis A. StHil.). Previous studies have shown that CGA has a significant neuroprotective effect and can improve global CI/R injury. However, the underlying molecular mechanism of CGA in CI/R injury has not been fully revealed.
Materials: In this study, CI/R rat model was constructed. The rats were randomly divided into nine groups with ten in each group: Control, CGA (500 mg·kg-1), CI/R, CI/R + CGA (20 mg·kg-1), CI/R + CGA (100 mg·kg-1), CI/R + CGA (500 mg·kg-1), ML385 (30 mg·kg-1), CI/R + ML385 (30 mg·kg-1), CI/R + CGA + ML385. Cerebral infarction volume was detected by TTC staining. Brain pathological damage was detected by H&E staining. Apoptosis of cortical cells was detected by TUNEL staining. The expression of related proteins was detected by RT-qPCR and Western blotting.
Results: Step-down test and Y maze test showed that CGA dose-dependently mitigated CI/R-induced brain damage and enhanced learning and spatial memory. Besides, CGA promoted the expression of BDNF and NGF in a dose-dependent manner and alleviated CI/R-induced nerve injury. Moreover, CGA increased the activity of SOD and the level of GSH, as well as decreased production of ROS and LDH and the accumulation of MDA. Notably, CGA attenuated oxidative stress-induced brain injury and apoptosis and inhibited the expression of apoptosis-related proteins (cleaved caspase 3 and caspase 9). Additionally, CGA reversed CI/R induced inactivation of Nrf2 pathway and promoted Nrf2, NQO-1 and HO-1 expression. Nrf2 pathway inhibitor ML385 destroyed this promotion.
Discussion: All the data indicated that CGA had a neuroprotective effect on the CI/R rats by regulating oxidative stress-related Nrf2 pathway.
Keywords: cerebral ischemia/reperfusion injury, chlorogenic acid, oxidative stress, neuroprotection, NF-E2-related factor 2 pathway
Introduction
The brain is an important organ with high perfusion, elevated oxygen consumption, high metabolism and low energy reserve. Cerebral ischemia and hypoxia can cause ischemic stroke, which accounts for about 80% of strokes.1,2 Ischemic stroke has a catastrophic impact on people’s life and has an extremely high incidence and mortality rate worldwide.3 Cognitive impairment is one of the most common complications of a stroke. Clinically, intravenous thrombolysis combined with alteplase or intra-arterial thrombectomy is an effective strategy for the treatment of ischemic stroke therapy.4 However, it may also aggravate the injury by inducing cerebral ischemia-reperfusion (CI/R)5. Hence, it is of profound significance to find new drugs with high efficiency and low toxicity for the prevention and treatment of CI/R injury. Chlorogenic acid (CGA, 5-O-caffeoylquinic acid) is a polyphenol component isolated from Coffea canephora, Coffea arabica L. and Mate (Ilex paraguariensis A. StHil.). Studies have shown that CGA has many physiological functions, such as neuroprotection,6 neuronutrition,7 anti-oxidation8 and anti-inflammatory.9 Clinical studies have shown that CGA relieved mental fatigue and headaches and had a positive effect on patients’ mood.10 In addition, CGA increased the survival of dopaminergic neurons11 and improved spatial learning and memory.12 Moreover, CGA enhanced the therapeutic effect of tissue plasminogen activator (tPA)13 and reduced oxidative stress and neuroinflammation caused by MPTP.14
Oxidative stress (OS) is one of the core processes of CI/R.15 Numerous studies have shown that NF-E2-related factor 2 (Nrf2) pathway is the most important antioxidant stress system in vivo and plays an important role in regulating oxidative stress-induced apoptosis and CI/R16–18. Negative regulatory nuclear transcription factor Nrf2 is a transcription factor that regulates the expression of a large number of antioxidant protein genes.19 Endogenous antioxidant enzymes induced by Nrf2 play an important role in many diseases.20 Previous studies have shown that CGA improved osteoporosis by activating Nrf2/HO-1 pathway. However, whether CGA can improve CI/R injury by regulating Nrf2/HO-1 pathway remains to be further studied.
In this study, we elaborated the role of CGA in CI/R injury in rats and its molecular mechanism. All data suggest that CGA attenuates CI/R injury by reducing oxidative stress through the Nrf2 signaling pathway.
