Back to Journals » Research and Reports in Urology » Volume 9
Prophylactic furosemide infusion decreasing early major postoperative renal dysfunction in on-pump adult cardiac surgery: a randomized clinical trial
Authors Fakhari S, Mirzaei Bavil F, Bilehjani E, Abolhasani S, Mirinazhad M, Naghipour B
Received 30 October 2016
Accepted for publication 16 December 2016
Published 19 January 2017 Volume 2017:9 Pages 5—13
DOI https://doi.org/10.2147/RRU.S126134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Solmaz Fakhari,1 Fariba Mirzaei Bavil,2 Eissa Bilehjani,1 Sona Abolhasani,3 Moussa Mirinazhad,2 Bahman Naghipour2
1Department of Anesthesiology, 2Department of Physiology, 3Tabriz University of Medical Sciences, Tabriz, Iran
Introduction: Acute renal dysfunction is a common complication of cardiac surgery. Furosemide is used in prevention, or treatment, of acute renal dysfunction. This study was conducted to evaluate the protective effects of intra- and early postoperative furosemide infusion on preventing acute renal dysfunction in elective adult cardiac surgery.
Methods: Eighty-one patients, candidates of elective cardiac surgery, were enrolled in this study in either the furosemide (n=41) or placebo (n=40) group. Furosemide (2 mg/h) or 0.9% saline was administered and continued up to 12 hours postoperatively. We measured serum creatinine (Scr) at preoperative and on the second and fifth postoperative days. Then calculated estimated glomerular filtration rate (eGFR) at these times. An increase in Scr of >0.5 mg/dL and/or >25%–50%, compared to preoperative values, was considered as acute kidney injury (AKI). In contrast, an increase in Scr by >50% and/or the need for hemodialysis was regarded as acute renal failure (ARF). At the end we compared the AKI or ARF incidence between the two groups.
Results: On the second and fifth postoperative days, Scr was lower, and the eGFR was higher in the furosemide group. AKI incidence was similar in the two groups (11 vs 12 cases; P-value 0.622); however, ARF rate was lower in furosemide group (1 vs 6 cases; P-value 0.044). During the study period, Scr was more stable in the furosemide group, however in the placebo group, Scr initially increased and then decreased to its preoperative value after a few days.
Conclusion: This study showed that intra- and early postoperative furosemide infusion has a renal protective effect in adult cardiac surgery with cardiopulmonary bypass. Although this protective effect cannot be discovered in mild renal dysfunctions, it apparently reduces the rate of the more severe renal dysfunctions. A more multidisciplinary strategy may be needed in reducing the milder renal damage.
Keywords: furosemide, postoperative acute kidney injury, postoperative acute renal failure, cardiac surgery, cardiopulmonary bypass
Introduction
Acute renal dysfunction occurs in up to 30% of the patients undergoing cardiac surgery.1,2 Renal failure requiring hemodialysis may arise in up to 1% of all patients. The development of postoperative acute renal failure (ARF) is independently associated with very high morbidity and mortality.3–5 It was also reported that mild renal injury might also increase postoperative complications and mortality.1,6 No universal definition method has been established thus far for classifying renal dysfunction. RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classification has been employed since 2004,7 however, owing to a number of difficulties RIFLE classification was dealing with, the modern Acute Kidney Injury Network (AKIN) classification method was introduced in 2007.8 Based on AKIN criteria, postoperative renal damage is classified in three stages: stage 1 is when postoperative serum creatinine (PScr) rises up to 1.5-fold or ≥0.3 mg/dL; stage 2 is when PScr rises up to 2-fold; stage 3 is when PScr rises up to 3-fold or ≥0.5 mg/dL if the baseline Scr>4.0 mg/dL. Patients receiving renal replacement therapy are considered to have met stage 3 criteria, irrespective of the stage in which they are.9
