Back to Journals » Clinical Ophthalmology » Volume 17
Prospective Study Comparing Quantitative Self-Monitoring Metamorphopsia Measurement Tools in Myopic Choroidal Neovascularization (mCNV)
Authors Hoffmann L, Müller S, Bachmann LM, Claessens D, Hatz K
Received 3 November 2022
Accepted for publication 28 April 2023
Published 9 May 2023 Volume 2023:17 Pages 1347—1355
DOI https://doi.org/10.2147/OPTH.S395989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Laura Hoffmann,1,2,* Susanne Müller,1,3,* Lucas M Bachmann,4,5 Daniela Claessens,6 Katja Hatz1,7
1Medical Retina & Research Department, Vista Eye Clinic Binningen, Binningen, Switzerland; 2Department of Ophthalmology, University Hospital Charité - Campus Benjamin Franklin, Berlin, Germany; 3Department for Health Sciences, Donau University Krems, Krems, Austria; 4Oculocare Medical, Medignition AG, Zurich, Switzerland; 5Faculty of Medicine, University of Zurich, Zurich, Switzerland; 6Gemeinschaftspraxis Augenheilkunde Lindenthal, Cologne, Germany; 7Faculty of Medicine, University of Basel, Basel, Switzerland
*These authors contributed equally to this work
Correspondence: Katja Hatz, Vista Augenklinik, Hauptstrasse 55, Binningen, 4102, Switzerland, Tel +41 61 426 60 79, Fax +41 61 426 60 01, Email [email protected]; [email protected]
Purpose: To assess the ability of two self-monitoring digital devices to detect metamorphopsia in myopic choroidal neovascularization (mCNV) and compare their usability.
Patients and Methods: This was a 12-month prospective observational study at a tertiary care eye hospital, Switzerland. Twenty-three Caucasian patients with mCNV were recruited, 21 eyes were analyzed. Primary and secondary outcome measures: Primary outcome measures were the metamorphopsia index scores as assessed by the two self-monitoring digital devices (Alleye App and AMD – A-Metamorphopsia-Detector software) at baseline, at 6 and 12 months and individual optional visits in between. Secondary outcome measures included best corrected visual acuity and morphological parameters (including disease activity) as evaluated by spectral-domain optical coherence tomography and fundus autofluorescence imaging. Location of mCNV was graded using the Early Treatment of Diabetic Retinopathy Study grid overlay. A usability questionnaire was administered at 12 months. Bland-Altman plots evaluated the limits of agreement of both devices. Linear regression analysis assessed the correlation between the difference and the average of the two scores.
Results: A total of 202 tests were performed. Disease activity of mCNV was observed at least once in 14 eyes. Both scores concordantly detected metamorphopsia exhibiting a displaced scale of measurement yielding a coefficient of determination of 0.99. Concordance rate for pathological scores was 73.3%. Both scores were not significantly different in active and inactive mCNV. Overall, the usability scores were higher for the Alleye App than the AMD – A-Metamorphopsia-Detector software (4.61± 0.56 vs 3.31± 1.20; p< 0.001). In subjects aged > 75 years, scores were slightly lower (4.08± 0.86 vs 2.97± 1.16; p= 0.032).
Conclusion: Whilst both self-monitoring devices concordantly identified metamorphopsia, they might act as an adjunct to hospital visits, but due to slight reactivations in mCNV and presence of metamorphopsia also in inactive disease the ability of detecting early mCNV activity might be limited.