Materials and Methods
Animal
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by Luoyang Central Hospital Affiliated to Zhengzhou University. A total of 70 Sprag-Dawley rats (male, 250–280 g) were obtained from the Animal Center of Luoyang Central Hospital Affiliated to Zhengzhou University and housed in a controlled environment at 25 ± 3°C, humidity 60%, 12-h light/dark cycle with free access to food and water.
Grouping
Rats were randomly divided into nine groups with ten in each group: Control group; CGA (500 mg·kg−1) group, rats were administered with 500 mg·kg−1 CGA; CI/R group orally; CI/R + CGA (20 mg·kg−1) group, CI/R rats were administered with 20 mg·kg−1 CGA orally; CI/R + CGA (100 mg·kg−1) group, CI/R rats were administered with 100 mg·kg−1; orally CI/R + CGA (500 mg·kg−1) group, CI/R rats were administered with 500 mg·kg−1 orally;21 ML385 (30 mg·kg−1) group, rats were intraperitoneal injected with 30 mg·kg−1 ML385; CI/R + ML385 (30 mg·kg−1) group,22 CI/R rats were intraperitoneal injected with 30 mg·kg−1 ML385, a Nrf2 pathway inhibitor. CI/R + CGA + ML385 group, CI/R rats were intraperitoneal injected with 30 mg·kg−1 ML385 30 mins before CGA (500 mg·kg−1) administration.
Model
The rats were anesthetized with sodium pentobarbital (0.84 mL) and fixed supine after 1 hr of gavage on day 7. The bilateral common carotid arteries (CCA) were separated and clamped with a micro-arterial clamp for 10 mins, released for 10 mins, and clamped for another 10 mins.23 The rats in the control group received the same surgery, but no clamp. The rats that woke up from anesthesia were put back in their cages and given a random diet and water.
Step-Down Test
The Step-down test was employed to observe the learning and memory ability of rats. The Step-down test was carried out in accordance with the method of Longa et al24 In brief, the rats were placed on a diving platform to familiarize themselves with the environment for 3 mins, and then powered on for 5 mins. After training, the rats were received the same treatment, and the error times to jump off the platform within 5 mins was observed.
Y Maze Test
The Y maze experiment was applied to evaluate spatial memory and spatial reference memory of rats. Y maze is randomly divided into three arms: the new arm, starting arm and the other arm. The Y maze experiment includes two steps. In the first step, the new arm was closed, and the rats were allowed to explore freely the other two arms of 10 mins. Second, open all the arms and let the rats move freely among the three arms for 5 mins. Video software tracking system was used to track the time and number of times the rats stayed in the new arm to evaluate the spatial recognition, memory ability and active ability of the rats.
2,3,5-Triphenyltetrazolium Chloride (TTC) Assay
The brain tissue was removed, washed with saline and cut into 2-mm thick sections. Subsequently, the sections were exposed to 1% TTC solution (Oxoid, Hampshire, UK) at 37°C for 30 mins in the dark, and then fixed in 10% formaldehyde for 24 hrs and photographed. Unstained areas were defined as infarcted areas. Actual corrected edema infarct volume was calculated by dividing the infarct volume by the edema index, which was calculated by dividing the total volume of the left hemisphere by the total volume of the right hemisphere.25 The total wet weight of brain tissue was measured, and the normal brain tissue and infarct tissue were placed in a 105°C oven for 24 hrs and the dry weight was measured, respectively. Brain water content (%) = (total wet weight of brain–total dry weight of brain)/total wet weight of brain × 100%; brain Index (%) = brain weight/body weight × 100%
Neurological Function Assessment
According to the Zea-Longa scoring system,26 the neurological scores of rats in each group were as follows: 0 = no symptoms of neurological impairment; 1 = unable to extend the contralateral forepaws; 2 = turn to the hemiplegic side when walking; 3 = dumping to the hemiplegic side; 4 = unable to walk spontaneously, unconscious.
