Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Protective Effect of Panax notoginseng Extract Fermented by Four Different Saccharomyces cerevisiae Strains on H2O2 Induced Oxidative Stress in Skin Fibroblasts
Authors Wang Z, Fang J, Zu S, Sun Q, Song Z, Geng J, Wang D, Li M , Wang C
Received 8 October 2023
Accepted for publication 16 January 2024
Published 13 March 2024 Volume 2024:17 Pages 621—635
DOI https://doi.org/10.2147/CCID.S443717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Ziwen Wang,1,2 Jiaxuan Fang,1,2 Shigao Zu,1,2 Qianru Sun,1,2 Zixin Song,1,2 Jiman Geng,1,2 Dongdong Wang,1,2 Meng Li,1,2 Changtao Wang1,2
1Beijing Key Laboratory of Plant Resource Research and Development, Beijing Technology and Business University, Beijing, 100048, People’s Republic of China; 2School of Light Industry Science and Engineering, Beijing Technology and Business University, Beijing, 100048, People’s Republic of China
Correspondence: Dongdong Wang; Changtao Wang, Beijing Technology and Business University, Beijing, 100048, People’s Republic of China, Tel/Fax +86 158 1135 7756, Email [email protected]; [email protected]
Purpose: To produce Panax notoginseng extract as a cosmetic ingredient through Saccharomyces cerevisiae fermentation.
Methods: We first compared the total sugar content, polysaccharide content, reducing sugar content, total phenolic content, total saponin content, DPPH free radical, ABTS free radical, hydroxyl free radical scavenging ability and ferric reducing antioxidant power (FRAP) of Panax notoginseng fermented extract (pnFE) and unfermented extract (pnWE). Their potential correlations were analyzed by Pearson’s correlation analysis. Then, the oxidative stress model of H2O2-induced MSFs was used to evaluate the effects of different pnFE on MSF viability, reactive oxygen species (ROS), malondialdehyde (MDA), and the activities of catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) to explore their protective effects on MSFs subjected to H2O2-induced cellular oxidative damage. Finally, their safety and stability were evaluated by using the red blood cell (RBC) test and hen’s egg test-chorioallantoic membrane (HET-CAM) assay, and changes in pH and content of soluble solids, respectively.
Results: Compared with pnWE, pnFE has more active substances and stronger antioxidant capacity. In addition, pnFE has a protective effect on H2O2-induced oxidative stress in MSFs with appropriate safety and stability.
Conclusion: PnFE has broad application prospects in the field of cosmetics.
Keywords: Panax notoginseng, Saccharomyces cerevisiae, fermentation, oxidative stress, fibroblast cells, cosmetic ingredients
Introduction
Traditional Chinese medicine (TCM) can date back several thousand years, which carries valuable experience and theoretical knowledge of the ancient Chinese people when they fought against all kinds of diseases. Panax notoginseng (Burk.) F.H. Chen is a perennial herb of the family Araliaceae, and its roots and rhizomes are the main herb components used in TCM, which are traditionally used for dispersing blood stasis and hemostasis, reducing swelling and calming pain. Panax notoginseng saponins are considered the main active components of Panax notoginseng, which also contains active substances such as polysaccharides, flavonoids, phytosterols, polyacetylenes, amino acids and cyclopeptides.1,2 Recent research has found that Panax notoginseng has various effects such as treating cardiovascular and cerebrovascular diseases, anti-tumor, anti-oxidation, anti-aging, anti-inflammatory, neuroprotective, and anti-diabetic effects and improving the blood circulation.2–9
However, the long and high-temperature decocting process may result in the loss of active ingredients in TCMs.10 To improve the extraction efficiency of active substances from Panax notoginseng, it is necessary to develop new extraction methods. Previous studies have shown that microbial fermentation can digest plant cell walls, resulting in the release or synthesis of various antioxidants.11 In our previous study, it was found that polysaccharides from the fermented roots of Panax notoginseng can alleviate oxidative stress in human dermal fibroblast cells and may promote type I pro-collagen synthesis through the TGF-β/Smad signaling pathway.12 Another study found that solid-state fermentation of Aspergillus cristatus could increase the content of protopanaxadiol and protopanaxatriol in Panax notoginseng and exert anti-aging activity.13 However, Panax notoginseng has various types of active ingredients, and the content of active substances, antioxidant capacity and safety of Panax notoginseng fermented by Saccharomyces cerevisiae have not been evaluated.