Diagnosis of acute kidney injury (AKI) requires an easily-measured criteria that can be widely applied to clinical practice. Several groups are working on developing valid biomarkers of kidney injury, which may be used in the future for diagnosis and prognosis of AKI; currently, however, there is not any sensitive or specific biomarker for kidney injury in clinical practice. Scr levels and changes in urine output are the most commonly applied measures of renal function.10,11 According to AKIN criteria, diagnostic criteria for acute kidney injury is currently defined as an absolute increase in Scr by ≥0.3 mg/dL, a percentage increase in Scr of ≥50% (1.5-fold from baseline), or a reduction in urine output (<0.5 mL/kg per hour for more than 6 hours). Recently, AKI and ARF terms have been commonly used for defining postoperative renal dysfunction. There is a common consensus that AKI increases the creatinine level more than 0.5 mg/dL or >25% of its preoperative level early postoperatively, furthermore, ARF is diagnosed when creatinine is boosted by 50–100%, or there is a need for artificial renal modalities.4
Many hemodynamic, inflammatory, and nephrotoxic factors are involved in the pathogenesis of the acute postoperative renal failure. Cardiopulmonary bypass (CPB) is a physiological extreme causing hemodynamic instability and inflammatory stimulation. Clinical studies have identified numerous risk factors involved in CPB, which induce renal injury, and have demonstrated strategies for renal protection; however, there is not any single strategy for renal protection. Similar to any other organ, the main key in renal protection is manipulating the supply/demand equation, ie, decreasing demand in parallel with increasing kidney supply. Many investigations have studied the factors possibly contributing to supply/demand equation. Theoretically, loop diuretics such as furosemide may be renal-protective by decreasing the energy/oxygen demand in kidneys (by blocking the K/Na/2Cl cotransporter process in the ascending limb of the loop of Henle). Nuutinen and Tuononen12 reported that the prophylactic use of furosemide infusion had a beneficial impact on renal function in cardiac surgery, other studies, however, could not support this finding.2,13 Only a few studies supported the efficiency of furosemide, and some evidence has even indicated its disadvantages.2,13–15 In a double-blind, randomized, controlled trial, Lassnigg et al2 reported that furosemide might even potentiate renal dysfunction after cardiac surgery. Recently, in a single-blind, randomized, controlled trial in patients who had undergone elective coronary artery bypass grafting (CABG) using CPB, Bayat et al16 reported that compared to placebo or fixed-dose furosemide groups, intermittent furosemide administration method may cause detrimental renal effects. In the present study, we investigated the renal protective effects of the intraoperative furosemide continuous infusion on adult patients undergoing open heart surgery using CPB.
Materials and methods
Type of study and patients
This study was a double-blind, randomized, clinical trial, registered in the Iranian Registry of Clinical Trials with registration number of IRCT138706091127N1 (www.irct.ir). The sampling was performed through consecutive sampling method. Regarding the wide range and high prevalence of the AKI after on-pump cardiac surgery (1–30%), the average prevalence of 15%, the study strength of 0.8, and the confidence level of 0.95%, the sample size was calculated at 38 patients for each group. Considering a 10% loss of follow-up rate, we planned to study 82 patients in furosemide (n=41) and placebo (n=41) groups.
Inclusion and exclusion criteria
All the patients, who over 18 years old and were candidates for elective cardiac surgery using CPB in Tabriz University of Medical Sciences Hospital (Tabriz, Iran), qualified to be included in this study.
Patients with the following were not permitted to participate in the study: emergency or redo surgeries; preoperative Scr level of >1.5 mg/dL; any previous non-cardiac diseases (diabetes mellitus, respiratory, neurologic, etc); patients who had been exposed to intravenous radiocontrast media during the previous week; left ventricle ejection fraction of <0.4; history of unstable hemodynamic in the previous month; preoperative anemia (hemoglobin <10 g/dL); patients who had received any extra doses of furosemide pre-, intra-, or postoperatively.