RT-qPCR
The mRNA levels of BDNF and NGF were detected by RT-qPCR. In brief, total RNA (1μL) was isolated from ischaemic brain tissue using the RNeasy Midi kit (Qiagen, Valencia, CA) and reversely transcribed to cDNA using FastKing One Step RT-PCR Kit (Tiangen, Beijing, China). Then, qPCR was performed with SYBR Premix Ex Taq II enzyme (Takara, Shanghai, China) on BioRad CFX96 Sequence Detection System (BioRad, Shanghai, China). GAPDH was employed as normalization. Products were detected by agarose gel electrophoresis and stained with GoldView (Solarbio, Beijing, China). Fold change is analyzed by comparative Ct value method. Primers were provided below:
BDNF, F, 5ʹ- CCGTAACGATACGACTACG −3ʹ; R, 5ʹ- GTACATGCAACTGCTGAC - 3ʹ NGF, F, 5ʹ- TGTGCACATGCTACGTCCT’ - 3ʹ; R, 5ʹ- ACTGCTCGACCAAGCCGC - 3ʹ GAPDH, F, 5ʹ- TCATGCATGCTGACGCTAC - 3ʹ; R, 5ʹ- TTGTACTGCCTGCACTGC - 3ʹ
Western Blotting
Protein was extracted from Ischaemic brain tissues using a Tissue protein Extraction Kit (PHYGENE, Beijing, China) with protein phosphatase inhibitors. The protein concentration was determined using the BCA protein assay kit (Takara, Dalian, China). After that, the protein (20 μg) was separated by 10% SDS-PAGE and then transferred onto a PVDF membrane (Roche, Beijing, China). Then, the sample was cultured with the diluted primary antibodies anti-BDNF (ab108319, 1:5000, Abcam, UK), anti-NGF (ab6199, 1:5000, Abcam, UK), anti-Actin (ab179467, 1:5000, Abcam, UK), anti-Caspase 3 (ab13847, 1:5000, Abcam, UK), anti-cleaved caspase 3 (ab2302, 1:5000, Abcam, UK), anti-Caspase 9 (ab202068, 1:5000, Abcam, UK), anti-cleaved caspase 9 (ab2324, 1:5000, Abcam, UK), anti-Nrf2 (ab62352, 1:5000, Abcam, UK), anti-NQO-1 (ab28947, 1:5000, Abcam, UK) and anti-HO-1 (ab13243, 1:5000, Abcam, UK) overnight at 4°C. The next day, the samples were incubated with the secondary antibody at room temperature for 2 hrs. The protein signal was detected by the ECL kit (Millipore Corp. Bedford, MA, USA).
Hematoxylin and Eosin (HE) Staining
H&E staining was applied to detect the histopathological lesion. Hippocampal neurons were fixed with 4% paraformaldehyde, embedded in paraffin and cut into slices. The histological changes of brain tissue were observed by TissueFAXS (TissueGnostics, Vienna, Austria) at 400× magnification.
TUNEL Staining
Paraffin-embedded brain cortical tissue was fixed in xylene, hydrated with a gradient of ethanol, and permeabilized with proteinase K for 30 mins. Subsequently, brain tissue was subjected to TUNEL reagent for 1 hr at 37°C, followed by photography, and the brown-labeled cells were TUNEL-positive cells.27
Detection of Oxidative Stress
ROS production in ischaemic brain tissues was detected by Reactive Oxygen Species Assay Kit (Beyotime, Shanghai, China). Malondialdehyde (MDA) was measured by Lipid Peroxidation MDA Assay Kit (Beyotime, Shanghai, China) and glutathione peroxidase (GSH-Px) was detected by Glutathione Reductase Assay Kit (Beyotime, Shanghai, China). Superoxide dismutase (SOD) were determined by Total Superoxide Dismutase Assay Kit with NBT (Beyotime, Shanghai, China), and LDH was measured according to the method of Zhu et al.28
Statistical Analysis
All data are presented as mean ± standard deviation (SD). GraphPad prism 5 and one–way ANOVA followed by Tukey’s post hoc test were employed for data analysis. P < 0.05 was deemed as statistically significant difference.