The skin is the largest organ in the human body and the organ that displays the most obvious signs of aging. When the skin is affected by exogenous factors (ultraviolet light, environmental toxins, etc.) and endogenous factors, intracellular reactive oxygen species (ROS) levels increase. ROS are byproducts of the electron transport chain in mitochondrial aerobic metabolism, and mainly include superoxide anion (O2·-), hydroxyl radical (·OH) and H2O2. When ROS exceed a certain limit, the redox balance in the body is broken down, leading to the occurrence of oxidative stress. Oxidative stress can lead to damage in lipids, proteins, nucleic acids and organelles. In this state, the content of lipid peroxidation products such as malondialdehyde (MDA) increases, and the level of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) decreases, which is one of the core mechanisms that mediate skin aging.14–17 H2O2 is a ROS produced by normal cell metabolism that can stimulate the formation of ·OH through the Haber-Weiss reaction catalyzed by transition metals, which causes lipid peroxidation and DNA damage, inhibits cell proliferation, and induces cell senescence and death. Since H2O2 can increase the free radical load of cells, it is widely used to induce oxidative stress in in vitro cell models.18,19
To explore the influence of Saccharomyces cerevisiae fermentation on the antioxidation and safety of Panax notoginseng in this study, we first determined the contents of five active substances (total sugars, polysaccharides, reducing sugars, total phenols, total saponins) and four antioxidant capacities (DPPH, ABTS, hydroxyl radical, and ferric reducing antioxidant power) in the extracts of Panax notoginseng fermented by four strains of Saccharomyces cerevisiae and unfermented, and discussed the correlation between the changes in its active components and anti-oxidant capacity. Second, we explored the protective effect of Saccharomyces cerevisiae fermentation of Panax notoginseng on H2O2-induced oxidative stress in MSF cells by comparing the activities of antioxidant enzymes (SOD, CAT, and GSH-Px) and the content of MDA and ROS with and without the addition of pnFE1, pnFE2, pnFE3, and pnFE4 lyophilized powders. Finally, the safety of the four fermentation broths were evaluated through the red blood cell (RBC) test and hen’s egg test on chorioallantoic membrane (HET-CAM), and the storage stability of the fermentation broths was investigated through changes in the pH value and soluble solid content. These observations provide a foundation for the application of Saccharomyces cerevisiae-fermented Panax notoginseng in the cosmetic, health care and food industries.
Materials and Methods
Materials
Panax notoginseng powder was purchased from Beijing Tongrentang Co., Ltd. (Beijing, China), and the Saccharomyces cerevisiae strains FP413, FP524, FP419 and FP444 were stored in our laboratory. Mouse skin fibroblasts (MSFs) were obtained from the Chinese Academy of Sciences (Beijing, China). Fertilized chicken embryos of white Leghorn chickens were purchased from the Beijing Guichuan Yashen Breeding Center (Beijing, China). Yeast extract powder, peptone and glucose were purchased from Beijing Obstar Biotechnology Co., Ltd. (Beijing, China). DMEM and pancreatin were purchased from Gibco Life Technology Company (USA). 1,1-Diphenyl-2-trinitrophenylhydrazine (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and Folin-Ciocalteu were purchased from Beijing Biotopped Technology Co., Ltd. (Beijing, China). The reactive oxygen species assay kit (S0033S), lipid peroxidation MDA assay kit (S0131S), total superoxide dismutase assay kit with WST-8 (S0101S), catalase assay kit (S0051), and total glutathione peroxidase assay kit with NADPH (S0058) were purchased from Beyotime Biotechnology Co., Ltd. (Beijing, China). All other chemicals used were of high-purity biochemistry grade or analytically pure.
Preparation of Different Saccharomyces Cerevisiae Fermentation Broths of Panax Notoginseng
The Saccharomyces cerevisiae strain uses 1% yeast extract, 2% peptone, and 2% glucose as the growth medium. Panax notoginseng powder was mixed with water at a solid‒liquid ratio of 1:20 (g/mL), sterilized at 121°C for 30 min, and inoculate the activated Saccharomyces cerevisiae strains FP413, FP524, FP419 or FP444 on an ultraclean bench. Then, the samples were cultured on a shaker (180 r/min) at 28°C for 72 h, sterilized at 121°C for 30 min, and centrifuged (5050×g) for 30 min to obtain the Panax notoginseng fermentation broths, which were marked as pnFE1, pnFE2, pnFE3 and pnFE4. The samples that used the same procedure but were not inoculated were called pnWE, and the lyophilized powders made by vacuum freeze-drying the samples were marked with the same names.
Determination of the Active Substance Content
Total Sugar Content
Total sugar content was measured by following the instructions of the Solarbio Total Sugar Assay Kit (Product number: BC2710).
Polysaccharide Content and Reducing Sugar Content
The polysaccharide content was calculated by subtracting the reducing sugar content from the total sugar content. The reducing sugar content was determined according to the instructions of the Solarbio reducing sugar content assay kit (product number: BC0235).
Total Phenol Content
Total phenols were determined by the Folin-Ciocalteu method and were adjusted appropriately.20 One hundred microliters of gallic acid standard solution (0.02–0.10 mg/mL) or properly diluted sample was placed into a 1.5 mL centrifuge tube, and 200 μL of 10% Folin-Ciocalteu reagent was prepared, mixed evenly, and added to each centrifuge tube. Then, 800 μL of 700 mM Na2CO3 solution was added to each centrifuge tube, incubated at room temperature for 2 h and then 200 μL of the liquid was transferred into a 96-well plate. The absorbance value was measured at a wavelength of 765 nm. The results were expressed as milligrams of gallic acid equivalent per gram of sample dry weight (mg GAE/g DW).