Study design
In order to investigate the effect of intraoperative furosemide infusion on renal protection from CPB-induced damage, we studied 82 adult patients who were candidates for elective cardiac surgery using CPB. The study was conducted in a 6-month period from May to November 2014. Written informed consent was obtained from all of the patients to participate in this study. The patients were allocated into either the furosemide (n=41) or the placebo (n=41) group, according to a randomization list that was prepared, using online software at a 1:1 ratio. The list was coded (A or B) and preprinted in sealed envelope packets. All except one of the authors were blinded to the treatment solution for every patient during the study. For all the patients, the blood urea nitrogen (BUN) and creatinine concentrations were measured on the day before as well as the morning of the operation day, and the average values were calculated. Preoperative estimated glomerular filtration rate (eGFR) was calculated through the Cockcroft-Gault formula. Premedication was performed, using oral diazepam (10 mg) and intramuscular morphine (0.1 mg/kg) plus promethazine (0.5 mg/kg). Anesthesia was induced with 5–7 μg/kg of fentanyl, 10–15 μg/kg of midazolam, and 0.15 μg/kg of cisatracurium and was maintained with the infusion of 5–10 μg/kg/h of fentanyl, 10 μg/kg/h of midazolam, and 100μg/kg/h of cisatracurium. In all of the patients, temperature, invasive arterial blood and central venous pressure monitoring, end-tidal capnography, pulse oximetry, electrocardiogram monitoring, and arterial blood gas analysis were performed intra- and postoperatively. Anesthesia, CPB, and surgery were managed as routine local practice and without any special intervention. After the induction of anesthesia, the responsible coworker started the infusion of the treatment solution at the rate of 2 mL/h and continued up to 12 hours postoperatively. This treatment solution contained 50 mg of furosemide in 50 mL of saline 0.9% or pure saline 0.9% in furosemide or placebo groups, respectively. At the end of the surgery, all patients were transferred to the postcardiac surgery intensive care unit (ICU), while they were still anesthetized and intubated, and the monitoring was continuing in almost the same way as the operation room. On the early morning of the second and fifth postoperative days, Scr and BUN concentration were measured, and eGFR was calculated again. An increase in Scr of >0.5 mg/dL and/or >25%–50%, compared to preoperative values, was considered as AKI. In contrast, an increase in Scr of >50% and/or the need for hemodialysis was regarded as ARF.
The collected data were as follows: demographics, intra- and postoperative arterial and central venous blood pressure, surgical, CPB, and aortic cross-clamp times, intraoperative and first postoperative 24-hour urinary output, the data on the needs for inotropic agents (for more than 60 minutes) and diuretics, bleeding, complications, ventilator support, ICU stay duration, and eGFR. At the end of the study, serum BUN and creatinine, eGFR changes, AKI or ARF incidence, and postoperative complications were compared between the two groups.
Ethical considerations
This study was conducted after achieving the approval of the Ethics Committee of Tabriz University of Medical Sciences, providing the patients with a full explanation of the study, as well as obtaining written informed consent to participate in the study, and filling the consent form by the subjects. The patients were allowed to leave the study at any point. It must be noted that the consumption of different doses of furosemide in this study was completely routine and had no unusual adverse effects.
Statistical analysis
Statistical analysis was performed, using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The data were presented by descriptive methods (frequency, percentage, mean ± standard deviation). The categorical data were compared between the two groups through chi-square test (Fisher’s exact test if n<5). The normality of distribution was tested by Kolmogorov-Smirnov test. In addition, the two independent-sample t-test was employed for comparing the parametric variables between the two groups. The paired sample t-test was applied to analyze the serum BUN and creatinine and eGFR changes in each group. P-values less than 0.05 were considered statistically significant.
Results
Of the 82 patients who enrolled in this study, one patient was excluded from the study (because of problems in urine collection), and the data obtained from 81 patients (41 patients in the furosemide group and 40 patients in the placebo group) were analyzed and compared between the two groups (Figure 1). There were more male participants than female ones (58% vs 42%).
  | Figure 1 Flow diagram of the study. |
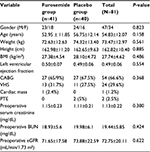
Table 1 shows the demographic and preoperative characteristics of the patients studied as furosemide and placebo groups. As shown in Table 1, the two groups had the same demographic data and distribution of the various types of surgery. Preoperative left ventricular ejection fraction was the same in both groups, and in general, the CABG surgery was the most common type of surgery (66.6%). Preoperative eGFR in furosemide and placebo groups was similar (71.65 ± 17.58 mL/min/1.73m2 vs 73.88 ± 22.59 mL/min/1.73m2), and preoperative serum creatinine (Scr) and BUN concentrations were the same in both groups.
