Back to Journals » Medical Devices: Evidence and Research » Volume 13
Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants
Authors Patel V , Kovalsky D , Meyer SC, Chowdhary A, Lockstadt H, Techy F, Langel C, Limoni R, Yuan PS, Kranenburg A, Cher D , Tender G, Hillen TJ
Received 13 March 2020
Accepted for publication 27 May 2020
Published 16 June 2020 Volume 2020:13 Pages 173—182
DOI https://doi.org/10.2147/MDER.S253741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Vikas Patel,1 Don Kovalsky,2 S Craig Meyer,3 Abhineet Chowdhary,4 Harry Lockstadt,5 Fernando Techy,6 Casey Langel,7 Robert Limoni,8 Philip S Yuan,9 Andy Kranenburg,10 Daniel Cher,11 Gabriel Tender,12 Travis J Hillen13
1Department of Orthopedics, University of Colorado, Aurora, CO, USA; 2Orthopaedic Center of Southern Illinois, Mt. Vernon, IL, USA; 3Columbia Orthopaedic Medical Group, Columbia, MO, USA; 4Overlake Medical Center, Bellevue, WA, USA; 5Bluegrass Orthopaedics, Lexington, KY, USA; 6ClinTech Center for Spine Health, Johnstown, CO, USA; 7The B.A.C.K. Center, Melbourne, FL, USA; 8BayCare Clinic Orthopedics & Sports Medicine, Green Bay, WI, USA; 9Memorial Orthopaedic Surgical Group, Long Beach, CA, USA; 10South Oregon Orthopedics, Medford, OR, USA; 11SI-BONE, Inc., Santa Clara, CA, USA; 12Department of Neurosurgery, Louisiana State University, New Orleans, LA, USA; 13Department of Musculoskeletal Radiology, Washington University St. Louis, St. Louis, MO
Correspondence: Vikas Patel Tel +1 303 724-8936
Email [email protected]
Background: Prior trials provide strong evidence supporting minimally invasive sacroiliac joint (SIJ) fusion using triangular titanium implants (TTI) for chronic SIJ dysfunction.
Objective: To assess the safety and effectiveness of SIJF using a 3D-printed TTI.
Methods: Fifty-one subjects with carefully diagnosed SIJ dysfunction underwent SIJF with 3D TTI. Subjects completed pain, disability and quality of life questionnaires at baseline and 3, 6 and 12 months postoperatively. Functional tests were performed in the clinic at each visit. Pelvic CT scans were independently evaluated for radiolucency, bridging bone and other endpoints.
Results: Ninety percent had 12-month follow-up. Dysfunction due to pain (Oswestry Disability Index [ODI]) decreased from 52.8 at baseline to 27.9 at 12 months (p< .0001 for change, p=.004 for non-inferiority primary hypothesis). SIJ pain scores improved from 78 preoperatively to 21 at 12-month follow-up (P< .0001). Ninety-six percent experienced an improvement of 20 points or more in VAS SIJ pain by month 12. The percentage of subjects reporting minimal difficulty performing physical activities typically impaired by back/SIJ pain improved significantly for all activities. The proportion of subjects taking opioids for SIJ pain decreased from 57% to 22%. Three physical function tests improved markedly from baseline to 1 year. Positive radiographic findings were observed, including a 70% and 77% rate of bone bridging observed at 6 and 12 months, respectively. There was no evidence of device breakage, migration or subsidence.
Conclusion: In this prospective multicenter trial, SIJF with 3D-printed TTI markedly improved pain, disability and quality of life. Results are consistent with 3 prior prospective multicenter trials of a milled implant but suggest accelerated bony fusion with the newer implant. Physical function improved, and high rates of opioid cessation were observed.
Level of Evidence: Level II.