Total Saponin Content
The total saponin content was determined by the vanillin-sulfuric acid method,21 add 250 μL of extraction solvent, standards in extraction solvent, and sample in extraction solvent to the test tubes respectively, and then add 250 μL of 8% vanillin ethanol solution and 2500 μL of 72% sulfuric acid aqueous solution to all test tubes. The tubes were then sealed and placed in a 60°C water bath for 15 minutes. Subsequently, the tubes were cooled at room temperature for 5 min and mixed properly, and 200 μL of the solution in each test tube was pipetted into a 96-well plate. The absorbance values at a wavelength of 560 nm were measured. The standard solution of ginsenoside Re (0.1–1.0 mg/mL) was prepared in ethanol, the samples were appropriately diluted with water, and the reagent blank was carried out with water and ethanol. The results are expressed as milligrams of total saponin equivalents per gram of sample dry weight (mg/g DW).
Evaluation of the in vitro Antioxidant Capacity
DPPH Free Radical Scavenging Ability
The DPPH free radical scavenging ability experiment was adjusted according to the method of Blois.22 Two hundred microliters of DPPH in DMSO solution (2×10−4 moL/L) was mixed with 200 μL of appropriately diluted sample solution, denoted as A1. Two hundred microliters of DPPH in DMSO solution (2×10−4 moL/L) was mixed with 200 μL of water, denoted as A2. Two hundred microliters of DMSO was mixed with 200 μL of appropriately diluted sample solution, denoted as A3. The reaction was carried out in the dark for 30 min, and the absorbance values were measured at 517 nm. The inhibition rate of DPPH radicals was calculated as follows:
DPPH free radical scavenging rate (%)=(A2+A3-A1)/A2×100%
Using ascorbic acid as a positive control, the results were expressed as milligrams of ascorbic acid equivalent corresponding to the dry weight of 1 g of the sample (mg AAE/g DW).
ABTS Free Radical Scavenging Ability
The free radical scavenging ability experiment of ABTS was performed according to a previous method with some modifications.23 First, 0.8 mL of ABTS working solution was mixed with 0.2 mL of sample solution, denoted as A. Second, 0.8 mL of ABTS working solution was mixed with 0.2 mL of sample solvent, denoted as A0. After the reactions incubated in the dark for 6 min, the absorbance values were measured at 734 nm. The ABTS free radical scavenging rate is calculated as follows:
ABTS free radical scavenging rate (%)=(A0-A)/A0×100%
Taking ascorbic acid as a positive control, the results are expressed as milligrams of ascorbic acid equivalent corresponding to the dry weight of 1 g of the sample (mg AAE/g DW).
Hydroxyl Free Radical Scavenging Ability
The hydroxyl radical scavenging ability experiment was performed according to the method cited in Zhuang et al24 with some modifications. The reaction system was constructed according to Table 1:
 |
Table 1 Reaction System for Assessment of the Hydroxyl Radical Scavenging Ability |
The mixture was shaken, placed at 37°C for 30 min, and centrifuged, and the absorbance value of each group was measured at a wavelength of 510 nm. The clearance rate was calculated according to the following formula:
Hydroxyl radical scavenging activity (%)= ((A-C)-(B-D))/(A-C)×100%
Using ascorbic acid as a positive control, the results are expressed as milligrams of ascorbic acid equivalent corresponding to the dry weight of 1 g of the sample (mg AAE/g DW).
Ferric Reducing Antioxidant Power
The FRAP was measured using the Solarbio Total Antioxidant Capacity (T-AOC) Detection Kit (FRAP method) (Product number: BC1310) according to the instructions.
Cell Culture
MSFs was cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin, with the incubator set at 37°C and 5% CO2. The medium was changed every 2 days. When the cell confluence reached 80–90%, 0.25% Trypsin–EDTA was used for digestion, and the cells were passaged at a volume ratio of 1:3.
Cell Viability Analysis
The fibroblasts were made into a cell suspension with a concentration of 7×104 cells/mL, and 100 μL of the cell suspension was added to each well of a 96-well plate. After incubation for 12 hours, replace the previous liquid with samples of different concentrations prepared in serum-free DMEM medium and continue incubation for 24 hours. Each well was filled with 10 μL of CCK-8 solution. After the reaction incubated for 2–4 h, the absorbance was measured at 450 nm.
Measurement of the ROS Content
The assay was performed according to the instructions of the Beyotime Reactive Oxygen Species Assay Kit (product number: S0033S).
Enzyme-Linked Immunosorbent Assay
The levels of MDA, SOD, CAT, and GSH-Px in cells were detected by enzyme-linked immunosorbent assay (ELISA), and the detection protocol was carried out in strict accordance with the kit manufacturer’s instructions.