Results
CGA Mitigates Brain Damage in Rats with CI/R
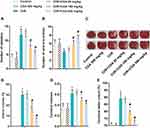
The learning and memory ability of rats was reflected by Step-down test. As shown in Figure 1A, the number of mistakes in the CI/R group was significantly higher than that in the control group and the CGA group, while different doses of CGA (20, 100 and 500 mg/kg) significantly reduced the number of mistakes caused by CI/R. Secondly, the Y maze test was used to detect spatial memory impairment. As shown in Figure 1B, compared with the control group and the CGA group, the number of the times the CI/R rats entered the new arm was decreased, while the number of times the CGA-treated CI/R rats entered the new arm was increased in a dose-dependent manner. In addition, compared with the control group and the CGA group, CI/R treatment also caused an increase in cerebral infarction volume, cerebral water content and cerebral index in rats. Interestingly, CGA treatment prevented CI/R from promoting brain injury in a dose-dependent manner (Figure 1C and D). In summary, these results suggest that CGA mitigates brain damage in CI/R rats.
CGA Alleviates Nerve Injury in Rats with CI/R
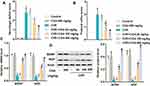
Next, this study explored the effect of CGA on neurological impairment. As shown in Figure 2A and B, neurological deficit scores were increased in the CI/R group compared with the control group and the CGA group, while decreasing in the CGA-treated CI/R rats in a dose-dependent manner. Besides, RT-qPCR and Western blotting showed that CI/R treatment inhibited the expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) at both RNA and protein levels, while CGA treatment significantly reversed the inhibitory effects (Figure 2C and D). In summary, these results indicate that CGA alleviates nerve injury in CI/R rats.
CGA Alleviates Oxidative Stress Induced by CI/R in Rats
This study examined the effect of CGA on oxidative stress. As shown in Figure 3A–E, compared to the control group and the CGA group, CI/R treatment reduced the activity of SOD and GSH, and promoted the release of LDH and the production of MDA and ROS. It is worth noting that after treatment with different doses of CGA (20, 100 and 500 mg/kg), the activities of SOD and GSH in CI/R rats were increased, while the contents of LDH, MDA and ROS were decreased in a dose-dependent manner. These results indicate that CGA alleviates oxidative stress induced by CI/R in rats.
CGA Improves the Pathological Damage of Hippocampal Neurons and Reduces Apoptosis in Rats with CI/R
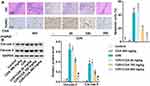
Pathological damage of hippocampal neurons in rats was measured by H&E staining, and apoptosis rate of cerebral cortex cells was detected by TUNEL staining. As shown in Figure 4A, hippocampal neurons in the control group and CGA group were structurally complete and closely arranged. The nuclei were normal, and the nucleoli were clear with a uniform staining. However, in the CI/R group, the structure of hippocampal neurons was severely damaged, with nuclear deformation, shrinkage, hyperchromia and even disappearance. Besides, the cells were loosely arranged and began to form cavities. It is worth noting that CGA treatment improved the structural damage of hippocampal neurons induced by CI/R and reduced the cavity structure in rats. In addition, TUNEL staining showed that the number of brown labeled apoptotic cells in CI/R group was significantly higher than that in control group and CGA group. Notably, CGA treatment prevented cortical cell apoptosis in a dose-dependent manner. Similarly, Western blotting analysis showed that CI/R treatment promoted the expression of cleaved caspase 3 and cleaved caspase 9. On the contrary, CGA treatment abolished the promotional effect on the expression of cleaved caspase 3 and cleaved caspase 9 (Figure 4B). All these data suggest that CGA improves the pathological damage of hippocampal neurons and reduces apoptosis of cerebral cortex cells in CI/R rats.