Keywords: sacroiliac joint pain, sacroiliac joint arthrodesis, chronic low back pain, triangular titanium implants
Background
Up to 30% of cases of chronic low back pain may have a contribution from the sacroiliac joint (SIJ).1–5 The term SIJ dysfunction describes limitations related to SIJ pain. The evidence base supporting long-term responses from non-surgical treatments of chronic SIJ dysfunction is very limited. In contrast, minimally invasive SIJ fusion (SIJF) is gaining popularity as a safe and effective treatment for this condition, with supportive results from prospective trials6–8 and case series.9–14 These studies showed immediate and sustained improvement in pain, disability and quality of life following minimally invasive SIJF. Most published studies report outcomes using porous triangular titanium implants (TTI, iFuse Implant System, SI-BONE, Santa Clara, California, USA).
Prior studies of TTI reported the use of solid (milled) implants with porous surfaces created by a plasma spray coating process. Orthopedic implants with porous surfaces provide a scaffold for osteoconduction and osteointegration with host bone so as to promote bony ongrowth and ingrowth, and decrease the incidence of migration, subsidence, and pseudoarthrosis. Porous surfaces may also improve bony ongrowth in patients with osteoporosis or in those who smoke.15,16
Titanium plasma sprays typically create surfaces with low interconnectivity and small pore sizes (100–150 μm). In addition, the porous surface depth can vary significantly secondary to the manufacturing process. 3D printing of metal surfaces (also called additive manufacturing) allows the creation of implants with optimized and consistent microstructures. 3D-printed implants can have surface features with characteristics matching more closely those of cancellous bone. 3D-printed TTI, developed in 2015, support the growth of human osteoblasts, with higher calcium production compared to solid implants with porous spray surfaces.17 3D implants show higher bony ongrowth and bony ingrowth through the implant (not possible with a solid implant) in an animal model.18
3D-printed TTI (called iFuse-3D) were cleared by the US Food and Drug Administration in 2017 for SIJF. Herein we report one-year safety, effectiveness and radiographic outcomes from a prospective multicenter trial of 3D-printed TTI in patients with SIJ dysfunction.
Methods
Design and Site Participants
SALLY (Study of Bone Growth in the Sacroiliac Joint after Minimally Invasive Surgery with Titanium Implants, NCT03122899) is a prospective multicenter single-arm clinical trial. 11 sites in the United States participated. Site participation required a trained surgeon who had completed at least 10 pre-trial cases and a dedicated study coordinator for subject assessments and data collection.
Study Goals
The study’s goals were (1) to compare clinical responses after SIJF using 3D-printed TTI to those from previous studies that used the milled implant, (2) to evaluate the change in physical function tests after SIJF, (3) to evaluate the use of “opioid contracts” and self-efficacy reminders on use of opioids, and (4) to determine whether 3D-printed TTI may accelerate bony fusion of the SIJ compared to the prior implant.
Patient Population
Enrollment criteria were described previously;19 a complete listing of criteria is available on ClincalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03122899). Patients were enrolled if they were adults (age 21–70) with SIJ pain for at least 6 months inadequately responsive to conservative care, an Oswestry Disability Index (ODI) score of at least 30%, and an average SIJ pain score of at least 50 (0–100 mm visual analog scale). SIJ pain diagnosis was made using a standardized algorithm, that included history, physical examination and a 50% or more decrease in SIJ pain 30 or 60 minutes after SIJ block. All blocks were performed under image guidance; sites used local anesthetic of their choice (typically lidocaine or bupivacaine). Patients were excluded for a variety of reasons, including: bilateral SIJ pain but the patient was unwilling to undergo bilateral treatment, pregnancy, severe back or hip pain from other causes, inflammatory sacroiliitis, sacral fracture, recent trauma, osteoporosis, allergy to titanium, and other conditions.
Study data were collected using an electronic case report form system. Data entered into case report forms were 100% source-verified. Qualified requestors may obtain study data through Yale University’s Yale Open Data Access (YODA) platform. Data provided will include de-identified subject data, the study protocol and annotated case report forms.
Interventions
Prior to each subject’s SIJF, coordinators collected medical history, opioid medication use, and quality of life questionnaires. Coordinators also oversaw physical function testing (see Physical Function Tests below). Subjects underwent index side SIJF using iFuse-3D within 30 days of baseline assessments. Surgeons were allowed to use bone allograft (demineralized bone matrix) or autograft (bone fragments obtained from drilling, the first step in SIJF); when used these were typically placed onto/into implants immediately before they were inserted. Investigators recorded any device deficiencies, complications or procedure-related adverse events on case report forms. Subjects who entered the study with bilateral SIJ pain were asked to undergo contralateral SIJF with the same device/technique within 90 days after the first-side procedure.
