Back to Journals » Clinical Ophthalmology » Volume 17
Prospective Randomized Single-Masked Study of Bilateral Isofocal Optic-Design or Monofocal Intraocular Lenses
Authors Ang RET , Stodulka P , Poyales F
Received 28 June 2023
Accepted for publication 25 July 2023
Published 4 August 2023 Volume 2023:17 Pages 2231—2242
DOI https://doi.org/10.2147/OPTH.S425352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Robert Edward T Ang,1 Pavel Stodulka,2 Francisco Poyales3
1Cataract and Refractive Surgery Department, Asian Eye Institute, Makati City, Philippines; 2Cataract and Refractive Surgery Department, Gemini Eye Clinic, Zlín and Gemini Eye Clinic, Prague, Czech Republic; 3Cataract and Refractive Surgery Department, Miranza IOA, Madrid, Madrid, Spain
Correspondence: Robert Edward T Ang, Cataract and Refractive Surgery Department, Asian Eye Institute, 8th Floor PHINMA Plaza, Rockwell Center, Makati City, Philippines, Email [email protected]
Purpose: To assess refractive and visual outcomes of bilateral implantation of an isofocal optic-design intraocular lens (IOL) or a monofocal IOL following cataract surgery.
Methods: A total of 127 patients were recruited into a prospective, single-masked, randomized trial. Sixty-five patients bilaterally implanted with the Isopure Isofocal IOL and 62 patients with the Micropure Monofocal IOL were followed for 4– 6 months. Refraction, monocular and binocular uncorrected-distance-visual acuity, corrected-distance-visual acuity (CDVA), uncorrected-intermediate-visual acuity and distance-corrected-intermediate-visual acuity (DCIVA, 66/80 cm), uncorrected-near-visual acuity, and distance-corrected-near-visual acuity (DCNVA, 40 cm) were evaluated. Binocular defocus curve, binocular contrast sensitivity (photopic, mesopic with/without glare), and glare and halo phenomena were also measured.
Results: 99.23% of eyes were within ± 1.00D and 84.62% of eyes within ± 0.50D for the Isopure patients and 98.39% and 82.26% for the Micropure patients, respectively. The mean spherical-equivalent was − 0.06 ± 0.36D and 0.10 ± 0.32D for the Isopure and Micropure patients, respectively. 98.5% and 100% of patients implanted with the Isopure and Micropure IOLs showed a cumulative binocular CDVA value ≥ 20/20, respectively. 80% and 67.70% of patients implanted with the Isopure presented a binocular DCIVA ≥ 20/25 at 80 and 66 cm, respectively. These percentages were 46.8% and 40.3% with the Micropure IOL, respectively. For Isopure, 7.7%, 30.8%, and 58.5% of patients presented a DCNVA ≥ 20/25, ≥ 20/32 and ≥ 20/40, respectively. These values were lower for the Micropure: 1.6%, 19.4% and 46.8%, respectively. Defocus curves showed similar good visual acuity at distance for both lenses with better intermediate vision for the Isopure. Both groups presented good contrast sensitivity, and the size and intensity of halo and glare phenomena were similar between the two. No adverse-events were reported.
Conclusion: Our trial shows that both IOLs provide excellent visual acuity and contrast sensitivity for far vision with similar photic phenomena, and the Isopure IOL improved unaided intermediate vision performance.
Keywords: intraocular lens, phacoemulsification, cataract, visual acuity, defocus curve
Introduction
A recently published meta-analysis and systematic review based on 21 randomized controlled trials with 2951 patients comparing multifocal intraocular lenses (IOLs) with monofocal IOLs concluded that patients undergoing multifocal IOL implantation are more likely to be spectacle free but have a higher risk of glare, halos, and lower contrast sensitivity.1 Another study concluded that extended depth-of-focus IOLs provide better intermediate and near visual acuities than monofocal IOLs but increase the risk of contrast reduction and more frequent halos.2 In this sense, a report by the American Academy of Ophthalmology evaluating the efficacy and safety of presbyopia correcting IOLs pointed out that most patients implanted with multifocal and extended depth-of-focus IOLs, compared with patients implanted with a control monofocal IOL, showed that patient-reported spectacle independence was superior to that of the monofocal one.3 This report indicates that patients implanted with this type of IOL reported decreased contrast sensitivity and showed more visual phenomena as compared with control participants who received monofocal IOLs.
