Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Propensity score matching comparison of laparoscopic versus open surgery for rectal cancer in a middle-income country: short-term outcomes and cost analysis
Authors Tayar DO , Ribeiro Jr U , Cecconello I , Magalhães TM , Simões CM , Auler Jr JOC
Received 11 May 2018
Accepted for publication 19 June 2018
Published 12 September 2018 Volume 2018:10 Pages 521—527
DOI https://doi.org/10.2147/CEOR.S173718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Daiane Oliveira Tayar,1 Ulysses Ribeiro Jr,2 Ivan Cecconello,2 Tiago M Magalhães,3 Claudia M Simões,4 José Otávio C Auler Jr1
1Department of Anesthesia and Critical Care, University of São Paulo, Faculty of Medicine, São Paulo, Brazil; 2Department of Gastroenterology, University of São Paulo, Faculty of Medicine, São Paulo, Brazil; 3Department of Statistics, Institute of Exact Sciences, Federal University of Juiz de Fora, Juiz de Fora, Brazil; 4Department of Anesthesia and Critical Care, Cancer Institute of the State of São Paulo, São Paulo, Brazil
Background: Laparoscopic surgery for rectal cancer is associated with improved postoperative outcomes compared to open surgery; however, economic studies have yielded contradictory results. The aim of this study was to compare the clinical and economic outcomes of laparoscopic versus open surgery for patients with rectal cancer.
Methods: Propensity score matching analysis was performed in a retrospective cohort of patients who underwent elective low anterior resection for rectal cancer treatment by laparoscopic and open surgery in a single Brazilian cancer center. Matched covariates included age, gender, body mass index, pTNM stage, American Society of Anesthesiologists score, type of anesthesia, neoadjuvant chemoradiotherapy, and interval between neoadjuvant chemoradiotherapy and index surgery. The clinical and economic outcomes were evaluated. The follow-up period was within 30 days of the index procedure. The clinical outcomes were reoperation, postoperative complications, operative time, length of stay in the intensive care unit, and postoperative hospital stay. For economic outcomes, a cost analysis was used to compare the costs.
Results: Initially, 220 patients were evaluated. After propensity score matching, 100 patients were included in the analysis (50 patients in the open surgery group and 50 patients in the laparoscopic surgery group). There were no differences in patients’ baseline characteristics. Operative time was longer for laparoscopic surgery (247 minutes vs 285 minutes, P=0.006). There were no significant differences in other clinical outcomes. The hospital costs were similar between the two groups (Brazilian reais 21,233.15 vs Brazilian reais 21,529.28, P=0.115), although the intraoperative costs were higher for laparoscopic surgery, mainly owing to the surgical devices and the theater-related costs. The postoperative costs were lower for laparoscopic surgery, owing to lower intensive care unit, ward, and reoperation costs.
Conclusion: Laparoscopic surgery for rectal cancer is not costlier than open surgery from the health care provider’s perspective, since the intraoperative costs were offset by lower postoperative costs. Open surgery tends to have a longer length of stay.
Keywords: rectal cancer, laparoscopy, open surgery, propensity score matching, health care costs, cost analysis
Introduction
Colorectal cancer is the cancer with the third highest incidence in Brazil for both men and women, with approximately 34,000 new cases expected for 2016, which represents 8.1% of total estimated newly diagnosed cancer cases.1
Surgical treatment is indicated for most rectal cancer cases. In Brazil, the standard and authorized surgical treatment in the public health care system is open resection, although reference centers also perform laparoscopic surgery.