Follow-Up
In-clinic follow-up visits occur at 3, 6, 12, 24, and 60 months after surgery. Herein we report 12-month follow-up. At each visit, subjects were assessed for adverse events and completed questionnaires. Each adverse event was rated by site investigators as to severity, relatedness to the study device and procedure, other procedures used to treat SIJ pain and pre-existing conditions.
Study Questionnaires
The following questionnaires were performed in the clinic at baseline and follow-up. SIJ pain (both sides) using a visual analog pain score using a 100 mm scale, where 0 = no pain and 100 = worst imaginable pain. Oswestry Disability Index,20,21 a commonly used questionnaire of disability due to back pain. EuroQOL-5D,22 a generic quality of life assessment tool. EuroQOL-5D scores estimate health state utility (0=death, 1=perfect health). Pain maps wherein the subject checks boxes according to areas currently causing pain and to indicate the ability to perform 22 daily activities (sitting, getting out of car, walking, etc.). Level of confidence in ability to stop taking opioids (i.e., self-efficacy23). All but 2 sites collected questionnaires using an online system; 2 sites used paper versions of questionnaires.
Physical Function Tests
At baseline and follow-up visits, coordinators oversaw physical function testing as described previously.19 Briefly, subjects were asked to perform: 1) active straight leg raise test (ASLR)24 2) five times sit-to-stand, and 3) transitional timed up-and-go. For the latter 2 tests, the measure of interest was the time required to perform the task. Videos showing physical function tests are available at: http://siboneclinical.com/video/. These videos were shown to study subjects at each study visit immediately before they performed the tests.
Opioid Contract and Self-Efficacy
Prior to surgery, subjects signed an opioid contract. The contract noted several aspects of opioid use, including: opioids used for SIJ pain would be stopped if they are not effective or being misused, opioids should be taken only as prescribed, and the subject should obtain opioids only from the study investigator.
CT Scans
Subjects underwent high-resolution pelvic computed tomography (CT) at either 6 or 12 months after SIJF (assigned at random via a pre-programmed secure website). An independent radiologist [TH] analyzed CT scans for the following endpoints: 1) Degree of apposition of bone to the implant within the sacrum (rated as 0–30%, 30–50%, 50–90%, >90%), 2) presence of radiolucency in the sacral or iliac bones suggestive of implant loosening (and, if present, extent of lucency rated as <15%, 15–50%, 50–100% along bone-implant interface), 3) bridging bone within the treated joint (and, if present, location of bridging [adjacent, distant, both] and extent of bridging (<5%, 5–15%, 15–30%, 30–100% of SI joint volume), 4) evidence of positive bone remodeling, defined as presence of increased bone density/sclerosis adjacent to the implant that appears to be due to new bone formation and/or an increase in mechanical demand at or distant from the bone-implant interface, 5) adverse bone reaction, defined as presence of erosions, cysts, signs of infection, osteolysis or other findings in the bone adjacent to the implant, 6) device failure (breakage), 7) device migration, and 8) heterotopic bone, defined as islands of bone within soft tissue or bone spurs continuous with native bone.
Endpoints
As noted previously,19 the primary success endpoint in the study was improvement in ODI score at 6 months compared to baseline. The endpoint was deemed positive if the ODI change score was non-inferior (10-point non-inferiority margin) to the 25-point ODI improvement in 3 prior trials of the earlier device in the same target patient population.6–8 Assuming a standard deviation of 20 points, a total of 50 subjects provided sufficient (>80%) power to conclude non-inferiority. The primary radiographic endpoint was the bone apposition to at least 30% of the devices’ surface area within the sacrum.
The study included several secondary endpoints: improvement in SIJ pain, ODI and EQ-5D time trade-off index at other study visits, improvement in physical function tests, decrease in proportion of subjects using opioids for SIJ pain, and rate of procedure or device-related serious adverse events.