Therefore, new IOL designs aiming to provide patients with better visual acuity at intermediate distances should prevent the reduction of contrast sensitivity or the appearance of photic phenomena, being comparable to those outcomes found in patients implanted with monofocal IOLs. The Isopure 1.2.3. IOL (Beaver-Visitec International, Inc. [BVI], Waltham, USA) is a new aspheric lens with an optic design based on an isofocal concept4 to provide high-quality distance vision with improved functional intermediate vision. Recent publications analyzing this lens have reported good outcomes at different distances.5–9 Thus, in order to fully analyze this lens to ascertain possible differences with a monofocal IOL, this study aimed to assess refractive outcomes, visual acuity at different distances, contrast sensitivity under different lighting conditions and photic phenomena in patients with bilateral randomized implantation of the Isopure 1.2.3. IOL or its monofocal counterpart IOL (Micropure 1.2.3., with the same material and haptic design) following cataract surgery.
Methods
Study Design
This was a multicentric, prospective, randomized, controlled, single-blind post-market clinical follow-up trial conducted at three sites. The trial protocol was reviewed and approved by the Ethics Committee of the Asian Eye Institute in Philippines, Eticka Komise Ocni Kliniky Gemini in Republic Czech, and Hospital Clínico San Carlos in Spain following the tenets of the Helsinki Declaration. Written informed consent from all patients was obtained, and the study was registered at www.clinicaltrial.gov with the following number: NCT04249492. The main inclusion criteria considered cataract eyes (no comorbidity), IOL power available within the range of the study IOLs, patients aged ≥45 years, corneal regular astigmatism ≤1.0D and clear intraocular media (other than cataracts). The key exclusion criteria were the following: irregular astigmatism, difficulty to cooperate in the study, patients with degenerative visual disorders such as macular degeneration, patients with suspected age-related macular degeneration or who may be expected to require during the study retinal laser treatment, previous corneal or intraocular surgery, traumatic cataracts, abnormal pupils or expected complicated surgery or significant dry eye among others.
After being enrolled in the study and successfully completing the screening process, patients were randomized to either study group and bilaterally implanted with Isopure or bilaterally implanted with Micropure lenses. The ratio of randomization between the study group and control group is 1:1 and cannot be influenced by the researcher. This study was designed as a single-blind study. During the duration of the study, the patients were not informed if they had been implanted with the Isopure lens or the Micropure lens. This design was chosen to have the best possible objectivity in comparing the outcomes.
Intraocular Lenses and Surgical Procedure
The Isopure 1.2.3. IOL is a glistening-free hydrophobic IOL (n = 1.53, Abbe number = 42) with blue-light and ultraviolet filters with micro 4-closed loops and posterior angulated haptics platform. The aspheric optic design of the lens is based on an isofocal concept.4 The optic diameter of the lens is 6.00 mm (from 10 to 24.5D) and 5.75 mm (from 25 to 30D), and the overall diameter is 11.00 mm (from 10 to 24.5D) and 10.75 mm (from 25 to 30D). The Micropure 1.2.3. IOL (Beaver-Visitec International, Inc. [BVI], Waltham, USA) is made of the same material with the same haptic design, but the optic is biconvex aspheric monofocal. The optic diameter of the lens is 6.00 mm (from −10 to 24.5D) and 5.75 mm (from 25 to 35D), and the overall diameter is 11.00 mm (from −10 to 24.5D) and 10.75 mm (from 25 to 35D). Standard phacoemulsification technique for cataract surgery with a 2.2 mm incision was done considering a capsulorhexis of 5.5 mm diameter. IOL power was targeted for emmetropia using the IOLMaster 700 optical biometer (Carl Zeiss Meditec, Jena, Germany) using the Barrett formula.