Several studies have evaluated the differences between surgical approaches for rectal cancer.2–11 There seems to be a consensus regarding the association between the use of laparoscopy and lower hospital stay owing to faster recovery of peristalsis and earlier resumption of oral diet.2–9 However, for other short-term outcomes, the studies remain controversial. While some show the superiority of the laparoscopic approach, including lower hospital readmission and mortality,2–7,12 others have shown that the two techniques present similar results regarding complications.8,9
Another factor indicated as a determinant of short-term postoperative outcome is the operative time, which directly influences complications such as postoperative ileus and surgical site infections,13 and is an independent risk factor for postoperative complications, even for laparoscopic surgery.14,15 The surgeon’s experience is a key factor in shortening operative time for laparoscopic surgeries and decreasing postoperative complications associated with longer operative time.12,17–19
While laparoscopy can reduce costs because it promotes faster nutritional and immune recovery conditions and decreases the inflammatory response to surgical stress,5,6,16 the intraoperative technical difficulty may require longer operative time.12,17–19 Moreover, the use of laparoscopic medical devices may further increase the costs of surgical treatment.20
Thus, laparoscopic surgery may be more or less costly than open surgery. On one hand, it can increase costs relating to operative time and medical devices. On the other hand, laparoscopic surgery may decrease costs relating to postoperative hospital stay and postoperative complications.16,20 The cost-effectiveness may be altered by the rate of inclusion and implementation of the technique in the country, as well as the volume of procedures performed by surgeons and institutions.
In this context, a cost analysis was performed from the health care provider’s perspective to compare the costs of surgical treatment of laparoscopic versus open surgery for rectal cancer at a high-volume, single-center institution.
Thus, the aim of this study was to compare laparoscopic and open low anterior resection (LAR) using propensity score matching (PSM) analysis. For the economic evaluation, a cost analysis was performed to compare the costs of the two techniques in relation to the short-term outcome.
Materials and methods
After obtaining approval from the local institutional Ethics Review Board of the Faculty of Medicine of University of São Paulo (FMUSP), a retrospective review of 220 consecutive patients who underwent an operation for rectal cancer between 2008 and 2012 at the Cancer Institute of the State of São Paulo (Instituto do Cancer do Estado de São Paulo [ICESP] HC-FMUSP) was conducted. The intraoperative and 30-day postoperative data were obtained by medical records review between 2013 and 2014. As all the data analyzed were obtained from the routine care of the patients and were made anonymous by the researchers, no informed consent was required.
Inclusion criteria
To be included in the study analysis, patients had to meet the following criteria: adenocarcinoma of the rectum confirmed by histopathologic examination; elective LAR with no additional procedures; and experienced digestive surgeons performed all resections. During the study period, 405 rectal cancer procedures were performed by the digestive surgeons, including open and laparoscopic procedures. Patients who underwent laparoscopy with extracorporeal anastomosis were excluded from the study.
Preoperative assessment
All patients were evaluated by clinical and proctologic examination, colonoscopy with tumor biopsy, abdominal and thoracic computed tomography scan, and pelvic magnetic resonance imaging to determine the preoperative stage.
Data collection
The following baseline characteristics were assessed for each patient: age, gender, body mass index (BMI), neoadjuvant chemoradiotherapy (CRT), interval between neoadjuvant CRT and surgery, American Society of Anesthesiologists (ASA) physical status score, type of anesthesia, and operative approach.
The following outcomes were evaluated: operative time, 30-day mortality after the procedure, length of stay in the intensive care unit (ICU) and on the ward after surgery, and 30-day postoperative complications according to the Clavien–Dindo classification.21
Cost estimation
A cost analysis was performed from a health care provider’s perspective. Costs were obtained in Brazilian reais (BRL), provided by the hospital costs report of August 2011. These include the costs of professional fees, prescription drugs, imaging, and laboratory tests. Cost estimations were based on the reference costs per hour of surgery, ICU stay per day, hospital stay per day, and surgical device costs for each type of procedure. All costs were inflation adjusted to the value of Brazilian reais in February 2016. The follow-up period for inpatient costs was 30 days from the index operation and, in the case of reoperation, the costs related to the operative time, the surgical devices authorized by the public health care system, and the ICU and hospital stay were added to the total inpatient costs.
The costs associated with medical devices were calculated manually. For open LAR, the following surgical devices authorized by the Brazilian public health care system were considered: circular stapler, linear stapler, and linear cutter stapler. For laparoscopic LAR procedures, the following surgical devices were considered: one Veress needle, two trocars, one flexible endoscopic stapler, one circular stapler, and one harmonic scalpel.