Statistical Analysis
All statistical analysis was performed using R.25 Continuous variables were summarized as mean, standard deviation and range. Binary outcomes were summarized as percentages with exact 95% confidence intervals. Non-inferiority was evaluated with student’s T-test. Change from baseline in continuous measures across time was evaluated using repeated measures analysis of variance. Binary outcomes were assessed using Fisher’s or McNemar’s test.
Trial Oversight
The study was sponsored by the device manufacturer (SI-BONE,Inc., Santa Clara, CA, USA). The study was conducted according to the Declaration of Helsinki and relevant items from ISO 14,155:2011. All sites obtained written approval from a local or central institutional review board (IRB) prior to first enrollment. All subjects provided written informed consent prior to participating. Subjects were paid nominal amounts (approved by the IRB) for their time and expense to complete study assessments. IRBs overseeing the study are: Aspire IRB, 11,491 Woodside Ave, Santee, CA 92071, USA; Louisiana State University Health Sciences IRB, Office of Research Services, 433 Bolivar Street, Room 206, New Orleans, LA 70ss112, USA; Providence Health Services, 5251 NE Glisan, Building A, 3rd Floor, Portland, OR 97213, USA.
Results
One hundred seventy-six patients were screened and 51 study subjects underwent SIJF between October 2017 and January 2019. Three subjects were enrolled despite not meeting eligibility criteria: in one, pain improvement after SIJ block did not exceed the 50% threshold; in one subject, block was not performed to confirm SIJ pain; in one subject patient age was slightly above the upper age criteria of 70 years. All 3 deviations were approved by the study sponsor.
Baseline Characteristics
Mean subject age was 53 years and mean pain duration was 8 years (range 0.4–44, Table 1). 76% were women. SIJ pain etiologies were: SIJ disruption (20 sides) and degenerative sacroiliitis (42 sides). 50/51 (98%) subjects had prior physical therapy and 50 (98%) had undergone prior SIJ corticosteroid injections; 8 (16%) had prior radiofrequency ablation of lateral branches of the sacral nerve roots. 16 (31%) had a history of prior lumbar fusion and 5 (10%) had prior SIJ fusion contralateral to the affected side.
 |
Table 1 Baseline and Surgical Characteristics of SALLY Participants (n=51). Data from Index Procedure Reported |
Procedure
Forty-six subjects underwent unilateral SIJ fusion. Five subjects (10%) underwent staged bilateral SIJ fusion. In one bilateral case, the second-side procedure took place 1 year after the first side (the study protocol required a time interval of <90 days); the delay was related to inability to take time off from work. For the purposes of analysis, this subject was analyzed as a unilateral case. Two subjects were enrolled with bilateral SIJ pain but decided to have unilateral SIJF only.
Mean procedure time was 52 minutes. Bone graft (autograft or allograft) was used in 45% of cases. Blood loss was low (estimated mean 37 cc, range 0–100 cc). No technical complications, device malfunctions or adverse events during the procedure were reported. Mean length of stay was 0.8 days. 25 (49%) of subjects were discharged from the hospital/surgical facility on the day of surgery and length of stay was 2 or fewer days in 45 (88%) subjects.
Follow-Up
Twelve-month follow-up was available in 46 (90%) cases. Two subjects were lost to follow-up (1 soon after hospital discharge and one after the month 3 visit); one subject withdrew prior to the 3-month visit; a 12-month study visit was not done in 1 subject due to coronavirus fears and in a second subject due to competing health issues. One subject had moved out of state and completed 12-month questionnaires only but not physical function testing.