Table 2 presents the intra- and postoperative characteristics of the studied patient groups. In 57 patients (70.37%), the surgery was performed with aortic crass clamping that was similar in furosemide and placebo groups (68.3% vs 72.5%, P-value 0.432). Aortic crass clamp, CPB, and surgical duration were not different between groups. Mannitol usage was the same in the two groups and was utilized in 72.8% of the patients during CPB. Intraoperative needs for inotropic agents (for more than 60 minutes) and blood products were the same in both groups. In the postoperative period, the need for inotropic agents (for more than 60 minutes), blood products, ventilatory support, complication rate, and ICU stay were the same in the two groups. Postoperative complications were observed in six patients and were atrial fibrillation (AF) (3 cases), myocardial infarction (MI) (2 cases), and pulmonary edema (1 case). No patient required additional diuretic therapy during intraoperative or ICU stay period.
The urinary output at pre-, on, and postpump periods or throughout the intra-operative period was the same in both groups, however, on the first postoperative day, it was significantly higher in the patients in the furosemide group compared to the placebo group (2.25±0.66 ml/kg/h vs 1.89±0.59 ml/kg/h, P-value 0.012). Data analysis of Scr, BUN, and eGFR on the second and the fifth postoperative days showed that compared to the patients in the placebo group, postoperative Scr and eGFR were significantly lower in the patients in the furosemide group, however, there was no difference regarding the postoperative BUN between the two groups. The rates of AKI and ARF incidence are given in Table 2 and Figure 2. The rate of AKI incidence was almost the same in both furosemide and placebo groups (11 cases [26.8%] vs 12 cases [25%], respectively), however, the rate of ARF incidence was higher in the placebo group (6 [15%] cases vs 1 case [2.4%], P-value 0.044). None of the patients required any kind of dialysis or renal replacement therapy up to their discharge from the hospital.
The trend of changes in Scr concentration, BUN, eGFR and hourly urinary output at various periods of the study are demonstrated in Figure 3. Scr was stable in the furosemide group during the study, however, it increased in the placebo group on the second postoperative day and then retreated to its preoperative value again on the fifth postoperative day. Compared to preoperative value, BUN decreased in both groups postoperatively. In spite of the furosemide group (eGFR increased postoperatively), eGFR decreased in the placebo group postoperatively in a progressive manner. Table 3 depicts the postoperative AKI and ARF incidence in relation to intra- and postoperative usage of inotropic agents, blood products, and mannitol during CPB. There was no correlation between renal complications and usage of the inotropic agents, blood products, and mannitol.