The estimated cost for a ward bed was BRL 1,150 per day, that for an ICU bed was BRL 3,856 per day, and the theater cost was BRL 1,026 per hour. Considering an exchange rate of BRL 1= USD 0.27, we obtained the following costs in dollars: the cost for a ward bed was USD 310 per day, that for an ICU bed was USD 1,041 per day, and the theater cost was USD 277 per hour. For the costs related to surgical devices for open and laparoscopic surgery, the prices paid by ICESP HC-FMUSP in 2016 were considered.
Statistical analysis
All patient data were collected on a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA) and were divided into open and laparoscopic groups according to the surgical approach. To minimize selection bias derived from a retrospective observational study, PSM was performed to generate two groups considering the following covariates: age, gender, BMI, histopathologic TNM stage, ASA physical status score, type of anesthesia, neoadjuvant CRT, and interval between neoadjuvant CRT and index surgery.22 The two matched groups were compared regarding clinical and economic outcomes. Logistic regression for the abovementioned covariates, considering nearest-neighbor one-to-one matching, were performed to determine propensity scores. The patient characteristics for each group were analyzed to determine group parity. Fisher’s exact test was used for categorical variables, and the Mann–Whitney U test, Brunner–Munzel t test, or Student’s t test was used for continuous variables, as appropriate. All analyses were performed using the statistical software package R version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria). A P-value less than 0.05 was considered statistically significant.
Patient and public involvement
Given the retrospective nature of this study, no patients were involved during this study.
Results
Patient baseline characteristics
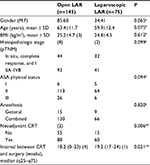
Between December 2008 and December 2012, a total of 220 patients underwent laparoscopic LAR (n=75) or open LAR (n=145). The patient baseline characteristics are shown in Table 1. There were significant differences between the two groups regarding use of neoadjuvant CRT rate and interval in the weeks between CRT and surgery, although the histopathologic TNM stages were similar between the groups.
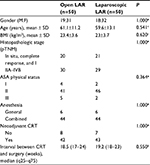
After PSM, two samples of 50 patients each were obtained. An analysis of the baseline characteristics was performed to evaluate the accuracy of the method, as shown in Table 2. There were no significant differences between the two matched groups regarding patient baseline characteristics.
Clinical outcomes
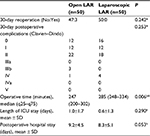
Clinical outcomes are shown in Table 3. There were no significant differences in patient morbidity. The group undergoing laparoscopic LAR had a longer operative time than the group undergoing open LAR (laparoscopic LAR 285 [248–334] minutes vs open LAR 247 [200–302] minutes, P=0.006). There were four reoperations among three patients in the open LAR group and no reoperations in the laparoscopic LAR group.
Economic outcomes
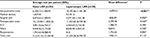
The cost analysis results for the two groups of patients after PSM are shown in Table 4. Regarding inpatient costs, the intraoperative costs were significantly higher for laparoscopic surgery (open LAR BRL 6,100.15 vs laparoscopic LAR BRL 10,195.66, P<0.001) owing to increased operative time costs (open LAR BRL 4,470.65 vs laparoscopic LAR BRL 5,077.54, P=0.006) and the costs of the surgical devices used.
The postoperative costs for patients undergoing laparoscopic surgery were significantly lower compared to those for patients undergoing open surgery (open LAR BRL 15,133.00 vs laparoscopic LAR BRL 11,333.62, P=0.020) owing to decreased costs associated with the shorter ICU and hospital stay and also reoperation costs, although these differences were not significant.
Therefore, a balance was observed between intraoperative and postoperative costs, and the overall hospital costs did not differ significantly between laparoscopy and open surgery (open LAR BRL 21,233.15 vs laparoscopic LAR BRL 21,529.28, P=0.115).