Efficacy
Mean ODI improved from 53 at baseline to 28 at 12 months (improvement of 25 points [49%], p<.0001). The 6-month ODI change met the study’s non-inferiority endpoint (p=.0004). Improvement in pain, disability (ODI) and quality of life (EQ5D-TTO index) scores was rapid and sustained. Each improvement was statistically significant (p<.0001 for each endpoint at each postoperative time point, see Figure 1). The figure shows that change scores in the current study (green lines) were similar to those observed in 3 prior studies (two multicenter randomized trials6,8 and one single-arm multicenter trial7) that used the solid TTI. Mean SIJ pain improved by 58 points at 12 months compared to baseline. By month 12, 96% experienced an improvement of 20 points or more in VAS SIJ pain on the target side and 67% experienced an improvement of 15 points or more in ODI. These thresholds are commonly used as clinically important responses.26,27 67% had both a 20-point improvement in pain score and a 15-point improvement in ODI. Quality of life, assessed with EuroQOL-5D time trade-off index, improved from 0.47 at baseline to 0.74 at month 12 (p<.0001).
The proportion of subjects reporting difficulty with activities of daily living improved substantially at each postoperative timepoint (Figure 2) with all tests showing statistically significant improvement (McNemar test p<.05) compared to baseline. Satisfaction rates were high throughout follow-up (69–78% reporting “very satisfied” and 92–98% reporting “satisfied or very satisfied”). 75–83% of subjects would have the procedure again.
Opioid Use
At baseline, 57% of subjects were taking opioids for SIJ pain. At 12 months, only 22% were using opioids for SIJ pain (p<.0001 vs. baseline). Daily mean oral morphine equivalents (OME) decreased from 27.6 mg/day at baseline to 17.6 mg/day at 12 months (reduction in OME by Wilcoxon test, p=0.0118). For subjects who continued opioid use, mean OME was similar at 12 months (80 mg/day) compared to baseline (81 mg/day). No subject started taking opioids anew during follow-up.
Physical Function Tests
At all follow-up time points, physical function tests improved (Figure 3). Active straight leg raise test improved for both the most painful and least painful side (p<.0001). Five times sit-to-stand mean times improved from 23.4 seconds at baseline to 17.8 seconds at 12 months, an improvement of 6.6 seconds (p=.0053). One subject, who could not perform this task preoperatively due to pain, was able to perform it at 12 months. Transitional up-and-go decreased from a mean of 22.6 seconds at baseline to 15.6 seconds at 12 months, an improvement of 7.4 seconds (p<.0001). Two subjects who were unable to perform this task preoperatively due to pain were able to perform it at 6 and 12 months.
Ambulatory and Work Status
At baseline, 80% reported being able to walk fully without any assistance; at 12 months 80% were fully ambulatory (McNemar p=1 for 12-month change in ambulatory status). 4 subjects who were fully ambulatory at baseline were not so at 12 months (2 due to recurrent SIJ pain, one due to a flare-up of knee pain related to a motor vehicle accident and one due to trochanteric bursitis and overexertion related to L5-S1 degeneration). 5 subjects who required an assistive device for ambulation at baseline reported being able to walk fully without assistance at 12 months.
At baseline, 31% of subjects were working full-time. The proportion working full-time at 12 months was similar (35%, McNemar p=.7055). At baseline, 14% were working less than full-time because of SIJ pain and 6% were not working because of SIJ pain; at 12 months, these statistics were 9% and 2%, respectively.
Adverse Events
By month 12, 112 adverse events were reported in 43 subjects. Of these, one was definitely related to the device; this subject experienced postoperative pain in the L5 distribution. CT scan showed that the superior implant had breached the superior alar border near the left L5 nerve root. The subject was taken back to the OR, the implant was repositioned such that the distal end was fully in the sacrum, which resulted in immediate pain improvement. However, the subject subsequently experienced intermittent leg and foot pain, inability to wear shoes, and foot discoloration with symptoms consistent with reflex sympathetic dystrophy. Two nerve blocks produced short-term pain relief. She underwent placement of a spinal cord stimulator with improvement but not complete resolution of symptoms. Stimulator lead migration led to an episode of pain recurrence. One subject experienced skin rash rated as definitely related to bandages placed in the OR. An additional 5 events were rated as probably related to the procedure, including 1 case each of: muscular dysfunction of the hip related to L3/4 disc herniation, anemia, temporary surgical site pain, trochanteric bursitis and a small wound dehiscence. None of these 5 events were deemed severe. 5 additional events in 4 subjects met criteria for serious adverse event: Contralateral SIJ pain (n=3, one case related to a motor vehicle accident), ipsilateral SIJ pain (n=1, related to the same motor vehicle accident) and aspiration pneumonitis (n=1). One of these subjects underwent revision SIJF and one underwent contralateral SIJF.