Keywords: myopic CNV, metamorphopsia, disease activity, self-monitoring, optical coherence tomography
Introduction
Metamorphopsia as a leading symptom of worsening macular pathology is frequently used in therapy management.1 Due to the global rise of myopia a considerable increase in the associated social and economic burden is to be expected in the coming years.2 Pathological myopia (PM) as a possible outcome of high myopia can lead to degenerative changes and the development of myopic choroidal neovascularization (mCNV).3 In contrast to macular neovascularization (MNV) caused by neovascular age-related macular degeneration (nAMD), mCNV is characterized by considerably less retinal edema and subretinal fluid, hence requiring highly sensitive measurements to detect mCNV activity.4 Multimodal imaging including optical coherence tomography angiography may provide quantitative analysis regarding the therapeutic effect of intravitreal anti-vascular endothelial growth factor for myopic CNV.5
Nowadays, retinal pathologies such as nAMD and diabetic macular edema (DME) are typically treated by intravitreal injections of anti-vascular endothelial growth factor (VEGF) using a treat-and-extend regimen (TER). For the treatment of active mCNV, however, several authors have recommended one to three initial monthly injections followed by additional injections in a pro re nata (PRN) treatment scheme5–7 due to the occurrence of recurrences at irregular intervals.
Since treatment delay may lead to a diminished improvement in final visual acuity (VA) outcome,8 early detection of recurrences is mandatory to prevent future functional impairment. In spectral domain optical coherence tomography (SD-OCT) guided treatment of mCNV with ranibizumab, a previous study showed that outer retinal integrity, fluctuation in central retinal thickness (CRT) and baseline visual acuity were predictors for VA outcomes at 12 months.9 Furthermore, female gender and baseline external limiting membrane integrity have been described as a positive predictive factor to treatment response.10 In its active form mCNV may present with a decrease in central VA, metamorphopsia or a central scotoma depending on the position of the CNV.
Especially in the context of the COVID-19 pandemic and in consideration that mCNV typically affects subjects of working age, patient self-monitoring for a timely detection of visual deterioration would be desirable to limit economic burden and to save hospital resources. The traditionally employed paper based Amsler grid, which has been around since the late 1940ies, may increasingly be replaced by smartphone and computer-based home monitoring solutions. They have shown to reliably identify diseased subjects and monitor progression of several macular diseases.11–13 However, to date these devices have mainly been evaluated for nAMD and DME but not exclusively for mCNV. To fill this gap, we investigated the usability and concordance of two digital applications for metamorphopsia, the Alleye app (Oculocare medical AG, Switzerland) and the AMD – A-Metamorphopsia-Detector (MI; app4eyes GmbH & Co. KG, Germany), in mCNV.
Materials and Methods
This prospective, observational study included patients treated for a mCNV at the Vista Augenklinik Binningen, Switzerland, between October 2019 and February 2021. The study was approved by the local ethics approval board (Ethikkommission Nordwestschweiz (EKNZ 2019–01602)) and adhered to the tenets of the Declaration of Helsinki and Good Clinical Practice (ICH – GCP). The study is registered at ClinicalTrial.gov (NCT 04112524). Informed consent was obtained of all participants prior to the enrolment.
Eligible patients had to fulfill the following inclusion criteria: diagnosis of fluorescein angiography confirmed mCNV with leakage involving the juxtafoveal/foveal region, best-corrected visual acuity (BCVA) of at least 35 letters Early Treatment of Diabetic Retinopathy Study (ETDRS) evaluated at a distance of 4m, age ≥18 years and ability to follow the study procedures. Included were both freshly diagnosed mCNV lesions as well as eyes with currently inactive lesions but prior mCNV activity and previous anti-VEGF injection. Excluded were eyes with a history of vitreoretinal surgery or exhibiting a macular hole or epiretinal membrane with significant distortion of the retinal architecture.