RBC Test
Dilute fresh rabbit blood with phosphate buffer solution (PBS) in a volume ratio of rabbit blood: PBS=2:5. At room temperature, centrifuge the diluted solution at 1500 x g for 10 minutes, carefully remove the supernatant and white blood cell layer after centrifugation, then add PBS and repeat the washing and centrifugation steps three times. After the last centrifugation, the precipitated cells were diluted with PBS to a 2% concentration of RBC suspension. Mix 750 μL samples of different concentrations with 250 μL RBC suspension, incubate for 60 minutes in a 150rpm shaker at room temperature, and then centrifuge at 10,000 × g for 1 minute to terminate the incubation. PBS was used as a negative control, and deionized water was used as a complete hemolysis control. The absorbance of the supernatant was measured at 560 nm, and calculate the hemolysis rate according to the following equation:
Hemolysis rate (%) = (ODsample - ODnegative)/(ODcomplete hemolysis - ODnegative) × 100%
Het-Cam
Fertilized white Leghorn chicken embryos were purchased from the Beijing Guichuan Yashen Breeding Center (Beijing, China). At 37°C and 60–70% humidity, the white Leghorn chicken eggs were rotated in an egg incubator for 9 days, and the normally developed eggs were selected for treatment on the 9th day. The eggshell was opened on the air chamber side 2 mL of 0.9% NaCl solution was used to wet the chorioallantoic membrane (CAM). Each substance was tested on 6 eggs using the endpoint evaluation method. Three hundred microliters of the test substance (negative control, positive control and Panax notoginseng fermentation broth stock solution) was added directly into the CAM, and the membrane was immediately placed under the microscope to observe the changes in blood vessels within the first 3 min. Three end points (coagulation, Haemorrhage and vessel lysis) were recorded, and an end point score (ES) was calculated. Sodium chloride solution (0.9%) was used as the negative control, and 0.1 mol/L sodium hydroxide solution was used as the positive control. The injury evaluation criteria and scoring criteria of the stimulation are shown in Tables 2 and 3.
 |
Table 2 Vascular Injury Evaluation |
 |
Table 3 Irritation Classifications Based on ES |
Stability Test
Determination of the pH Value
The pH value of Panax notoginseng fermentation broth at 0, 1, 2, 3, and 4 weeks was measured with a precision pH meter (Shanghai Sanxin Instrument Factory, Shanghai, China).
Determination of the Soluble Solid Content
A digital refractometer (Kyoto Electronics Manufacturing Co., Ltd., Kyoto, Japan) was used to determine the content of soluble solids in the fermentation broth of Panax notoginseng at 0, 1, 2, 3 and 4 weeks.
Statistical Analysis
Each experiment was repeated at least three times, and the experimental results are expressed as the means ± standard deviations (SDs). IBM SPSS Statistics 26, GraphPad Prism 9 and ChiPlot (https://www.chiplot.online/) were used for data analysis and plotting. One-way ANOVA was used for the significance test, and P<0.05 was considered a statistically significant difference.
Results
Active Substance Content
To understand the influence of four different kinds of Saccharomyces cerevisiae strains on the extraction rate of active substances from Panax notoginseng, we first measured the content of total sugars, reducing sugars, polysaccharides, polyphenols and saponins in fermented Panax notoginseng extracts pnFE1, pnFE2, pnFE3, pnFE4 and unfermented pnWE. The experimental results are shown in Table 4. After fermentation by Saccharomyces cerevisiae, the content of active substances in Panax notoginseng changed to varying degrees. Compared with the unfermented pnWE, the total sugar content of the four fermentation broths decreased to varying degrees, and the reducing sugar content was significantly decreased, indicating that Saccharomyces cerevisiae utilized sugars during the fermentation process, possibly converting them into other compounds. Compared with the pnWE, the polysaccharide content of pnFE1 was significantly increased, the total phenolic content of pnFE1, pnFE2, and pnFE3 was significantly increased, and the total saponin content of pnFE1 and pnFE2 was also increased to a certain extent.
 |
Table 4 Content of Active Substances in Different Extracts of Panax Notoginseng |
In vitro Antioxidant Capacity
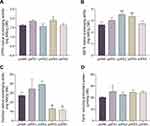
Fermentation has a positive effect on increasing the antioxidant capacity of plants.11 We analyzed the unfermented Panax notoginseng extract pnWE and four kinds of Panax notoginseng fermentation broths pnFE1, pnFE2, pnFE3, and pnFE4 and found that the antioxidant activity of most of the fermentation broths was higher than that of pnWE, increasing to varying degrees (Figure 1). The DPPH free radical scavenging ability of pnFE3, the ABTS free radical scavenging ability of pnFE2 and pnFE3 and the ferric reducing antioxidant power of pnFE1 were significantly higher than those of pnWE. The results showed that fermentation of Saccharomyces cerevisiae enhanced the antioxidant capacity of Panax notoginseng extracts to different degrees. In addition, compared with pnWE, the hydroxyl radical scavenging capacity of pnFE3 and pnFE4 decreased significantly, which may be related to the change in active substance content. Next, we further analyzed the correlation between them.