Radiographic Analysis
CT scans were available in 46 subjects (23 at 6 months and 23 at 12 months). Due to an error at the site, one subject underwent CT at 6 months despite being assigned to CT at 12 months; for the purposes of this analysis, this subject was reclassified into the 6-month group. Subjects in whom CTs were unavailable had withdrawn from the study. CTs imaged 52 treated sides (6 subjects with CTs had undergone bilateral SIJF, one outside of the study). Of 52 sides, 1 had 4 implants, 6 had 2 implants and the remaining 45 had 3 implants.
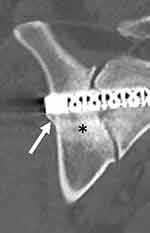
The primary endpoint, apposition of bone to at least 30% of the devices’ surface area within the sacrum, occurred in all (100%) treated sides. No radiolucencies were observed. Bridging bone could not be assessed due to imaging artifact or implants in close approximation in 8 sides. In the remaining 44 sides, evidence of bridging bone was seen in 16 (70%) sides at 6 months and 17 (77%) sides at 12 months. When bridging was present, it was typically directly adjacent to the implant and involved <5% of the total SI joint surface area (see Figure 4). In a small number of cases, bridging was present distant from implants. Evidence of positive bone remodeling (Figure 5) was seen in all but 2 treated sides. Not seen were evidence of adverse bone remodeling, device failures, or device migration. Small foci of heterotopic bone production (almost always less than <1 cm, see Figure 5) were observed in 18 sides abutting the implant at the iliac bone.
Discussion
Most evidence supporting the safety and efficacy of SIJF reports use of milled (solid) triangular titanium implants with a plasma spray-coated surface. Evidence includes 2 randomized trials,6,8 prospective cohorts,7,28 and retrospective studies.9–13,29 The current study provides strong prospective evidence that a newer 3D-printed fenestrated triangular titanium implant designed with optimized surface characteristics to promote bony ongrowth and ingrowth results in improvements in pain, disability and quality of life and satisfaction levels that are similar to those seen in clinical trials of the prior device. The safety profile of SIJF using iFuse-3D was reasonable with no unanticipated events. One subject underwent a revision surgery due to implant malposition and one subject underwent late revision after a motor vehicle accident. These revision rates are consistent with those observed in post-market surveillance for iFuse-3D.30
In comparison to prior studies, the current study has several additional contributions.
Daily Activities
Self-reported ability to perform daily activities suspected to be impacted by SIJ pain improved markedly from baseline to 6 and 12 months after SIJF; all categories of activities improved.
Functional Tests
We incorporated 3 functional tests into our study: active straight leg raise test, 5 times sit-to-stand and a transitional variant of the timed up-and-go test. The up-and-go test involved a twisting maneuver that was thought to be particularly difficult in patients with chronic SIJ pain. All 3 tests showed statistically significant improvement. For 5-times sit-to-stand, mean times to complete the task improved from 23.4 to 17.8 seconds. Compared to healthy volunteers, who could perform the same task in a mean of 9.6 seconds (Data on file, SI-BONE), the 6.8-second decrease represents an improvement of approximately 49% (=6.8/(23.4–9.6)) towards normal values. Similarly, compared to healthy volunteers, who could perform the transitional timed up-and-go test in a mean of 10.6 seconds, the improvement in study subjects from 22.1 to 15.6 seconds represents an improvement of approximately 64% towards normal (=7.4/(22.1–10.6)).
Reduced Opioid Use
Our study contained an intervention designed to promote opioid cessation, namely opioid contracts and periodic reinforcement of self-efficacy for discontinuing opioids. In combination with a device and procedure that reliably and markedly reduces pain and disability, we observed marked decreases in opioid use for SIJ pain from 57% to 22% at 12 months. We observed a concomitant decrease in total opioid morphine equivalents. Opioid cessation without pain relief is very challenging. Our study showed that with high levels of pain relief and appropriate opioid prescribing practices, opioid cessation can be commonly achieved, limiting the strong negative potential associated with chronic opioid use.