  | Figure 3 The changes in (A) serum creatinine concentration, (B) blood urea nitrogen (BUN), (C) estimated glomerular filtration rate (eGFR), and (D) hourly urinary output, in various periods. |
Discussion
As in any major surgery, many factors may affect the postoperative renal function. In the present study, we tried to manage these confounding factors. Data analysis showed that the two groups had the same background properties (demographic and preoperative characteristics). This may help to strengthen the findings validity; however, many types of operation could negatively affect the validity of the results. Since Nuutinen and Tuononens’ study in 1976,12 many other studies have been performed to define the furosemide prophylactic or therapeutic role in the renal injury of postcardiac surgery. Nuutinen et al reported that prophylactic use of furosemide infusion for more than 60 minutes had a beneficial effect on renal function, however, this regime in a period shorter than 60 minutes may be harmful owing to the increased excretion of water, sodium, and potassium. This may be related to the severity of the stresses imposed on kidneys in cardiac surgery, especially with CPB.13 We used furosemide infusion for up to 12 hours postoperatively, and our study supported the furosemide renal protective effect occurrence. Intraoperative diuretics induce diuresis, which possibly causes postoperative hypovolemia and augments the postoperative renal dysfunction. It is a common practice for anesthesiologists to induce diuresis and administer volume expanders liberally during the perioperative period. Furosemide-induced diuresis can be considered as a method to decrease kidney oxygen demand. Compared to normal daily diuresis (1–1.5 mL/kg/h), intra- and postoperative diuresis in our study were higher (up to 11.5 mL/kg/h). This furosemide-induced diuresis along with liberal volume administration (maintaining kidney supply) may be the main reasons for furosemide renal protective effect in our study. The dehydrated patients’ response to furosemide may be blunted.17 Our study was performed on patients with normal preoperative renal function, probably they had enough renal reserve for carrying surgical stress. This can account for the similarities in the majority of intraoperative and postoperative patients’ properties (needs for inotropic agents, blood products, ventilator support, complication rate, and ICU stay). The same AKI rate may point out the fact that patients with normal renal function can easily tolerate CPB-induced stress and do not require any extra hemodynamic support, however, the higher ARF rate in the placebo group may indicate that some patients with undefined low preoperative renal reserve or complicated surgery may need other renal protective modalities such as furosemide. The same urinary output at prepump, on-pump, and postpump periods, or totally intraoperative period may point out the fact that the renal protective effect of furosemide is not solely due to induced diuresis; however, this effect is presented as steady large postoperative diuresis or low creatinine in the study group. Our results are not in agreement with Lassnigg et al who reported that preoperative furosemide might even potentiate renal dysfunction after cardiac surgery.2 Compared to Lassnigg et al study, we utilized a large dose of furosemide (approximately 2-fold) with apparent induced intraoperative diuresis (in his study, Lassnigg et al did not measure the intraoperative urine output and excluded patients with large postoperative diuresis). Due to the supply/demand balance, it is logical to state that in stressful conditions, it is better to induce good diuresis with liberal volume replacement.
In our study, the rate of AKI incidence was the same in both furosemide and placebo groups; however, ARF incidence was significantly lower in the placebo group. It is predicted that similar to many other conditions, the total body metabolic rate increases during cardiac surgery. Thus, in patients with limited renal function, blood creatinine and BUN may increase. In our study, creatinine concentration was stable in the furosemide group during the study; nevertheless, in the placebo group, it increased on the second postoperative day and then declined to its preoperative value again on the fifth postoperative day. This may reflect the renal protective effect of furosemide that helps kidneys tolerate the high metabolic rate easily. As any other organ, it is important to manage kidney supply/demand preoperatively. By blocking the luminal K/Na/2Cl co-transporter type 2 in the thick ascending limb of the loop of Henle cells, furosemide may decrease the demand in supply/demand equation. Approximately 98% of furosemide bounds to plasma proteins and the GFR of the drug is very low.18,19 Hence, for exerting this effect, it is necessary that furosemide should be actively secreted from the proximal tubule via the organic anion transporter-1.20,21 In point of fact, the response to furosemide is directly related to its urinary concentrations.20 At severe renal injury, this