While in each patient fixed study visits were performed at baseline, after 6 and 12 months individual supplementary optional study visits according to mCNV activity took place in between. At baseline, after 6 and 12 months patients underwent a standardized refraction protocol measuring ETDRS BCVA at 4m and completed the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25). At each visit the two metamorphopsia tests were performed as described below followed by biomicroscopic fundus examination, fundus autofluorescence imaging (FAF) and SD-OCT (Spectralis, Heidelberg Eng., Heidelberg, Germany), using the following scans: horizontal volume scan 19 sections, horizontal 6 mm scan in follow-up mode and macular star 9 sections as well as FAF imaging acquired with confocal scanning laser ophthalmoscope (HRA, Heidelberg Engineering, Germany). The ETDRS grid was centered on the fovea and the automated segmentation lines were corrected manually. Scans were evaluated by two physicians (KH, LH) to determine mCNV activity (defined as intra- or subretinal fluid or a CNV with fuzzy demarcation), a pigment epithelial detachment (PED) and subretinal fibrosis. Scans were graded dichotomously depending on the presence of those lesions in the central 1 mm, 3 mm and 6 mm of the ETDRS ring. The size of the pigment epithelial atrophy was measured based on FAF using the built-in measuring tool. At 12 months a manageability questionnaire rating was administered. In case of mCNV activity an intravitreal injection with ranibizumab was administered based on the standard treatment protocol in our clinic.
At each visit, the two metamorphopsia tests were performed always in the same order following an initial training and test instructions given by a single examiner (SM). The required time and any difficulties to complete the measurements were documented. The Alleye test is a CE-marked and Food and Drug Administration (FDA) approved mobile hyperacuity task intended for the detection and characterization of metamorphopsia in patients with AMD and diabetic retinopathy. It is based on a monocular hyperacuity task similar to the Amsler grid consisting in the alignment of a central dot to an imaginary straight line between two fixed outer dots, see Figure 1.12 While this task is repeated in 12 different positions and four axes, continuous fixation of the central dot is required, hence avoiding inadvertent saccades. The final score ranges between 0 and 100.
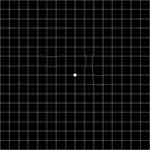
The AMD – A-Metamorphopsia-Detector based on the Amsler grid, is a software using the concept of a negative image so that a distorted image can be straightened by moving the mouse.13 White horizontal and vertical lines on a black background distant by one degree need to be straightened while fixing the central point monocularly, see Figure 2. The required distance between the eye and the screen is indicated on the display. The amplitude (d), the distance from the central fixation point (eccentricity) and the area (A) are transformed into a logarithmic metamorphopsia index (MI). The critical value of the score is defined by any result above zero.
Statistical Analysis
Statistical analysis was performed using SPSS statistical package version 24 (SPSS, Inc., Chicago, IL) and the Stata 16.1 statistics software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.). Mean and standard deviation (SD) or percentages of the data are reported, as appropriate. To assess the concordance of both scores, Bland-Altman diagrams plotting the differences between the two scores against their averages were generated. The upper and lower limits of agreement consisting in the mean difference +1.96 SD and −1.96 SD include 95% of the values. Subsequently, the concordance rate was calculated assuming a critical value for pathologic results of below 74 for the Alleye score and above 0 for the MI. To investigate proportionality bias, we performed linear regression analysis of the difference between scores (dependent variable) and the average of the two scores (independent variable). From this analysis, we calculated the slope and the coefficient of determination (R2) to assess the differences between the observed data and the fitted values.
The mean values of both scores of all visits without mCNV activity as graded on OCT and the mean metamorphopsia index changes to follow-up visits with mCNV activity, namely intraretinal or subretinal fluid or fuzzy CNV borders, were calculated for each eye individually. Given the logarithmically scaled nature of MI, a transformation by exponential function was performed before calculating the mean. A paired t-test was used to assess an association between the metamorphopsia index change of visits with disease activity from the mean of the inactive visits and presence of lesions in the different subfields for the two devices. One sample t-tests were used to test differences in the usability scores between the two tests. To evaluate the reliability of the scores to identify inactivity of the CNV, intraclass correlation coefficients (ICC) were computed to compare the inactive visits whereas an ICC above 0.9 indicates an excellent reliability based on a 95% confidence interval (CI).14 P-values <0.05 were considered statistically significant.