Correlations Between Active Substances and Antioxidant Capacity
According to the previously determined data of active substance content and antioxidant capacity, we further studied the correlation between them through Pearson correlation coefficient, and the results are shown in Figure 2. There was a significant positive correlation between the total sugar content and polysaccharide content (r=0.99, p<0.01), and the hydroxyl radical scavenging ability in Panax notoginseng extracts was significantly positively correlated with the polysaccharide content (r=0.90, p<0.05) and total saponin content (r=0.93, p<0.05). In addition, the reducing sugar content was negatively correlated with multiple values, suggesting that the fermentation process of Saccharomyces cerevisiae decreased the reducing sugar content and may have a potential connection with the increase in active substances in Panax notoginseng and the enhancement of its antioxidant capacity. The total phenol content was positively correlated with DPPH free radical scavenging ability (r=0.77), ABTS free radical scavenging ability (r=0.52) and FRAP (r=0.86), suggesting that the polyphenols in Panax notoginseng are an important antioxidant, but their effects on the antioxidant capacity of Panax notoginseng are not unique. There may be synergistic effects between these components. For example, the hydroxyl radical scavenging ability of Panax notoginseng extract is influenced by the content of polysaccharides and total saponins, while the ABTS radical scavenging ability is influenced by the content of total saponins (r=0.61) and total phenols (r=0.52). These results indicate that the correlation between the antioxidant capacity of Panax notoginseng and active substances is very complex. To further verify the antioxidant capacity of Panax notoginseng fermentation broth, we made pnFE1, pnFE2, pnFE3, and pnFE4 into freeze-dried powders and explored their protective effects on MSFs damaged by hydrogen peroxide.
 |
Figure 2 Pearson correlation coefficient between the active substances and antioxidant capacity of Panax notoginseng. |
Cell Viability Analysis
The CCK-8 assay was used to detect the effects of Saccharomyces cerevisiae-fermented Panax notoginseng lyophilized powders pnFE1, pnFE2, pnFE3, and pnFE4 on the MSF cell viability and the effect of H2O2 induction on cell viability. As shown in Figure 3A, there were significant differences in cell viability after adding different sample solutions at 1, 0.5, 0.25, 0.13, 0.06, and 0.03 mg/mL to MSF medium for 24 h. When the cell viability was 80% and above, the sample was considered to be less cytotoxic to cells. Therefore, for subsequent experiments, the concentration of all four samples and ascorbic acid as a positive control was set to 0.06 mg/mL. As shown in Figure 3B, the cell viability clearly depended on the H2O2 concentration gradient When modeling cellular oxidative damage, cell viability should be between 50% and 70%. When the cell viability is too low, it may cause irreversible damage; when the cell viability is too high, no obvious oxidative damage can be caused, so 1000 μmol/mL H2O2 was selected for further use.
ROS Content
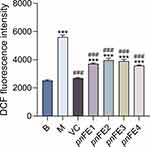
DCFH-DA (2’,7’-dichlorodihydrofluorescein diacetate) is a fluorescent probe for ROS detection that can freely pass through the cell membrane. After entering the cell, it can be hydrolyzed by intracellular esterase to generate DCFH (dichlorodihydrofluorescein diacetate), which cannot penetrate the cell membrane. Intracellular ROS can oxidize nonfluorescent DCFH into fluorescent DCF. Therefore, cellular ROS levels can be determined by measuring DCF fluorescence. As shown in Figure 4, compared with the blank control group, the intracellular ROS level was increased in the H2O2-treated group. Both the 0.06 mg/mL samples and VC decreased the content of ROS in MSFs (P<0.001), indicating that the four samples could inhibit the ROS induced by H2O2, especially pnFE1 and pnFE4.
MDA Content and SOD, GSH-Px, and CAT Activities
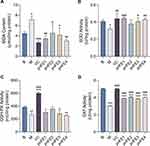
After adding 0.06 mg/mL of the four different pnFE to the cell culture medium for 24 h and then adding 1000 μmol/L H2O2 for 2 h, pnFE significantly reduced the MDA content (Figure 5A) and significantly increased the activities of the antioxidant enzymes SOD, GSH-Px and CAT (Figure 5B–D). Different samples have different antioxidant capacities. Compared with the model group, all four pnFE significantly reduced MDA content and increased CAT activity. pnFE1, pnFE3, and pnFE4 significantly increased SOD activity, while pnFE1, pnFE2, and pnFE3 had some beneficial effects on the improvement of GSH-Px activity. All four pnFE showed varying degrees of protection against H2O2-induced oxidative stress in fibroblasts.
Safety Evaluation of pnFE
RBC Test
The RBC test, one of the in vitro alternatives to the Draize eye irritation test, reflects membrane damage and protein denaturation of red blood cells and is suitable for evaluating hemolysis caused by certain chemicals and preparations.25,26 The results of the RBC test are shown in Figure 6. The centrifuge tubes are the positive control, negative control, and 8, 4, 2, 1, 0.5 and 0.25 mg/mL of different sample solutions from left to right. The hemolysis rate of all samples at 0.25–8 mg/mL is lower than 3%, indicating that there is no obvious occurrence hemolysis on the erythrocyte membrane caused by the four samples, and that they have high safety.
 |
Figure 6 Hemolysis rate of four samples pnFE1 (A), pnFE2 (B), pnFE3 (C) and pnFE4 (D) at different sample concentrations. PC: Positive control; NC: Negative control. |
Het-Cam
HET-CAM is a versatile and sensitive in vitro assay based on the response of the denervated and highly vascularized chorioallantoic membrane (CAM) to assess injury in a manner similar to mucosal and subcutaneous tissue. This method allows the evaluation of different types of substances, such as surfactants, cosmetic raw materials, and chemical products, and is another alternative to the Draize eye irritation test.27 It can be clearly seen from Figure 7 that the CAM vascular response of the positive control (0.1 M NaOH) included significant hemorrhage, coagulation and lysis of blood vessels. The negative control (0.9% NaCl) had no significant adverse vascular changes. The CAM vascular reaction of the four fermentation broths was similar to that of the negative control. Morphological observation and scoring were conducted for all samples, and the scoring results are shown in Table 5. The ES scores of pnFE1, pnFE2, pnFE3 and pnFE4 were all 0, and there was no coagulation, hemorrhage or lysis of blood vessels, indicating that the four samples did not stimulate blood vessels and were biologically safe.