Outcome Measures
Sphere, cylinder and the spherical equivalent [SE] were obtained pre- and at 4–6 months post-surgery. Monocular and binocular visual acuities were measured using the Clinical Trial Suite device (M&S Technologies, Niles, IL, USA) on a LogMAR scale: uncorrected- distance-visual acuity (UDVA), corrected-distance-visual acuity (CDVA), uncorrected-intermediate-visual acuity (UIVA), and distance-corrected-intermediate-visual acuity (DCIVA, at 66 and 80 cm), uncorrected-near-visual acuity (UNVA), and binocular distance-corrected-near-visual acuity (DCNVA, at 40 cm). Photopic binocular defocus curve at 4m (from −2.50 D to +1.50D in 0.5D steps) and binocular photopic, mesopic and mesopic with glare contrast sensitivity were performed using the same device. Halo and Glare simulator software (Eyeland Design Network GmbH company, Germany) was used to assess the subjective perception of photic phenomena. This test was done before and after surgery, with the preoperative values considered the baseline measurement. All subjects in the trial were followed for 4–6 months. Mean, standard deviation (SD) and range values were obtained for continuous variables, and count and percentages were considered for categorical and incidence measures.
Results
In this clinical trial, we enrolled 130 eyes from 65 patients implanted with the Isopure IOL, 39 being female (60%), and 124 eyes from 62 patients implanted with the Micropure IOL, 39 being female (62.9%). Table 1 indicates the patients’ demographics (age) and baseline characteristics (refraction, visual acuity, biometric parameters and IOL power implanted). In this trial, no surgical complications and no adverse events classified by the researcher to be potentially associated with the study device were reported.
 |
Table 1 Demographic Characteristics of Participants Shown as Means, Standard Deviations (SD) and Ranges of the Two Groups |
Refractive and visual acuity outcome graphs were plotted for the data obtained in the Isopure and Micropure IOL patients at 4–6 months. Figure 1 depicts the distribution of the SE prediction error (top). For the Isopure IOL, the highest percentage of eyes, 38.5%, was for the range between ±0.13 D followed by 23.8% for the −0.50 to −0.14 D range. For the Micropure IOL, 33.1% were for ±0.13 D and 32.3% for the 0.14 to 0.50 D range. 99.23% of the eyes (129) were within ±1.00 D and 84.62% of the eyes (110) within ±0.50 D for the Isopure IOL and 98.39% (122 eyes) and 82.26% (102 eyes) for the Micropure IOL, respectively. The mean postoperative SE was −0.06 ± 0.36D (range from 1.25 to −1.38 D) and 0.10 ± 0.32D (range from 1.38 to −0.50 D) for the Isopure and Micropure IOL patients, respectively. The same figure shows the distribution of the postoperative refractive cylinder (bottom). 96.2% of the eyes showed a cylinder ≤1.00 D and 74.6% ≤0.50 D for the Isopure IOL and 96.0% and 75% for the Micropure IOL, respectively. The mean postoperative refractive cylinder was −0.47 ± 0.37 D and −0.42 ± 0.36 D, respectively.