Results
While 23 eyes were initially recruited for the study, two eyes were excluded due to the development of interfering concomitant ocular diseases (macular hole and severe epiretinal membrane). 16 females and 5 males with a mean age of 68.14±16.29 years were included. At baseline mean BCVA was 76.86±10.82 ETDRS letters. Mean central retinal thickness (CRT) was 281.71±71.3 μm. The included subjects received a total of 57 intravitreal anti-VEGF-injections (ranibizumab, aflibercept) during follow-up with a mean of 2.71±2.64. Baseline characteristics are summarized in Table 1. The National Eye Institute Visual Function Questionnaire-25 assessing vision-related quality of life on a scale of zero to 100 was administered at baseline, 6 months and 12 months. Mean NEI-VFQ-25 score at baseline revealed a considerably diminished value of 74.10±18.56 and remained stable over the study period (74.75±18.70 at 6 months and 74.25±18.45 at 12 months).
 |
Table 1 Baseline Characteristics |
In total, 202 tests were performed whereof 126 tests took place at the fixed visits at baseline, at 6 and 12 months. The remaining measurements were performed at optional unscheduled visits according to individual mCNV activity. The mean time employed for the completion of the measurement decreased from 6.62±3.93 minutes for the Alleye score and 8.19±6.61 minutes for the MI at baseline to 3.14±2.12 respectively 3.29±3.77 minutes at the 12 months visit.
Bland-Altman plots (Figure 3) show a proportionality bias, indicating that the methods do not agree equally through the range of measurements. Hereby, a linear relation between the mean of the scores and their differences in the absence of a scattering was shown. A regression line yielding an excellent coefficient of determination (R2) of 0.99 was fitted. Interestingly, a fitting line was persistent when plotting the Alleye score against all of the 3 subscores of the MI (amplitude, eccentricity and area) (Figure 4). Concordance rate between the occurrence of pathological scores generated by both devices was 73.3% with a correlation coefficient of 0.463.
To assess the ability of the measures to reliably identify visits without mCNV activity, intraclass correlation coefficients (ICC) for the inactive visits were calculated. The ICC yielded excellent values for both the Alleye score with 0.98 (95% CI 0.94–0.99) and the MI with 0.97 (95% CI 0.91–0.99).
Fourteen eyes showed at least once a mCNV activity during the study period, whereof 9 eyes had an active lesion in the central 1 mm or 3 mm as well as three eyes in the central 6 mm. Figure 5 illustrates two OCT scans of an eye with mCNV activity in the central 1 mm and without activity, respectively. The mean metamorphopsia index changes between the visits with and without mCNV activity as graded on OCT with regard to the localization of the activity were calculated. The two function scores did not significantly correlate with the presence of morphological mCNV activity. There was a trend for differences between the MI scores of active and inactive mCNV (117.80±231.57 (p=0.079)), but no difference was found for the Alleye score (0.362± 17.16 (p=0.938)) when including all sites confounded (Table 2). The mean differences were higher for both scores upon inclusion of eyes with a mCNV activity solely in the central 1 mm (n=9), namely 128.70±272.94 (p=0.195) for the MI and −4.42±14.01 (p=0.372) for the Alleye score without reaching the significance level. Expectedly, the index changes were lower when considering eyes with an active lesion in the central 3 mm (50.41±125.91; p=0.264, respectively −0.12±19.18; p=0.985) and in the central 6 mm (70.67±155.16; p=0.513, respectively 1.43±11.82; p=0.853).
 |
Table 2 Comparison of Mean Index Changes Between Active and Inactive Visits |
Usability of the devices was assessed based on a self-developed questionnaire rating the difficulty, enjoyment, willingness to continuation and time span allocated to completion of the measurements. Subsequently, usability was estimated between zero (poor) and five (excellent). Whilst both scores reached positive appreciation, overall, the Alleye App obtained greater usability scores with a mean of 4.38±0.74 versus 3.16±1.16; p<0.001. Handling of the devices was more difficult for elderly subjects over 75 years (n=9) than younger ones (n=11) with nevertheless satisfactory values for the Alleye App (4.08±0.86 versus 4.61±0.56; p=0.114) compared to the MI (2.97±1.16 versus 3.31±1.20; p=0.530). In the subgroup of subjects aged >75 years, the scores of both tests were slightly lower (4.08±0.86; 3.31±1.20vs. 2.97±1.16; p= 0.032) but still in favor for the Alleye.