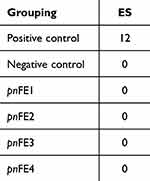 |
Table 5 Irritation Results of Different Samples on CAM |
 |
Figure 7 Representative results of chorioallantoic membrane treated with different samples. |
Stability of Four Types of Panax Notoginseng Fermentation Broths
Our previous experiments have proven that the fermentation broths of Panax notoginseng have suitable efficacy and safety. However, the impact of the storage stability on quality should be studied before largescale production. Previous studies have shown that sterilization treatment has no significant effect on the quality of the extract.28 To ensure safety, efficacy and stability, we measured the pH value (Figure 8A) and soluble solid content (Figure 8B) of four types of Panax notoginseng fermentation broth at different time periods. Due to the release of acid metabolites, such as acetic acid, lactic acid and CO2, the growth of bacteria is usually related to the decline in the pH value of the growth medium.29 Therefore, the pH value is one of the main indicators used to measure the quality of a fermentation liquid. The results showed that the pH value ranges of the Panax notoginseng fermentation broths were similar, ranging from 4.6 to 5.2, which is weakly acidic, and did not significantly change within 0–4 weeks. Moreover, the content of soluble solids can directly reflect the quality of the fermentation broth. The content of soluble solids in pnFE1 and pnFE2 was slightly higher than that in pnFE3 and pnFE4, but both remain in the lower range of 2.2–4.0 °Brix. The fermentation broth was clear, and there was no significant change within 0–4 weeks. The above results indicate that there is no microbial reproduction and metabolism in the fermentation broth, and pH values and the content of soluble solid solution content measured across 4 weeks indicate that they have favorable quality and storage stability.
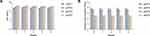 |
Figure 8 (A) pH values of Panax notoginseng fermentation broths at different time periods. (B) Content of soluble solids in Panax notoginseng fermentation broths at different time periods. |
Discussion
In recent years, research on Panax notoginseng has gradually increased due to its remarkable tonic effect and long history of use. In addition to its use as a clinical treatment, Panax notoginseng has also become an important source of various health, functional food and cosmetic products.30 In our study, four different Saccharomyces cerevisiae strains, FP413, FP524, FP419 and FP444, were used to ferment Panax notoginseng, and the differences between the content of active substances, antioxidant capacity and safety of its fermentation products were studied. During the fermentation process, Saccharomyces cerevisiae can produce biological enzymes such as amylase, β-glucosidase, sucrase, maltase, etc. These enzymes can disrupt plant cell walls, increase cell wall permeability, and facilitating the dissolution of active substances, which may be a possible explanation for the significantly higher total phenolic content in pnFE compared to pnWE. Another possible explanation is that during the fermentation process, microbial enzyme systems can promote the conversion and production of plant chemicals. β-Glucosidase can degrade phenolic compounds into aglycones, producing more free phenolic substances.11,31 During the fermentation process, Saccharomyces cerevisiae is able to convert glucose and fructose produced by the degradation of complex carbohydrates such as sucrose, maltose, and starch into carbon dioxide and ethanol.32 This may be the reason why the total sugar content and reducing sugar content of pnFE are significantly reduced compared to pnWE. Due to the complexity of fermentation systems, in future research, qualitative and quantitative analysis of active substances in fermented and unfermented products can be further carried out through techniques such as liquid chromatography-mass spectrometry to further elucidate their changes and molecular mechanisms.
Aging is an inevitable law of nature for all living things. In 1956, Denham proposed the farreaching free radical theory, which believed that aging is caused by the harmful effects of free radicals produced in the process of cell metabolism.33 ROS are the products of normal cell metabolism, including hydroxyl free radicals, superoxide anions, hydrogen peroxide and so on. Excessive production of ROS can lead to oxidative stress, a harmful process that causes lipid, protein and DNA damage.17 Skin aging is the result of natural aging and photoaging, and free radicals participate in these two aging processes. Therefore, scavenging free radicals by supplementing antioxidants is an effective way to delay skin aging.34 In our study, pnFE can reduce oxidative stress by inhibiting ROS production, reducing the content of MDA, and increasing antioxidant enzyme activity (SOD, GSH-Px, CAT), thus inhibiting the senescence of MSF cells. SOD, GSH-Px and CAT are three major enzymes in the cellular enzymatic antioxidant system. SOD reacts with O2·− to generate H2O2, which can be decomposed into water and oxygen by CAT and can also be converted into water by GSH-Px through glutathione (GSH) to prevent the formation of destructive ·OH free radicals. Moreover, GSH-Px can also transform the lipid peroxides that cause DNA damage into nontoxic products.14,17 Therefore, the activities of SOD, GSH-Px and CAT can be used as indicators of antioxidant capacity. Compared with the oxidative stress model group, the activity of SOD and CAT in MSFs after sample treatment increased, the activity of GSH-Px increased partially, and the content of ROS and MDA decreased, indicating that the products of Panax notoginseng fermented by Saccharomyces cerevisiae had antioxidant capacity, and specifically, the comprehensive performance of pnFE1 was superior.