Discussion
This retrospective analysis at a high-volume oncologic hospital showed no significant differences in clinical outcomes between the laparoscopic and open surgery groups during the period evaluated. However, the faster recovery among patients undergoing laparoscopies had a significant impact on the overall postoperative costs, offsetting the intraoperative costs associated with the surgical devices and the increased operative time for these procedures. These economic results are comparable with those of a study performed in Japan that compared open and laparoscopic colorectal procedures for colorectal cancer.23
A cost analysis was conducted to evaluate economically the use of laparoscopy in LAR compared to the treatment currently authorized by the Brazilian public health care system. Considering the current Brazilian scenario, the study shows that laparoscopic LAR does not result in higher total costs than open LAR.
This reduction in postoperative costs can be attributed to the advantages of minimally invasive surgery related to faster patient recovery. One possible explanation for the improved outcome in the postoperative period is the fact that laparoscopic surgery results in a less intense inflammatory response compared to open surgery, enabling faster recovery of peristaltic movements and decreased hospital stay.5,7,24,25
Although the outcomes in the postoperative period were better for laparoscopic procedures, most studies have shown that the laparoscopic procedure for rectal cancer is costlier than the open procedure.12,20,26–28 This increase is mainly explained by the higher cost of laparoscopic surgical devices and the longer operative time taken to perform laparoscopic procedures.
One study compared the costs of rectal laparoscopic surgery versus open surgery in a large randomized controlled clinical trial, the COLOR (COlorectal cancer Laparoscopic or Open Resection) II trial, using cost-minimization analysis.28 The study found that laparoscopic surgery is significantly costlier than open surgery in the short and medium term. However, in the long term, the costs are equal from a societal perspective.
A cost-effectiveness analysis comparing open and laparoscopic resection for rectal cancer showed that laparoscopy provides cost savings compared to open surgery, without showing differences in quality-adjusted life-years.29 However, a randomized controlled trial (the COREAN trial) showed that scores on quality-of-life questionnaires, including gastrointestinal and defecation problems, were significantly higher for laparoscopic surgery in the first 3 months after surgery compared to open surgery.26
Several studies have shown that, as the surgeon becomes more experienced, the operative time of laparoscopic surgery progressively decreases without affecting short- and long-term outcomes.17,18 A study conducted to determine the costs for the two surgical approaches for rectal cancer showed that laparoscopy had lower total costs compared to open surgery, and an important result differentiating this study from others was the shorter operative time of laparoscopic surgery compared to open surgery.2 This result may indicate that, as the operative time of the laparoscopic procedure decreases, the cost of laparoscopic approaches leads to cost savings compared to the open technique.
The combination of the laparoscopic technique with enhanced recovery programs, such as enhanced recovery after surgery (ERAS), could act synergistically to improve outcomes in the postoperative period, resulting in an additional contribution to cost savings.16,30,31
Regarding the cost of surgical devices as a proportion of theater costs, the weight of the cost of surgical devices to total intraoperative costs is higher in our study compared with a study conducted in England.32 In England, the cost of surgical devices corresponds to 9.9% and 17.6% of the total intraoperative cost for open and laparoscopic surgery, respectively, whereas in Brazil this percentage increases to 26.7% and 54.1%. The difference observed in the costs of surgical devices out of the total intraoperative costs can be attributed to the different rates per minute of surgery used in the two studies: the rate per minute in the English study was €26, six times higher than the rate per minute at the hospital evaluated in our study (exchange rate at 03/09/2016). However, in the English study,32 all anastomoses were performed manually, which can decrease considerably the weight of the surgical devices cost in the total intraoperative costs.
Strengths and limitations
Although there are publications comparing colorectal laparoscopic and open procedures regarding costs and clinical outcomes in the literature, data are still scarce in middle-income countries, where the financial burden might pose greater challenges, considering the stronger resource constraints in such countries. To our knowledge, this is the first study to compare clinical and economic outcomes for patients undergoing laparoscopic versus open LAR using PSM analysis in Latin America. PSM is currently the best statistical method to evaluate retrospective non-randomized controlled studies.22
The present study has several limitations. Although PSM is an accepted tool to decrease selection bias, the data were analyzed retrospectively. Some relevant factors for adequate patient matching may not have been considered. In addition, patients undergoing laparoscopic surgery with extracorporeal anastomosis were not included in the study because these surgeries were converted not by complications but by technical issues related to the surgeon’s experience with intracorporeal anastomosis. This factor may have resulted in some type of bias and confounding.