Discussion
The key findings of our study consist in a high concordance in metamorphopsia detection for both used measuring methods and satisfactory usability of both applications even in elderly patients. The evaluated metamorphopsia measurements reached comparable good test-retest-reliabilities in mCNV than other macular pathologies. Despite greatest correlation to disease activity in subfoveal location, the differences between scores in active and inactive disease did not reach significance. To the best of our knowledge, this is the first study to evaluate metamorphopsia self-assessment as a diagnostic tool in a population exclusively affected by mCNV.
Especially in the context of the COVID-19 pandemic efficient self-monitoring of macular pathology to optimally allocate health care resources remains an unmet clinical need.
The pathophysiology of metamorphopsia is not entirely elucidated. It may occur through a variety of mechanisms including lateral photoreceptor displacement, changes in axial length and as a consequence of scotoma.1 Perception of metamorphopsia is essentially a hyperacuity task relying upon spatial discrimination.15 Both metamorphopsia devices intend to capture the same biological phenomenon, yet they exhibit a quasi-ideal displaced scale of measurement. The Alleye consists in a hyperacuity task requiring the correction of a misaligned central dot between two fixed outer dots. In contrast, the MI yields a logarithmic summation score using the straightening of a distorted image. Interestingly, although the MI acts as a summation score, the concordance between both scores persists even for the subscores.
In the present study, both devices failed to detect disease reactivation in mCNV in contrast to their already proven usefulness in monitoring disease activity in nAMD and DME. Regarding the small number of affected individuals, our results might prevent false expectations in these patients. Myopic CNV is typically characterized by subtle morphological changes with often large preexisting retinal and pigment epithelial alterations as well as chorioretinal atrophies.4 Due to the foregoing alterations in retinal architecture and displacement of photoreceptors, metamorphopsia may therefore already occur in inactive disease. Recurrences of mCNV usually present with considerably less retinal edema and subretinal fluid as compared to nAMD and DME. Previously, the ability of the Alleye app to monitor disease activity in subjects with nAMD treated in a pro re nata (PRN) scheme was assessed,11 therefore usually implying large recurrences with extensive intra- or subretinal fluid. In contrast mCNV activity often presents as small lesions with modest exudation,4 therefore potentially limiting the sensitivity of the score. Whereas the MI was reported to moderately respectively highly correlate with CRT in macular edema due to nAMD and DME,16 in our study no correlation was observed.
Expectantly, both scores demonstrated improved correlation to disease activity in subfoveal location, for the MI just not reaching significance, may be due to the low number of participants. Contrary to nAMD, in our cohort, mCNV reactivations presented in parts exclusively extrafoveal (initial lesion extending into foveal/juxtafoveal areas). Since the Alleye covers a visual field of 12.7° and the MI of up to 40° depending on the test arrangement, detection of disease activity in extrafoveal location may be hindered.
Nevertheless, we consider it helpful to monitor metamorphopsia in mCNV patients as both scores could be shown to reliably identify timely spaced inactive visits with excellent intraclass correlation coefficients (ICC) (0.98 for the Alleye and 0.97 for the MI). Likewise, Claessens et al described an ICC of 0.97 for the MI in two repeated one hour spaced measurements in eyes with macular pathology beforehand.13
We consider the high test-retest-reliability of the inactive visits in our study as the basis of a useful metamorphopsia measurement device. The comparison to a healthy control group was not evaluated in this study but might point out a possibly altered metamorphopsia perception in active mCNV as opposed to a control group in future studies. Even in healthy subjects a surprising decrease in the Alleye score was observed, when the test was repeated rapidly several times12, attributed to a lack of motivation and fatigue.