Other studies have also shown that different active ingredients in Panax notoginseng can delay aging through different mechanisms. Zhai et al found that Panax notoginseng oligosaccharides isolated from Panax notoginseng could reverse fibroblast replicative senescence by promoting fibroblast proliferation, migration and CoL-I production.35 Panax notoginseng saponins can protect tumor necrosis factor-α-induced osteoarthritis rat chondrocytes from aging and apoptosis by regulating the PI3K-AKT-mTOR pathway, thereby delaying the destruction of cartilage by osteoarthritis.36 Moreover, Panax notoginseng polysaccharides can significantly prolong the average lifespan of wildtype Caenorhabditis elegans and may increase the survival rate of wildtype Caenorhabditis elegans under heat stress by increasing the activities of SOD and CAT and reducing the content of MDA.37 However, the fermentation product of Panax notoginseng is a mixed solution, and various components may have synergistic and antagonistic effects. The influence of Saccharomyces cerevisiae fermentation on the antioxidant effect of Panax notoginseng remains to be further studied. Current research has not been able to elucidate the specific active ingredients and their antioxidant mechanisms. Next, metabolomics analysis of different Saccharomyces cerevisiae fermentation broths of Panax notoginseng will be carried out to clarify the type and content of active substances and to study their antioxidant activity at the structural level. Moreover, we also found that the Panax notoginseng product fermented by Saccharomyces cerevisiae can protect fibroblasts from H2O2-induced oxidative stress. In the future, we can further clarify the molecular mechanism of its role through transcriptomics and other means and verify these findings in animal models.
Conclusion
The main focus of this study was exploring the cosmetic application potential of the extracts of Saccharomyces cerevisiae fermented Panax notoginseng. Measuring the total sugar content, polysaccharide content, reducing sugar content, total phenol content, total saponin content and four antioxidant capacities (DPPH, ABTS, FRAP, hydroxyl radical) proved that Saccharomyces cerevisiae fermentation can improve the extraction rate and antioxidant capacity of Panax notoginseng active substances and proved that there is a correlation between active substances and antioxidant capacity. Furthermore, through the H2O2-induced MSF oxidative stress model, it was proven that pnFE1, pnFE2, pnFE3, and pnFE4 freeze-dried powders can reduce the content of ROS and MDA in oxidatively stressed cells and increase the activities of the antioxidant enzymes SOD, CAT, and GSH-Px, indicating that the fermentation product has a protective effect on MSFs during oxidative stress. Finally, the RBC test and HET-CAM assay were used to verify that the four fermentation broths were not irritating and that there were no significant changes in pH value and soluble solid content within four weeks, which prove its stability. The above results prove that pnFE has favorable antioxidant activity, safety and stability and may delay skin aging, which provides a theoretical basis for the application of Saccharomyces cerevisiae fermented Panax notoginseng in cosmetics, health products and other industries.
Funding
This research was funded by the State Administration for Market Regulation Science and Technology Plan Project (No. 2022MK192).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang T, Guo R, Zhou G, et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. 2016;188:234–258. doi:10.1016/j.jep.2016.05.005
2. Zhao H, Han Z, Li G, et al. Therapeutic Potential and Cellular Mechanisms of Panax Notoginseng on Prevention of Aging and Cell Senescence-Associated Diseases. Aging Dis. 2017;8(6):721–739. doi:10.14336/AD.2017.0724
3. Duan L, Xiong X, Hu J, et al. Panax notoginseng Saponins for Treating Coronary Artery Disease: a Functional and Mechanistic Overview. Front Pharmacol. 2017;8:702. doi:10.3389/fphar.2017.00702
4. Sun Z, Wu H, Wu Y, et al. Comparative Analysis of Compatibility Influence on Invigorating Blood Circulation for Combined Use of Panax Notoginseng Saponins and Aspirin Using Metabolomics Approach. Front Pharmacol. 2021;12:544002. doi:10.3389/fphar.2021.544002
5. Liu YH, Qin HY, Zhong YY, et al. Neutral polysaccharide from Panax notoginseng enhanced cyclophosphamide antitumor efficacy in hepatoma H22-bearing mice. BMC Cancer. 2021;21(1):37. doi:10.1186/s12885-020-07742-z
6. Zhou N, Tang Y, Keep RF, et al. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21(10):1189–1195. doi:10.1016/j.phymed.2014.05.004
7. Wang Y, Shang Y, Tang F, et al. Self-Double-Emulsifying Drug Delivery System Enteric-Coated Capsules: a Novel Approach to Improve Oral Bioavailability and Anti-inflammatory Activity of Panax notoginseng Saponins. AAPS Pharm Sci Tech. 2023;24(4):90. doi:10.1208/s12249-023-02549-0