Radiographic Comparison to Prior Studies
The same independent radiologist who read CT scans for prior US studies of triangular titanium implants read CT scans from the current study. The rate of bone binding to the implant surface was very high (100%). Radiolucencies were not observed. In a prior study, radiolucencies were seen near the distal end of implants in 5% at 5 years.28 In the 5-year study, however, most radiolucencies were related to poor implant position with insufficient purchase into the sacrum. Importantly, the current study showed early evidence of bone growth within the SIJ at rates higher than prior studies. Specifically, in prior studies, bridging of bone was seen in 45% of sides at 12 months and 71% at 24 months. In the current study, the 6-month rate was 70% and the 12-month rate 77%, suggesting the new implant may accelerate fusion within the joint. High rates of bone apposition combined with relatively accelerated bone bridging suggest the implants may provide high levels of permanent fixation/fusion of the joint. Subsidence (settling or sinking of an implant into the bone) is occasionally observed with metallic implants placed in the intervertebral disk space (interbody fusion cages). This occurs as the implants are subjected to compressive vertical loads which can drive the implants into the caudal vertebral body. Subsidence was not observed with any of the iFuse-3D implants placed across the SI joint. This finding was expected as the iFuse-3D implants are subjected to loading in shear and rotation as opposed to vertical loading.
Advantages to our study include its prospective multicenter design that included scheduled assessments and visits, and similar conduct of assessments across sites (at most sites, questionnaires were administered via computer). All study data were monitored and source verified. 12-month follow-up was high and CT scans were read by an independent radiologist. Disadvantages include lack of randomization and lack of blinded treatment assignment. In light of two prior randomized trials of the initial device against non-surgical management,6,8 randomization and blinding were deemed not necessary.
Conclusions
In this prospective study, SIJF with 3D-printed triangular titanium implants resulted in marked, substantial and sustained improvement in pain, disability and quality of life, with both functional improvements and substantial opioid use reduction. Radiographic evidence of accelerated bony fusion was observed. No breakage, migration or subsidence were observed.
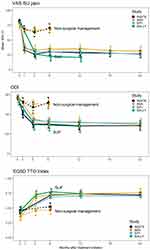 |
Figure 1 Improvement in scores over time comparing the current studies (INSITE (black, randomized trial of SIJF vs non-surgical management),6 iMIA (orange, randomized trial of SIJF vs conservative management)8 and SIFI (light blue, single-arm prospective trial of SIJF)).7 The top panel shows SIJ pain rated using a visual analog scale. The middle panel shows Oswestry Disability Index (ODI). The lower panel shows EuroQOL-5D time trade-off (TTO) index. |
 |
Figure 2 Proportion of subjects reporting minimal difficulty performing activities before (x-axis = 0) and 3, 6 and 12 months after SIJF. |
Disclosure
All authors conduct clinical research as part of prospective trials sponsored by SI-BONE. Abhineet Chowdhary, Travis J Hillen, Don Kovalsky, Andy Kranenburg, Harry Lockstadt, S. Craig Meyer, Vikas Patel and Gabriel Tender are paid consultants to SI-BONE. Daniel Cher is an employee of SI-BONE. Dr Vikas Patel reports grants from Pfizer, Orthofix, Globus, Medicrea, Mainstay and personal fees from DePuy-Synthes, SLACK Inc, Zimmer-Biomet, Aesculap, outside the submitted work. Dr S. Craig Meyer reports personal fees from Columbia Orthopaedic Group Clinical Research Foundation, during the conduct of the study. Dr Philip S Yuan reports personal fees from Choice Spine, outside the submitted work. Dr Travis J Hillen reports personal fees from Medtronic and Unltragenyx and is consultant for ERT, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;Apr(217):266–280. doi:10.1097/00003086-198704000-00029
2. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20(1):31–37. doi:10.1097/00007632-199501000-00007
3. Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889–1892. doi:10.1097/00007632-199608150-00012
4. Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86(1):37–44. doi:10.1097/PHM.0b013e31802b8554
5. Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi:10.1097/BRS.0b013e31818b8882
6. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:Article28. doi:10.14444/3028
7. Duhon BS, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T. Triangular titanium implants for minimally invasive sacroiliac joint fusion: 2-year follow-up from a prospective multicenter trial. Int J Spine Surg. 2016;10:Article 13. doi:10.14444/3013
8. Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Jt Surg. 2019;101(5):400–411. doi:10.2106/JBJS.18.00022
9. Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6(1):495–502. doi:10.2174/1874325001206010495
10. Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013;7:163–168. doi:10.2174/1874325001307010163
11. Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. doi:10.1186/1750-1164-6-13
12. Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. doi:10.1155/2013/536128
13. Cummings J
14. Schroeder JE, Cunningham ME, Ross T, Boachie-Adjei O. Early results of sacro–iliac joint fixation following long fusion to the sacrum in adult spine deformity. Hosp Spec Surg J. 2013;10(1):30–35. doi:10.1007/s11420-013-9374-4
15. Linden M-S-S, Paranhos L-R, De Carli J-P, et al. Influence of nicotine on machined- and anodized-surface implants. Histometric analysis. J Clin Exp Dent. 2017;9(10):e1207–e1211. doi:10.4317/jced.54127
16. Alsahhaf A, Alshagroud RS, Al-Aali KA, Alofi RS, Vohra F, Abduljabbar T. Survival of titanium-zirconium and titanium dental implants in cigarette-smokers and never-smokers: a 5-year follow-up. Chin J Dent Res. 2019;22(4):265–272. doi:10.3290/j.cjdr.a43737
17. MacBarb RF, Lindsey DP, Bahney CS, Woods SA, Wolfe ML, Yerby SA. Fortifying the bone-implant interface part 1: an in vitro evaluation of 3D-printed and TPS porous surfaces. Int J Spine Surg. 2017;11:15. doi:10.14444/4015
18. MacBarb R, Lindsey D, Woods S, Lalor P, Gundanna M, Yerby S. Fortifying the bone-implant interface part 2: an in vivo evaluation of 3D-printed and TPS-coated triangular implants. Int J Spine Surg. 2017;11(3):116–128. doi:10.14444/4016
19. Patel V, Kovalsky D, Meyer SC, et al. Minimally invasive lateral transiliac sacroiliac joint fusion using 3D-printed triangular titanium implants. Med Devices. 2019;12:203–214. doi:10.2147/MDER.S205812
20. Fairbank JC, Pynsent PB. The oswestry disability index. Spine. 2000;25(22):
21. Copay AG, Cher DJ. Is the oswestry disability index a valid measure of response to sacroiliac joint treatment? Qual Life Res. 2016;25(2):283–292. doi:10.1007/s11136-015-1095-3
22. EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9
23. Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–1184. doi:10.1037/0003-066X.44.9.1175
24. Mens JM, Vleeming A, Snijders CJ, Koes BW, Stam HJ. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26(10):1167–1171. doi:10.1097/00007632-200105150-00015
25. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available from:: http://Www.R-Project.Org/.
26. Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30(11):1331–1334. doi:10.1097/01.brs.0000164099.92112.29
27. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the oswestry disability index, medical outcomes study questionnaire short form 36, and pain scales. Spine J. 2008;8(6):968–974. doi:10.1016/j.spinee.2007.11.006
28. Whang PG, Darr E, Meyer SC, et al. Long-Term Prospective Clinical And Radiographic Outcomes After Minimally Invasive Lateral Transiliac Sacroiliac Joint Fusion Using Triangular Titanium Implants. Med Devices (Auckl). 2019;12:411–422. doi:10.2147/MDER.S219862
29. Graham Smith A, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14. doi:10.1186/1750-1164-7-14
30. Cher D, Wroe K, Reckling WC, Yerby S. Postmarket surveillance of 3D-printed implants for sacroiliac joint fusion. Med Devices. 2018;11:337–343. doi:10.2147/MDER.S180958
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.