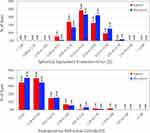 |
Figure 1 Distribution of predicted spherical equivalent error (top) and refractive cylinder (bottom) for the Isopure 1.2.3 and Micropure 1.2.3. IOL patients at 4–6 months post-surgery. |
Table 2 reports the monocular and binocular outcomes for UDVA, CDVA, UIVA and DCIVA (at 80 and 66 cm), and binocular DCNVA (at 40 cm) for Isopure and Micropure. IOL patients at 4–6 months post-surgery. Figure 2 depicts the cumulative monocular and binocular UDVA and CDVA values before (monocular values) and after 4–6 months post-surgery for the Isopure and Micropure IOLs. Under binocular conditions, 84.6% and 98.5% of patients implanted with the Isopure IOL showed cumulative binocular UDVA and CDVA values of 20/20 or better (≤0.00 logMAR), respectively, at 4–6 months post-surgery. These values were 93.5% and 100% for patients implanted with the Micropure IOL, respectively. All patients showed a binocular CDVA of 20/25 or better (≤0.10 logMAR). At intermediate vision, Figure 3 plots the cumulative monocular (left graphs) and binocular (right graphs) UIVA and DCIVA values after 4–6 months post-surgery both for 80 and 66 cm. Under binocular conditions, 80% and 67.70% of patients implanted with the Isopure IOL showed a binocular DCIVA of 20/25 or better (≤0.10 logMAR) at 80 and 66 cm, respectively. These percentages dropped to 46.8% and 40.3% with patients implanted with the Micropure IOL, respectively. At near vision (40 cm), Figure 4 provides the post-surgery cumulative binocular DCNVA values. For the Isopure patients, 7.7%, 30.8%, and 58.5% of patients presented a DCNVA ≤0.10 logMAR (20/25 or better), ≤0.20 logMAR (20/32 or better), and ≤0.30 logMAR (20/40 or better), respectively. These values were again lower for the Micropure patients: 1.6%, 19.4% and 46.8%, respectively.
Figure 5 shows the postoperative photopic defocus curve from 1.50 D to −2.50 D for the Isopure and Micropure IOL patients. In both groups of patients, there is a visual acuity peak at the expected distance focus (0D vergence/far vision) with the best visual acuity followed by reduced value (more pronounced for those patients implanted with the Micropure IOL) with increased negative vergence: intermediate (100 and 67 cm) and near (50 cm) distances. The maximum mean difference between groups was 0.06 logMAR at both −1.00D and −1.50D of vergence. It was 20/32 or better, up to about −1.75D in the Isopure IOL and up to −1.50D for the Micropure IOL.
Figure 6 plots the binocular mean photopic, mesopic and mesopic with glare contrast sensitivity values for both groups of patients. Both lenses showed similar and good contrast sensitivity outcomes at the different spatial frequencies assessed. The outcomes for the bright light conditions were slightly better than those found under low-lighting conditions with or without glare. Figure 7 shows the mean size and intensity of the photic phenomena on a 0–100 scale for both groups. At 4–6 months post-surgery, the mean values for the size and intensity of halo (from 18.31 to 27.32) and glare (from 10.34 to 18.76) phenomena were similar between the two groups.
 |
Figure 6 Mean binocular photopic (A), mesopic (B) and mesopic with glare (C) contrast sensitivity function for the Isopure 1.2.3 and Micropure 1.2.3. IOL patients at 4–6 months post-surgery. |
 |
Figure 7 Mean halo and glare simulation outcomes (intensity and size) for the Isopure 1.2.3 and Micropure 1.2.3. IOL patients before and at 4–6 months post-surgery. |
Discussion
Recent clinical literature on the refractive and visual outcomes of the isofocal optic design Isopure IOL considered several samples of eyes, follow-ups, comparators and different study designs. Table 3 shows a detailed description indicating the main conclusions found by these authors. The current proposal is the first prospective randomized single-blind study analyzing the outcomes of the Isopure IOL to be compared with its monofocal counterpart, the Micropure IOL.