Nrf2/NQO-1/HO-1 Pathway Is Involved in the Role of CGA in CI/R Rats
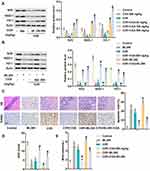
The potential molecular mechanism of CGA on CI/R was investigated by Western blotting analysis. As shown in Figure 5A, the expression of Nrf2 pathway proteins (Nrf2, NQO-1 and HO-1) was significantly inhibited in CI/R rats. However, levels of Nrf2, NQO-1 and HO-1 were dose-dependently up-regulated after treatment with different doses of CGA (20, 100 and 500 mg/kg). The Nrf pathway inhibitor ML385 (10 μ M) further confirmed that Nrf2 pathway was involved in the role of CGA. As shown in Figure 5B, protein levels of Nrf2, NQO-1 and HO-1 in healthy rats treated with ML385 alone were significantly lower than those in the control group. Similarly, the expression of Nrf2, NQO-1 and HO-1 was reduced in CI/R rats. Interestingly, CGA treatment reversed the decrease in CI/R-induced Nrf2 pathway-associated protein expression, whereas ML385 aggravated the inactivation of the Nrf2 pathway. Further functional experiments showed that ML385 aggravated CI/R-induced neuronal injury, apoptosis and oxidative stress in rats. However, CGA treatment counteracted the damage of ML385 to CI/R rats (Figure 5C and D). All these results suggest that Nrf2/NQO-1/HO-1 pathway is involved in the role of CGA in CI/R rats.
Discussion
The incidence of ischemic stroke has continued to increase in recent years.29 Cognitive impairment caused by learning and memory impairment is one of the most common complications of stroke.30 The restoration of blood flow in ischemic stroke will aggravate the injury and give rise to CI/R injury. The occurrence of CI/R injury is related to mitochondrial energy metabolism disorder, excitatory amino acid toxicity, ion balance imbalance, oxidative stress, inflammatory response, apoptosis and blood brain barrier destruction.31 Therefore, the prevention and treatment of CI/R injury is the focus of ischemic stroke therapy. CI/R injury usually result in neurological deficits, cerebral infarction, learning and memory impairment, edema, water content and high brain index. This study investigated the effect of CGA on CI/R injury. The results showed that CGA treatment reduced brain injury, nerve injury and cerebral infarction volume and inhibited neuron apoptosis by reducing CI/r-induced oxidative stress in rats.
In vitro and in vivo experiments have shown that CGA has obvious neuroprotective effects.32,33 Rebai et al pointed out that CGA inhibited endogenous ROS accumulation and restored mitochondrial membrane potential by activating the enzyme antioxidant system, and regulated intracellular Ca2+ concentration caused by glutamic acid over-stimulation, thereby protecting cortical neuron injury.33 Heitman et al believe that CGA protected against neurodegeneration and the resulting diseases associated with oxidative stress in the brain.34 Shan et al found that CGA showed a neuroprotective effect by inhibiting 6-ohda-induced ROS production and endoplasmic reticulum stress.35 In addition, Kumar et al investigated the effect of CGA on global CI/R injury. The results showed that CGA significantly reduced cerebral infarction area and Evans blue extravasation and restored cerebral water content. Moreover, CGA reduced the levels of calcium, nitrate and glutamate in the cortex, hippocampus cerebellum and cerebrospinal fluid. CGA significantly reduced the expression of TNF-α, iNOS and caspase-3.36 Similarly, this study found that CGA improved CI/R-induced brain tissue pathology and cortical cell apoptosis in a dose-dependent manner by reducing oxidative stress. In addition, we also found that the role of CGA in CI/R involves the activation of Nrf2 pathway.
CI/R causes brain injury, which is closely related to oxygen free radicals in the brain. CI/R-induced LDH release and MDA accumulation can directly reflect the severity of cerebral ischemia and the degree of lipid peroxidation. SOD and gsh-px are powerful free radical scavenging factors that can reduce CI/r-induced oxidative stress. Nrf2 pathway is the focus of antioxidant research in recent years.37 Nrf2 pathway shows an antioxidant effect by up-regulating a series of endogenous protective genes.38 HO-1 and NQO-1 are two major proteins that resist oxidative stress.39 HO-1 catalyzes the formation of biliverdin, bilirubin and ferritin, thus exhibiting antioxidant activity.40 NQO-1 plays an anti-oxidative stress role by preventing the production of ROS.41 Previous studies have shown that IL-1Ra significantly inhibits CI/R-induced oxidative stress by promoting the expression of Nrf2 and HO-1.42 Similarly, this study found that Nrf2 pathway was inactivated in CI/R rats. Interestingly, CGA reactivated Nrf2 pathway and promoted Nrf2, HO-1 and NQO-1 expression, which further increased SOD activity and reduced MDA level. In addition, CGA inhibited the apoptosis of cerebral cortex cells by reducing CI/R-induced oxidative stress and alleviated the brain pathological damage. As expected, Nrf2 pathway inhibitor ML385 destroyed the effect of CGA on CI/R and exacerbated CI/R-induced pathological damage. Overall, these results suggest that CGA appears to be protective in CI/R rats by regulating pathways related to oxidative stress.