Another interest in regular metamorphopsia assessment in mCNV lies in the possible adaption to metamorphopsia, both perceptual and structural.15 Prism adaption studies confirmed perceptional adaption in only 10 minutes taking place in different portions of the visual field.15 Therefore, sudden-onset retinal changes like nAMD are more likely to induce severe metamorphopsia than advanced atrophic conditions. Although the ability to adapt to metamorphopsia in chronic disease is limited, we consider the regular assessment of metamorphopsia in mCNV as a useful adjunct to clinical examinations. As shown in our study, vision-related quality of life was considerably diminished in mCNV despite a relatively preserved BCVA. The assessment of metamorphopsia might therefore complete the patients’ subjective impairment rather than being able to serve as an early indicator for mCNV reactivation using the two tested devices.
Limitations of the study lie in the small number of participants and hence number of recurrences, therefore limiting correlation to disease activity. Due to the rarity of the disease, subjects without access to an own smartphone or computer had to be included, therefore precluding the possibility of a frequent home monitoring. On the other hand, the perpetual execution of the measurements under supervision of a single instructor ensured an invariant high quality of the testing which might not be ensured in home monitoring. Furthermore, the execution of the tests in a predetermined order might have had an impact on the score results.
In view of our encouraging results regarding the detection of metamorphopsia, further studies including a greater study cohort of mCNV and a healthy control group should integrate regular home monitoring. In this manner, threshold alarms alerting the clinician could be provoked. Beforehand, the alarm generated by the Alleye app was established as a reliable indicator for the worsening of the disease and need for treatment.17 Besides, further studies should assess the cost efficiency of home monitoring as an adjunct to face-to-face clinical visits.
Since early treatment initiation upon recurrence of mCNV activity is essential for the functional outcome, regular home monitoring may provide immediate feedback to the patients and contribute to the reassurance of the affected individuals. Mobile health devices through patient’s empowerment may even lead to an increased treatment adherence.18 As this macular pathology frequently affects the working age population, self-monitoring seems particularly suited for mCNV to alleviate the associated economic burden related to hospital appointments and missed working hours. Younger age was previously associated with an increased participation rate in a self-monitoring initiative for macular pathology during the COVID-19 pandemic.19 Our study confirmed a satisfying usability of the devices particularly for the Alleye app especially in the younger patients. Likewise, higher user satisfaction and increased self-testing frequency was described for the smartphone-based application MacuFix when compared to the traditional Amsler grid in macular disease.20
Conclusion
Self-monitoring devices are a useful pillar of comprehensive, patient-centered therapy for mCNV. Despite a different mode of action, both scores concordantly identified metamorphopsia. Due to the good test-retest-reliabilities in mCNV we consider the monitoring of metamorphopsia as a useful adjunct to morphological examinations to fully understand the patients impairment. Nevertheless, we should be aware that due to slight reactivations in mCNV and presence of metamorphopsia also in inactive disease a significant correlation to disease activity could not be shown in this study.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request after approval of data transfer by the local ethics approval board.
Ethics Approval and Informed Consent
The study was approved by the local ethics approval board (Ethikkommission Nordwestschweiz (EKNZ 2019-01602)). Informed consent was obtained of all participants prior to the enrolment.
Acknowledgments
The study has been presented at the virtual European Association for Vision and Eye Research (EVER) Congress 2021 (02/10/2021).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Alleye licenses were provided by Bayer Switzerland AG. No further funding was provided to this study.