 |
Table 3 Clinical Studies Reporting Outcomes of Patients Implanted with the Isopure 1.2.3. Intraocular Lens |
Our refractive accuracy outcomes show excellent refractive values for both IOLs (Figure 1), with 99.23% of eyes within ±1.00 D and 84.62% of the eyes within ±0.50 D for the Isopure IOL patients, and 98.39% and 82.26%, for the Micropure IOL patients, respectively. These values are better than those found in the EUREQUO study, which analyzed more than 280,000 cataract and refractive surgeries (93% of eyes within ±1.00D and 72.7% of eyes within ±0.50D).10 The mean postoperative SE was less than a quarter of a diopter (Isopure: −0.06 ± 0.36D, Micropure: 0.10 ± 0.32D) and the mean postoperative refractive cylinder was less than half a diopter (Isopure: −0.47 ± 0.37D, Micropure: −0.42 ± 0.36D). In relation to other studies, for example, Stodulka and Slovak5 reported a mean SE of −0.16 ± 0.46 D (range from 0.50 to −1.38 D), 80.6% of eyes had an SE within ±0.50D, and in no eyes did the refraction deviate more than +1.00 D or −1.50D. Their mean postoperative refractive cylinder was −0.36 ± 0.45 D (ranging from 0 to −1.50D). Bernabeu-Arias et al7 found similar outcomes with 95.7% of eyes having a target SE within ±1.00 D and 73.2% of eyes within ±0.50 D, being the mean postoperative SE −0.12 ± 0.42 D (range from 1.13 to −1.50 D). Tomagova et al8 targeted their patients for mini-monovision of −0.50 D in the nondominant eye and emmetropia in the dominant eye and found that the mean SE was −0.15 ± 0.41D and −0.46 ± 0.35D, respectively. Lesieur and Dupeyre9 found a mean SE of −0.06 ± 0.32D for the Isopure, −0.51 ±0.28D for the Synthesis+ and 0.11 ±0.31D for the Lucidis IOLs. In this study, the distribution of SE revealed that 68%, 50% and 64% of eyes were within 0.50D of target refraction for the Isopure, Synthesis+ and Lucidis IOLs, respectively. These values changed to 100%, 100% and 95% for ±1.00D, respectively.
The cumulative proportion of eyes having a given monocular and binocular visual acuity value (for distance, intermediate and near) is depicted in Figures 2–4 with the mean values detailed in Table 2. Our results revealed good outcomes for distance vision, with 98.5% and 100% of patients implanted with the Isopure and Micropure IOLs, respectively, showing a cumulative binocular CDVA value of 20/20 or better (Figure 2). At intermediate vision (Figure 3), 80% and 67.70% of patients implanted with the Isopure IOL showed a binocular DCIVA of 20/25 or better at 80 and 66 cm, respectively. These percentages dropped to 46.8% and 40.3% with patients implanted with the Micropure IOL, respectively. In addition, for near vision (Figure 4), 30.8% and 19.4% of patients showed a DCNVA of 20/32 or better for the Isopure and Micropure IOL, respectively. The CDVA outcomes in our study are better than the data published in the EURQUO database.11 This database reports data on >368,000 cataract extractions: a CDVA of 20/40 or better and 20/20 or better was achieved in 94.3% and 61.3% of the cases, respectively. Stodulka and Slovak,5 with patients implanted with the Isopure lens, found, under binocular conditions, that 100% of patients achieved a cumulative CDVA value of 20/20 or better, 52.9% of patients a DCIVA value at 80 cm of 20/25 or better, and 17.6% of patients a DCIVA value at 66 cm of 20/25 or better. Our study showed better percentages for intermediate vision (see Figure 3). In our series, the postoperative mean values for binocular CDVA, and DCIVA at 80 and 60 cm, were −0.05 ± 0.07, 0.07 ± 0.09, and 0.11 ± 0.11 logMAR, respectively (Table 2). Stodulka and Slovak5 found similar values: −0.09 ± 0.06, DCIVA 0.14 ± 0.08, and 0.20 ± 0.11 logMAR. In another study, Bova and Vita6 assessed the visual outcomes after implantation of the Isopure IOL and compared it with the Tecnis PCB00 IOL (Johnson and Johnson Surgical Vision Inc., Santa Ana, USA). After surgery, both groups had similar acuity at distance without significant difference in the post-operative outcomes of binocular UDVA (p = 0.89) and CDVA (p = 0.90). They did, however, find that the Isopure IOL had a significantly better monocular and binocular UIVA (66 cm) than the PCB00 IOL (p < 0.01). The mean values of binocular UDVA, CDVA, UIVA and DCIVA were 0.03 ± 0.04, 0.01 ± 0.03, 0.22 ± 0.06, and 0.21 ± 0.07 logMAR, respectively. Bernabeu-Arias et al7 found slightly better outcomes in 74 patients bilaterally implanted with the Isopure, with mean values of 0.02 ± 0.08, −0.02 ± 0.06, and 0.13 ± 0.11 logMAR for UDVA, CDVA and DCIVA (66 cm), respectively. Our results were better too, being: 0.00 ± 0.07, −0.05 ± 0.07 and 0.11 ± 0.11, respectively.