8. Zhang C, Li C, Chen S, et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci Rep. 2017;7(1):41082. doi:10.1038/srep41082
9. Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28(4):510–516. doi:10.1002/ptr.5026
10. Li Z, Gu H, Song H, et al. Radix Angelica sinensis soup production by decocting process using high pressure. Int J Food Prop. 2017;20(sup1):S620–S631. doi:10.1080/10942912.2017.1306552
11. Hur SJ, Lee SY, Kim YC, et al. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi:10.1016/j.foodchem.2014.03.112
12. You S, Shi X, Yu D, et al. Fermentation of Panax notoginseng root extract polysaccharides attenuates oxidative stress and promotes type I procollagen synthesis in human dermal fibroblast cells. BMC Complement Med Ther. 2021;21(1):34. doi:10.1186/s12906-020-03197-8
13. Lee S, Reddy CK, Ryu JJ, et al. Solid-State Fermentation With Aspergillus cristatus Enhances the Protopanaxadiol- and Protopanaxatriol-Associated Skin Anti-aging Activity of Panax notoginseng. Front Microbiol. 2021;12:602135. doi:10.3389/fmicb.2021.602135
14. Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126(12):2565–2575. doi:10.1038/sj.jid.5700340
15. Gu Y, Han J, Jiang C, et al. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036. doi:10.1016/j.arr.2020.101036
16. Mittler R. ROS Are Good. Trends Plant Sci. 2017;22(1):11–19. doi:10.1016/j.tplants.2016.08.002
17. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi:10.1016/j.biocel.2006.07.001
18. Mo Q, Fu H, Zhao D, et al. Protective Effects of Mogroside V on Oxidative Stress Induced by H2O2 in Skin Fibroblasts. Drug Des Devel Ther. 2021;15:4901–4909. doi:10.2147/DDDT.S337524
19. Gille JJ, Joenje H. Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res. 1992;275(3–6):546.
20. Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–877. doi:10.1038/nprot.2007.102
21. Le AV, Parks SE, Nguyen MH, et al. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies. 2018;6(3):84. doi:10.3390/technologies6030084
22. Blois M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958;181(4617):1190–1200. doi:10.1038/1811199a0
23. Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi:10.1016/S0891-5849(98)00315-3
24. Zhuang H, Tang N, Yuan Y. Purification and identification of antioxidant peptides from corn gluten meal. J Funct Foods. 2013;5(4):1810–1821. doi:10.1016/j.jff.2013.08.013
25. Pape WJ, Pfannenbecker U, Argembeaux H, et al. COLIPA validation project on in vitro eye irritation tests for cosmetic ingredients and finished products (phase I): the red blood cell test for the estimation of acute eye irritation potentials. Present status. Toxicol In Vitro. 1999;13(2):343–354. doi:10.1016/S0887-2333(98)00085-X
26. Ma X, Wang H, Song Y, et al. Skin irritation potential of cosmetic preservatives: an exposure-relevant study. J Cosmet Dermatol. 2021;20(1):195–203. doi:10.1111/jocd.13502
27. Vinardell MP, Mitjans M. Alternative methods for eye and skin irritation tests: an overview. J Pharm Sci. 2008;97(1):46–59. doi:10.1002/jps.21088
28. Qu W, Breksa Iii AP, Pan Z, et al. Storage stability of sterilized liquid extracts from pomegranate peel. J Food Sci. 2012;77(7):C765–C772. doi:10.1111/j.1750-3841.2012.02779.x
29. Si Y, Grazon C, Clavier G, et al. Rapid and accurate detection of Escherichia coli growth by fluorescent pH-sensitive organic nanoparticles for high-throughput screening applications. Biosens Bioelectron. 2016;75:320–327. doi:10.1016/j.bios.2015.08.028
30. Li X, Liu J, Zuo TT, et al. Advances and challenges in ginseng research from 2011 to 2020: the phytochemistry, quality control, metabolism, and biosynthesis. Nat Prod Rep. 2022;39(4):875–909. doi:10.1039/d1np00071c
31. Leonard W, Zhang P, Ying D, et al. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol Adv. 2021;49:107763. doi:10.1016/j.biotechadv.2021.107763
32. Parapouli M, Vasileiadis A, Afendra AS, et al. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020;6(1):1–31. doi:10.3934/microbiol.2020001
33. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi:10.1093/geronj/11.3.298
34. Emerit I. Free radicals and aging of the skin. EXS. 1992;62:328–341. doi:10.1007/978-3-0348-7460-1_33
35. Zhai L, Xu X, Liu J, et al. A Novel Biochemical Study of Anti-Dermal Fibroblast Replicative Senescence Potential of Panax Notoginseng Oligosaccharides. Front Pharmacol. 2021;12:690538. doi:10.3389/fphar.2021.690538
36. Zhang Y, Cai W, Han G, et al. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int J Mol Med. 2020;45(4):1225–1236. doi:10.3892/ijmm.2020.4491
37. Feng S, Cheng H, Xu Z, et al. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int J Biol Macromol. 2015;81:188–194. doi:10.1016/j.ijbiomac.2015.07.057
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.