Disclosure
LMB and DC are founders of the self-monitoring tests described in this paper. LMB’s company Medignition is shareholder of Oculocare Medical, the company that developed the Alleye platform. DC reports App4eyes co-owner and has a patent US 10,588,506 B2, DE102015215557A1 issued to German Patent and Trademark Office, a patent DE10 2015 215557 issued to European Patent Office, a patent PCT/EP2016/069156) issued to United States Patent and Trademark Office. KH reports Bayer provided the AllEyeApp Licences for this study; contract research, advisory board fees from Novartis and Roche; advisory board fees from Bayer and Allergan/AbbVie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Simunovic MP. Metamorphopsia And Its Quantification. Retina. 2015;35(7):1285–1291. doi:10.1097/IAE.0000000000000581
2. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi:10.1016/j.ophtha.2016.01.006
3. Haarman AEG, Enthoven CA, Tideman JWL, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi:10.1167/iovs.61.4.49
4. Keane PA, Liakopoulos S, Chang KT, et al. Comparison of the optical coherence tomographic features of choroidal neovascular membranes in pathological myopia versus age-related macular degeneration, using quantitative subanalysis. Br J Ophthalmol. 2008;92(8):1081–1085. doi:10.1136/bjo.2008.138891
5. Cheng Y, Li Y, Huang X, Qu Y. Application of optical coherence tomography angiography to assess anti-vascular endothelial growth factor therapy in myopic choroidal neovascularization. Retina. 2019;39(4):712–718. doi:10.1097/IAE.0000000000002005
6. Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal neovascularization: review, guidance, and consensus statement on management. Ophthalmology. 2017;124(11):1690–1711. doi:10.1016/j.ophtha.2017.04.028
7. Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682–92.e2. doi:10.1016/j.ophtha.2013.10.023
8. Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology. 2015;122(6):1220–1227. doi:10.1016/j.ophtha.2015.01.025
9. Guichard MM, Peters G, Tuerksever C, et al. Outcome predictors of SD-OCT-driven intravitreal ranibizumab in choroidal neovascularization due to myopia. Ophthalmologica. 2020;243(2):154–162. doi:10.1159/000501040
10. Karasu B, Celebi ARC. The efficacy of different anti-vascular endothelial growth factor agents and prognostic biomarkers in monitoring of the treatment for myopic choroidal neovascularization. Int Ophthalmol. 2022;42(9):2729–2740. doi:10.1007/s10792-022-02261-1
11. Faes L, Islam M, Bachmann LM, et al. Correction: false alarms and the positive predictive value of smartphone-based hyperacuity home monitoring for the progression of macular disease: a prospective cohort study. Eye. 2021. doi:10.1038/s41433-021-01512-2
12. Schmid MK, Thiel MA, Lienhard K, et al. Reliability and diagnostic performance of a novel mobile app for hyperacuity self-monitoring in patients with age-related macular degeneration. Eye. 2019;33(10):1584–1589. doi:10.1038/s41433-019-0455-6
13. Claessens D, Schuster AK, Krüger RV, et al. Test-retest-reliability of computer-based metamorphopsia measurement in macular diseases. Test-retest-reliabilität der computergestützten metamorphopsiemessung bei makulaerkrankungen. Klin Monbl Augenheilkd. 2021;238(6):703–710. doi:10.1055/a-1252-2910
14. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
15. Hanumunthadu D, Lescrauwaet B, Jaffe M, et al. Clinical update on metamorphopsia: epidemiology, diagnosis and Imaging. Curr Eye Res. 2021;46(12):1777–1791. doi:10.1080/02713683.2021.1912779
16. Claessens D, Schuster AK. Correlation of quantitative metamorphopsia measurement and central retinal thickness in diabetic macular edema and age-related exsudative macular degeneration. Klin Monbl Augenheilkd. 2019;236(7):877–884. doi:10.1055/s-0043-125080
17. Islam M, Sansome S, Das R, et al. Smartphone-based remote monitoring of vision in macular disease enables early detection of worsening pathology and need for intravitreal therapy. BMJ Health Care Inform. 2021;28(1):e100310. doi:10.1136/bmjhci-2020-100310
18. McBride CM, Morrissey EC, Molloy GJ. Patients’ experiences of using smartphone apps to support self-management and improve medication adherence in hypertension: qualitative study. JMIR Mhealth Uhealth. 2020;8(10):e17470. doi:10.2196/17470
19. Teo KYC, Bachmann LM, Sim D, et al. Patterns and characteristics of a clinical implementation of a self-monitoring program for retina diseases during the COVID-19 pandemic. Ophthalmol Retina. 2021;S2468(21):55.
20. Claessens D, Ichhpujani P, Singh RB. MacuFix® versus Amsler grid for metamorphopsia categorization for macular diseases. Int Ophthalmol. 2021;22:1–10.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.