Although data including hospital stay, ICU stay, operative time, and data related to reoperation and complications were accurately measured, the costs were estimated based on the theater cost per hour, cost of ICU stay per day, and cost of ward bed stay per day. A detailed description of all direct costs related to the procedure and the hospital stay would be more accurate but was not possible to obtain retrospectively. Moreover, indirect and intangible costs, including loss of productivity, were not evaluated in the present study.
Finally, this study was performed at a single Brazilian institution, and thus, the results may not be generalizable to other institutions.
Conclusion
Taking into consideration that both techniques, open LAR and laparoscopic LAR, are equivalent in clinical outcomes, this cost analysis shows that there is no difference in total surgery-associated costs for the health care system during the first 30 days, despite the higher intraoperative costs for laparoscopic surgery.
Thus, laparoscopic LAR tends to have a shorter hospitalization without increasing the public health care system costs and is an attractive treatment option unless contraindicated.
Data sharing statement
The dataset is available from the Research Gate repository (DOI: 10.13140/RG.2.2.30161.63844). Further information is available from the corresponding author on request.
Acknowledgment
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
All authors had full access to all the data in the study and assumed responsibility for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. Furthermore, all authors were accountable for the parts of the work they did, able to identify which co-authors were responsible for specific other parts of the work, and confident in the integrity of the contributions of their co-authors. Daiane Oliveira Tayar affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. Daiane Oliveira Tayar, Ulysses Ribeiro Jr, Ivan Cecconello, Claudia M Simões, and José Otávio C Auler Jr made substantial contributions to the conception and design of the work; Daiane Oliveira Tayar, Ulysses Ribeiro Jr, Tiago M Magalhães, Claudia M Simões, and José Otávio C Auler Jr made substantial contributions to the data acquisition and/or analysis and interpretation of the data of the work; Daiane Oliveira Tayar and Tiago M Magalhães drafted the manuscript; Ulysses Ribeiro Jr, Ivan Cecconello, Claudia M Simões, and José Otávio C Auler Jr reviewed the article critically for important intellectual content. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors provided the final approval of the version to be published.
Disclosure
All authors have completed the ICMJE form for disclosure of potential conflicts of interest. Daiane Oliveira Tayar reports personal fees from Johnson & Johnson Medical Brazil, outside the submitted work. This study was conducted without any external influence. The authors report no other conflicts of interest in this work.
References
INCA. Estimative 2016, cancer incidence in Brazil [Internet]. Available from: http://www.inca.gov.br/wcm/dncc/2015/porincidencia.asp. | ||
Keller DS, Champagne BJ, Reynolds HL, Stein SL, Delaney CP. Cost-effectiveness of laparoscopy in rectal cancer. Dis Colon Rectum. 2014;57(5):564–569. | ||
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97(11):1638–1645. | ||
Inada R, Yamamoto S, Oshiro T, Takawa M, Fujita S, Akasu T. Case-matched comparison of the short-term outcomes between laparoscopic and open abdominoperineal resection for rectal cancer. Surg Today. 2014;44(4):640–645. | ||
Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255(2):216–221. | ||
Xu D, Li J, Song Y, et al. Laparoscopic surgery contributes more to nutritional and immunologic recovery than fast-track care in colorectal cancer. World J Surg Oncol. 2015;13(1):18. | ||
Vlug MS, Bartels SA, Wind J, et al. Which fast track elements predict early recovery after colon cancer surgery? Colorectal Dis. 2012;14(8):1001–1008. | ||