transporting capacity of proximal tubule may be affected extensively; moreover, at such patient the furosemide may not be able to reach to its action site. Accordingly, in patients with established ARF, loop diuretics are clearly ineffective. In such patients the nephrotoxic effect of furosemide may cause additional renal injury,22,23 but it may exert a renal protective effect if given before a potential renal insult.24–26 In their second meta-analysis (the review of 11 published studies), Ho and Power reported that current evidence could not support the efficacy of furosemide in improving renal function or mortality.21 They concluded that patients with mild AKI would respond to furosemide better than when they have severe AKI cases. In other words, this conclusion may support our finding, the renal protective effect of furosemide. In a recent systematic review Ahmed et al27 concluded that after AKI treatment with furosemide, the mortality, need for dialysis, and length of hospital stay are not reduced, and renal recovery is not improved. In a retrospective study Iyem et al28 reported the incidence of ARF in 185 out of 2,380 patients after elective open heart surgeries without primary renal failure. Studies have shown that if postoperative dialysis is required, the complications and mortality will strictly increase.5,8 In a review study, Gandhi et al29 noted that the existing studies revealed different results for the effect of furosemide on the need for post-cardiac surgery dialysis. The rate of ARF after cardiac surgery in Sirvinskas et al30 study was 10.6%, but none of the patients needed dialysis. Supporting Sirvinskas et als study, we found that none of our patients required any kind of dialysis or renal replacement therapy. This may support the renal protective effect of furosemide.28
Compared to its preoperative value, BUN decreased in both groups postoperatively in our study. However, we did not measure the fluid intake/output balance, BUN changes may be due to the common practice of liberal and over administration of fluids during the surgery that induces postoperative diuresis.31 None of our studied patients required any kind of dialysis or renal replacement therapy. This may again support the renal protective effect of furosemide. However, there are reports that furosemide is unable to affect mortality rate, in our study, there was not any case of mortality; thus, we could not discuss that.21 In a study which was similar to ours, Mahesh et al13 studied the preventive effect of furosemide infusion during cardiac surgery in high-risk patients (with low ejection fraction, high preoperative creatinine, and comorbid diseases). They concluded that although furosemide increases urinary output, it does not decrease postoperative ARF and AKI.13 The occurrence of many studies with contradictory results about furosemide may be due to the fact that the organ protection during cardiac surgery requires a multidisciplinary strategy, especially in high-risk patients. In addition, AKI is often only one part of a multi-system disease. In our study, furosemide had the capability of reducing the most severe renal damage, however, it is our conception that reducing the milder renal damage necessitates a more multidisciplinary strategy effort. Indeed, any aspects of surgical and anesthetic management (hemodynamic management, hemoglobin concentration, fluid balance, vasopressors, vasodilators, temperature, etc) can affect the patient’s final outcome.
In the present study, furosemide caused early postoperative diuresis. Mahesh et al reported the same finding.13 Kunt et al32 reported a higher postoperative urinary output in continuous versus intermittent furosemide administration during elective CABG, however, in analyzing some of the published studies, Gandhi et al observed no difference between the two methods due to the total urinary output.29 Kunt et al also concluded that furosemide appears to be effective in decreasing the need for renal replacement therapy.32 Postoperative diuresis may be useful in fluid balance and mechanical ventilation strategy.21 In our study, the time required for ventilatory support was the same in both groups; this may be owing to the fact that our patients were not high-risk patients. In our study the difference in the postoperative AKI prevalence in two groups was only 1.8%, this means that statistically for discovering this small difference between two groups we have to plan a more large size sample study.
Conclusion
This study supported the finding that furosemide infusion (during intra- and early postoperative period) has a renal protective effect during adult cardiac surgery using CPB. This protective effect presented itself by maintaining urinary output, eGFR, and Scr at normal value. However, it could not be discovered in mild renal dysfunctions (AKI), but it apparently reduced the rate of more severe renal dysfunctions (ARF). This may be due to the fact that reducing the milder renal damage necessitates a more multidisciplinary strategy effort.
Disclosure
The authors report no conflicts of interest in this work.