Bernabeu-Arias et al7 found 98.57% of patients with a cumulative binocular CDVA ≥20/25, 80.65% and 50.0% of patients with a binocular DCIVA ≥20/25 at 80 and 66 cm, respectively; and 41.94% of patients with a binocular DCNVA ≥20/40. In our study, these percentages were better: 100%, 80%, 67.7% and 58.5%, respectively (see Figures 2–4). Tomagova et al8 using mini-monovision found 77% of patients with binocular UDVA of ≥20/20, 58% of patients with binocular UIVA ≥20/25 and 51% of patients with binocular UNVA ≥20/40. They also indicated that on monocular testing, 84% and 98% of eyes had a CDVA ≥20/20 and ≥20/25, respectively, showing the safety of the Isopure lens. In addition, Lesieur and Dupeyre,9 for monocular conditions and eyes implanted with the Isopure IOL, reported a mean CDVA of 0.01 ± 0.02 logMAR with 82% of eyes with a CDVA ≥20/20 and 38% of eyes with DCNVA ≥20/20.
Figure 5 shows the binocular defocus curve for both groups of patients. The peak of the curve is found for distance vision (0D of vergence) and the other values reduce smoothly, with closer distances (negative defocus values) being more pronounced for patients implanted with the monofocal lens. The range for 0.2 logMAR visual acuity as per the American National Standard12 was from +1.25D to −1.75D for the Isopure IOL. Stodulka and Slovak5 also analyzed the binocular defocus curve showing a range from +1.0 to −1.50 D for this level of acuity, and Bova and Vita6 reported a similar range from about +0.75 D to −1.50D. These authors also analyzed the monofocal Tecnis PCB00 IOL and found that the Isopure group curve was flatter than the monofocal one, especially within the intermediate vergence levels. Bernabeu-Arias et al7 found a range from +0.75D to −1.50D in the binocular defocus curve for 0.2 logMAR. Tomagova et al8 found a higher range, varying from +0.90D to −1.60D in their binocular defocus curve (note that this sample considered mini-monovision eyes), and Lesieur and Dupeyre9 obtained a range from +0.75D to −1.25 in their monocular defocus curve. Considering our outcomes and those found by other studies, we believe that patients implanted with this lens show an acceptable visual acuity from distance up to 67 cm.
The outcomes for contrast sensitivity under different lightning conditions (see Figure 6) revealed good outcomes for both groups of patients. Stodulka and Slovak5 found outcomes within the normal range for monocular (at 1–2 months post-surgery) and binocular (at 4–6 months post-surgery) photopic and mesopic contrast sensitivities. Bova and Vita6 evaluated binocular photopic contrast sensitivity using sine-wave gratings from 1.5 to 18 cycles per degree and found no difference between Isopure and Tecnis PCB00 IOLs. They indicated that like a monofocal IOL, the Isopure uses all the light energy to extend the range of focus, not losing energy like diffractive designs and maintaining contrast sensitivity being comparable to a monofocal IOL. Furthermore, our results under mesopic conditions (with and without glare) support that the Isopure provides comparable contrast sensitivity to a monofocal IOL and can be an excellent option for patients without accepting possible reductions in distance visual quality. In this sense, Figure 7 shows the outcomes for photic phenomena (glare and halo) indicating that they were similar in both groups. This is in agreement with Bova and Vita,6 who found that none of their patients described night dysphotopsia, halos, or glare, and Lesieur and Dupeyre,9 who indicated that there were no-spontaneous dysphotopsia complaints such as halos and glare in their sample of patients. Stodulka and Slovak5 reported that 6 eyes (16.7%) of 3 patients required a posterior Nd:YAG capsulotomy. Bova and Vita6 indicated that no patients had a posterior capsular opacification requiring capsulotomy with Nd:YAG laser. Bernabeu-Arias et al7 did not report adverse events associated with the lens and Lesieur and Dupeyre9 (based on the retrospective nature of their study) excluded patients that required Nd:YAG posterior capsulotomy due to PCO.