Conclusion
In conclusion, this study elaborated the role of CGA in CI/R in rats and its underlying molecular mechanisms. All the data indicated that CGA had a neuroprotective effect on the CI/R rats by regulating oxidative stress-related Nrf2 pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19:166–187. doi:10.5853/jos.2016.01368
2. Jiang D, Sun X, Wang S, Man H. Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother. 2019;117:108941. doi:10.1016/j.biopha.2019.108941
3. Wang J, Chen T, Shan G. miR-148b regulates proliferation and differentiation of neural stem cells via Wnt/β-catenin signaling in rat ischemic stroke model. Front Cell Neurosci. 2017;11:329. doi:10.3389/fncel.2017.00329
4. Eryildiz ES, Ozdemir AO. Factors associated with early recovery after intravenous thrombolytic therapy in acute ischemic stroke. Noro Psikiyatri Arsivi. 2018;55:80–83. doi:10.29399/npa.22664
5. Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158. doi:10.1161/CIRCRESAHA.116.308022
6. Taram F, Winter AN, Linseman DA. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016;1648:69–80. doi:10.1016/j.brainres.2016.07.028
7. Ito H, Sun XL, Watanabe M, Okamoto M, Hatano T. Chlorogenic acid and its metabolite m-coumaric acid evoke neurite outgrowth in hippocampal neuronal cells. Biosci Biotechnol Biochem. 2008;72:885–888. doi:10.1271/bbb.70670
8. Yang L, Wang N, Zheng G. Enhanced effect of combining chlorogenic acid on selenium nanoparticles in inhibiting amyloid beta aggregation and reactive oxygen species formation in vitro. Nanoscale Res Lett. 2018;13:303. doi:10.1186/s11671-018-2720-1
9. Tsang MS, Jiao D, Chan BC, et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules. 2016;21:519. doi:10.3390/molecules21040519
10. Cropley V, Croft R, Silber B, et al. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl). 2012;219:737–749. doi:10.1007/s00213-011-2395-0
11. Shen W, Qi R, Zhang J, et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res Bull. 2012;88:487–494. doi:10.1016/j.brainresbull.2012.04.010
12. Han J, Miyamae Y, Shigemori H, Isoda H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169:1039–1045. doi:10.1016/j.neuroscience.2010.05.049
13. Lapchak PA. The phenylpropanoid micronutrient chlorogenic acid improves clinical rating scores in rabbits following multiple infarct ischemic strokes: synergism with tissue plasminogen activator. Exp Neurol. 2007;205:407–413. doi:10.1016/j.expneurol.2007.02.017
14. Singh SS, Rai SN, Birla H, et al. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front Pharmacol. 2018;9:757. doi:10.3389/fphar.2018.00757
15. Wu J, Chen Y, Yu S, et al. Neuroprotective effects of sulfiredoxin-1 during cerebral ischemia/reperfusion oxidative stress injury in rats. Brain Res Bull. 2017;132:99–108. doi:10.1016/j.brainresbull.2017.05.012
16. Xu X, Zhang L, Ye X, et al. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm Res. 2018;67:57–65. doi:10.1007/s00011-017-1095-6
17. Chen L, Cao J, Cao D, et al. Protective effect of dexmedetomidine against diabetic hyperglycemia-exacerbated cerebral ischemia/reperfusion injury: an in vivo and in vitro study. Life Sci. 2019;235:116553. doi:10.1016/j.lfs.2019.116553
18. Yoo JM, Lee BD, Sok DE, Ma JY, Kim MR. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017;11:592–599. doi:10.1016/j.redox.2016.12.034
19. Lou Y, Guo Z, Zhu Y, et al. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J Exp Clin Cancer Res. 2019;38:242. doi:10.1186/s13046-019-1255-3
20. Wang X, Saud SM, Zhang X, Li W, Hua B. Protective effect of Shaoyao Decoction against colorectal cancer via the Keap1-Nrf2-ARE signaling pathway. J Ethnopharmacol. 2019;241:111981. doi:10.1016/j.jep.2019.111981