References
Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–1605. | ||
Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11(1):97–104. | ||
Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–348. | ||
Leacche M, Rawn JD, Mihaljevic T, et al. Outcomes in patients with normal serum creatinine and with artificial renal support for acute renal failure developing after coronary artery bypass grafting. Am J Cardiol. 2004;93(3):353–356. | ||
Ostermann M, Taube D, Morgan C, Evans T. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med. 2000;26(5):565–571. | ||
Bove T, Monaco F, Covello R, Zangrillo A. Acute renal failure and cardiac surgery. HSR proceedings in intensive care & cardiovascular anesthesia. HSR Proceedings. 2009;1(3):13. | ||
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit care. 2004;8(4):R204–212. | ||
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):1. | ||
Olivero JJ, Olivero JJ, Nguyen PT, Kagan A. Acute kidney injury after cardiovascular surgery: an overview. Methodist DeBakey Cardiovasc J. 2012;8(3):31–6. | ||
Cruz DN, Goh CY, Haase-Fielitz A, Ronco C, Haase M. Early biomarkers of renal injury. Congest Heart Fail. 2010;16(s1):S25–S31. | ||
Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. | ||
Nuutinen L, Tuononen S. The effect of furosemide on renal blood flow and renal tissue oxygen tension in dogs. Ann Chir Gynaecol. 1976; 65(4):272–276. | ||
Mahesh B, Yim B, Robson D, Pillai R, Ratnatunga C, Pigott D. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial. Eur J Cardiothorac Surg. 2008;33(3):370–376. | ||
Gulbis BE, Spencer AP. Efficacy and safety of a furosemide continuous infusion following cardiac surgery. Ann Pharmacother. 2006;40(10):1797–1803. | ||
Levi T, Rocha M, Almeida D, et al. Furosemide is associated with acute kidney injury in critically ill patients. Braz J Med Biol Res. 2012;45(9):827–833. | ||
Bayat F, Faritous Z, Aghdaei N, Dabbagh A. A study of the efficacy of furosemide as a prophylaxis of acute renal failure in coronary artery bypass grafting patients: A clinical trial. ARYA Atheroscler. 2015;11(3):173–178. | ||
Gerber JG, Nies AS. Furosemide-induced vasodilation: importance of the state of hydration and filtration. Kidney Int. 1980;18(4):454–459. | ||
Brater DC. Resistance to diuretics. Drugs. 1981;22(6):477–494. | ||
Pichette V, Geadah D, du Souich P. Role of plasma protein binding on renal metabolism and dynamics of furosemide in the rabbit. Durg Metab Dispos. 1999;27(1):81–85. | ||
Ali SS, Sharma PK, Garg VK, Singh AK, Mondal SC. The target specific transporter and current status of diuretics as antihypertensive. Fundam Clin Pharmacol. 2012;26(2):175–179. | ||
Ho K, Power B. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283–293. | ||
Brown C, Ogg C, Cameron J. High dose frusemide in acute renal failure: a controlled trial. Clin Nephrol. 1981;15(2):90–96. | ||
Shilliday I, Quinn K, Allison M. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997;12(12):2592–2596. | ||
Heyman SN, Brezis M, Greenfeld Z, Rosen S. Protective role of furosemide and saline in radiocontrast-induced acute renal failure in the rat. Am J Kidney Dis. 1989;14(5):377–385. | ||
Brezis M, Rosen S, Silva P, Epstein FH. Transport activity modifies thick ascending limb damage in the isolated perfused kidney. Kidney Int. 1984;25(1):65–72. | ||
Driscoll DF, Pinson CW, Jenkins RL, Bistrian BR. Potential protective effects of furosemide against early renal injury in liver transplant patients receiving cyclosporine-A. Crit Care Med. 1989;17(12):1341–1343. | ||
Ahmed U, Iqbal H, Akbar S. Furosemide in acute kidney injury – a vexed issue. Austin J Nephrol Hypertens. 2014;1(5):1026. | ||
Iyem H, Tavli M, Akcicek F, Bueket S. Importance of early dialysis for acute renal failure after an open-heart surgery. Hemodial Int. 2009;13(1):55–61. | ||
Gandhi A, Husain M, Salhiyyah K, Raja SG. Does perioperative furosemide usage reduce the need for renal replacement therapy in cardiac surgery patients? Interact cardiovasc Thorac Surg. 2012; 15(4): 750–755. | ||
Sirvinskas E, Andrejaitiene J, Raliene L, et al. Cardiopulmonary bypass management and acute renal failure: risk factors and prognosis. Perfusion. 2008;23(6):323–327. | ||
Doherty M, Buggy D. Intraoperative fluids: how much is too much? Br J Anaesth. 2012;109(1):69–79. | ||
Kunt AT, Akgün S, Atalan N, Bitir N, Arsan S. Furosemide infusion prevents the requirement of renal replacement therapy after cardiac surgery. Anadolu Kardiyol Derg. 2009;9(6):499–504. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.