Future studies should increase the sample size and the follow-up of both groups of patients to properly analyze possible difference between lenses. In addition, other metrics such as wavefront aberrometry, quality of vision and/or patient satisfaction questionnaires should be included.
Conclusion
In summary, our results indicate that both IOLs provide good accuracy in postoperative refractive error, excellent visual acuity and contrast sensitivity for distance vision with similar photic phenomena, and, specifically, the Isopure IOL improved unaided intermediate vision performance.
Data Sharing Statement
Data of this trial are not available for sharing.
Funding
This trial was funded by Beaver-Visitec International, Inc. [BVI].
Disclosure
RET Ang obtained research grants from Acevision, Inc., Acufocus, Inc., Bausch&Lomb, Inc.; Beaver-Visitec International, Glaukos Corp., Ivantis, Inc., Johnson&Johnson Vision, and STAAR Surgical. P Stodulka obtained research grants from Bausch and Lomb. The authors report no other conflicts of interest in this work.
References
1. Cao K, Friedman DS, Jin S, et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64(5):647–658. doi:10.1016/j.survophthal.2019.02.012
2. Liu J, Dong Y, Wang Y. Efficacy and safety of extended depth of focus intraocular lenses in cataract surgery: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19(1):198. doi:10.1186/s12886-019-1204-0
3. Schallhorn JM, Pantanelli SM, Lin CC, et al. Multifocal and accommodating intraocular lenses for the treatment of presbyopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(10):1469–1482. doi:10.1016/j.ophtha.2021.03.013
4. Fernández Gutiérrez D, Barbero Briones S, Dorronso Díaz C, Marcos Celestino S. Refractive multifocal intraocular lens with optimised optical quality in a range of focus and method to produce it. Patent EP2941222A1; 2013.
5. Stodulka P, Slovak M. Visual performance of a polynomial extended depth of focus intraocular lens. Open J Ophthalmol. 2021;11(03):214–228. doi:10.4236/ojoph.2021.113017
6. Bova A, Vita S. Clinical and aberrometric evaluation of a new monofocal IOL with intermediate vision improvement. J Ophthalmol. 2022;2022:4119698. doi:10.1155/2022/4119698
7. Bernabeu-Arias G, Beckers S, Rincón-Rosales JL, Tañá-Rivero P, Bilbao-Calabuig R. Visual performance at different distances after implantation of an isofocal optic design intraocular lens. J Refract Surg. 2023;39(3):150–157. doi:10.3928/1081597X-20230124-02
8. Tomagova N, Elahi S, Vandekerckhove K. Clinical outcomes of a new non-diffractive extended depth-of-focus intraocular lens targeted for mini-monovision. Clin Ophthalmol. 2023;17:981–990. doi:10.2147/OPTH.S405267
9. Lesieur G, Dupeyre P. A comparative evaluation of three extended depth of focus intraocular lenses. Eur J Ophthalmol. 2023;5:11206721231154818. doi:10.1177/11206721231154818
10. Lundström M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282,811 cataract extractions reported to the European Registry of Quality Outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44(4):447–452. doi:10.1016/j.jcrs.2018.01.031
11. Lundström M, Barry P, Henry Y, Rosen P, Stenevi U. Visual outcome of cataract surgery; study from the European Registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2013;39(5):673–679. doi:10.1016/j.jcrs.2012.11.026
12. American National Standard Institute, Ophthalmics. Extended Depth of Focus Intraocular Lenses. ANSI Z80.35-2018. The Vision Council; 2018.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