21. Song J, Zhou N, Ma W, et al. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019;10:2947–2957. doi:10.1039/C8FO02599A
22. Sun J, Yu X, Huangpu H, Yao F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed Pharmacother. 2019;109:254–261. doi:10.1016/j.biopha.2018.09.002
23. Ghanbarabadi M, Falanji F, Rad A, et al. Neuroprotective effects of clavulanic acid following permanent bilateral common carotid artery occlusion in rats. Drug Dev Res. 2019. doi:10.1002/ddr.21595
24. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi:10.1161/01.STR.20.1.84
25. Hua F, Ma J, Ha T, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–108. doi:10.1016/j.brainres.2009.01.018
26. Zhao Y, Li D, Zhu Z, Sun Y. Improved neuroprotective effects of gallic acid-loaded chitosan nanoparticles against ischemic stroke. Rejuvenation Res. 2019. doi:10.1089/rej.2019.2230
27. Zuo L, Feng Q, Han Y, et al. Therapeutic effect on experimental acute cerebral infarction is enhanced after nanoceria labeling of human umbilical cord mesenchymal stem cells. Ther Adv Neurol Disord. 2019;12:1756286419859725. doi:10.1177/1756286419859725
28. Zhu Z, Li J, Zhang X. Salidroside protects against ox-LDL-induced endothelial injury by enhancing autophagy mediated by SIRT1-FoxO1 pathway. BMC Complement Altern Med. 2019;19:111. doi:10.1186/s12906-019-2526-4
29. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi:10.1371/journal.pmed.0030442
30. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862:915–925. doi:10.1016/j.bbadis.2016.01.015
31. Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46:1650–1667. doi:10.1159/000489241
32. Yao J, Peng S. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. Biofactors. 2019;45:616–626. doi:10.1002/biof.1507
33. Rebai O, Belkhir M, Sanchez-Gomez MV, Matute C, Fattouch S, Amri M. Differential molecular targets for neuroprotective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem Res. 2017;42:3559–3572. doi:10.1007/s11064-017-2403-9
34. Heitman E, Ingram DK. Cognitive and neuroprotective effects of chlorogenic acid. Nutr Neurosci. 2017;20:32–39. doi:10.1179/1476830514Y.0000000146
35. Shan S, Tian L, Fang R. Chlorogenic acid exerts beneficial effects in 6-hydroxydopamine-induced neurotoxicity by inhibition of endoplasmic reticulum stress. Med Sci Monit. 2019;25:453–459. doi:10.12659/MSM.911166
36. Kumar G, Mukherjee S, Paliwal P, et al. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1293–1309. doi:10.1007/s00210-019-01670-x
37. Jiang LJ, Zhang SM, Li CW, Tang JY, Che FY, Lu YC. Roles of the Nrf2/HO-1 pathway in the anti-oxidative stress response to ischemia-reperfusion brain injury in rats. Eur Rev Med Pharmacol Sci. 2017;21:1532–1540.
38. Wu G, Zhu L, Yuan X, et al. Britanin ameliorates cerebral ischemia-reperfusion injury by inducing the Nrf2 protective pathway. Antioxid Redox Signal. 2017;27:754–768. doi:10.1089/ars.2016.6885
39. Tanaka N, Ikeda Y, Ohta Y, et al. Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 2011;1370:246–253. doi:10.1016/j.brainres.2010.11.010
40. Lee JY, Park JM, Hong JA, Lee DC, Im JA, Lee JW. Serum ferritin is differentially associated with anti-oxidative status and insulin resistance in healthy obese and non-obese women. Korean J Fam Med. 2012;33:205–210. doi:10.4082/kjfm.2012.33.4.205
41. Siegel D, Gustafson DL, Dehn DL, et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi:10.1124/mol.65.5.1238
42. Jin C, Fu W-L, Zhang DD, et al. The protective role of IL-1Ra on intestinal ischemia reperfusion injury by anti-oxidative stress via Nrf2/HO-1 pathway in rat. Biomed J. 2019;42:36–45. doi:10.1016/j.bj.2018.11.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.