Kusano T, Inomata M, Hiratsuka T, et al. A comparison of laparoscopic and open surgery following pre-operative chemoradiation therapy for locally advanced lower rectal cancer. Jpn J Clin Oncol. 2014;44(4):305–310. | ||
Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–2229. | ||
Asoglu O, Balik E, Kunduz E, et al. Laparoscopic surgery for rectal cancer: outcomes in 513 patients. World J Surg. 2013;37(4):883–892. | ||
Bartels SA, Vlug MS, Hollmann MW, et al. Small bowel obstruction, incisional hernia and survival after laparoscopic and open colonic resection (LAFA study). Br J Surg. 2014;101(9):1153–1159. | ||
Morneau M, Boulanger J, Charlebois P, et al. Laparoscopic versus open surgery for the treatment of colorectal cancer: a literature review and recommendations from the Comité de l’évolution des pratiques en oncologie. Can J Surg. 2013;56(5):297–310. | ||
Miki C, Inoue Y, Mohri Y, Kobayashi M, Kusunoki M. Site-specific patterns of surgical site infections and their early indicators after elective colorectal cancer surgery. Dis Colon Rectum. 2006;49(10 Suppl):S45–S52. | ||
Hida K, Yamaguchi T, Hata H, et al. Risk factors for complications after laparoscopic surgery in colorectal cancer patients: experience of 401 cases at a single institution. World J Surg. 2009;33(8):1733–1740. | ||
Evans C, Lim J, Gatzen C, Huang A. Factors influencing laparoscopic colorectal operative duration and its effect on clinical outcome. Surg Laparosc Endosc Percutan Tech. 2012;22(5):437–442. | ||
Biondi A, Grosso G, Mistretta A, et al. Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg. 2013;13 Suppl 2(Suppl 2):S12. | ||
Kuo LJ, Hung CS, Wang W, et al. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183(2):524–530. | ||
Balik E, Asoglu O, Saglam S, et al. Effects of surgical laparoscopic experience on the short-term postoperative outcome of rectal cancer. Surg Laparosc Endosc Percutan Tech. 2010;20(2):93–99. | ||
Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg. 2009;13(2):275–281. | ||
Tanis PJ, Buskens CJ, Bemelman WA. Laparoscopy for colorectal cancer. Best Pract Res Clin Gastroenterol. 2014;28(1):29–39. | ||
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. | ||
Lonjon G, Boutron I, Trinquart L, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg. 2014;259(1):18–25. | ||
Hayashi H, Ozaki N, Ogawa K, et al. Assessing the economic advantage of laparoscopic vs. open approaches for colorectal cancer by a propensity score matching analysis. Surg Today. 2018;48(4):439–448. | ||
Kennedy RH, Francis EA, Wharton R, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol. 2014;32(17):1804–1811. | ||
de’Angelis N, Landi F, Vitali GC, et al. Multicentre propensity score-matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc. 2017;31(8):3106–3121. | ||
Son H-J, Lee H-Y, Park JW, Choi HS, Jeong S-Y, Oh JH. Cost-comparison of laparoscopic and open surgery for mid or low rectal cancer after preoperative chemoradiotherapy: data from a randomized controlled trial. World J Surg. 2013;37(1):214–219. | ||
Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50(4):464–471. | ||
Gehrman J, Björholt I, Angenete E, Andersson J, Bonjer J, Haglind E. Health economic analysis of costs of laparoscopic and open surgery for rectal cancer within a randomized trial (COLOR II). Surg Endosc. 2017;31(3):1225–1234. | ||
Jensen CC, Prasad LM, Abcarian H. Cost-effectiveness of laparoscopic vs open resection for colon and rectal cancer. Dis Colon Rectum. 2012;55(10):1017–1023. | ||
Feng F, Li XH, Shi H, et al. Fast-track surgery combined with laparoscopy could improve postoperative recovery of low-risk rectal cancer patients: a randomized controlled clinical trial. J Dig Dis. 2014;15(6):306–313. | ||
Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100(8):1108–1114. | ||
Widdison AL, Barns V, Prescott O, Pollard A. A cost-minimization analysis of first intention laparoscopic compared to open right hemicolectomy for colon cancer. Ann Med Surg. 2016;5:23–28. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.