Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Promotion of Hair Regrowth in Androgenetic Alopecia with Supplemented Erzhi Wan: Exploring Its Mechanism Using Network Pharmacology and Molecular Docking
Authors Ji C , Ma J, Feng C, Zhu H, Gao Y, Huang J, Shen H, Wei Y
Received 19 July 2023
Accepted for publication 20 September 2023
Published 24 October 2023 Volume 2023:16 Pages 2995—3022
DOI https://doi.org/10.2147/CCID.S425295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Chen Ji,1 Jun Ma,2 Chengcheng Feng,1 Hongliu Zhu,3 Yanwei Gao,1 Jun Huang,1 Hui Shen,1 Yuegang Wei4
1Department of Dermatology, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, People’s Republic of China; 2Department of Dermatology, the Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, People’s Republic of China; 3Department of Dermatology, Jiangyin Hospital of Traditional Chinese Medicine, Wuxi, People’s Republic of China; 4First College of Clinical Medicine, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Hui Shen, Department of Dermatology, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, 4 Kangle Street, Suzhou, Jiangsu Province, 215600, People’s Republic of China, Email [email protected] Yuegang Wei, First College of Clinical Medicine, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing, Jiangsu Province, 210023, People’s Republic of China, Email [email protected]
Purpose: Supplemented Erzhi Wan (SEZW) is a Traditional Chinese Medicine commonly used in the treatment of androgenetic alopecia (AGA). This study aims to verify the effectiveness of SEZW for the treatment of AGA in mice and explore the potential molecular mechanisms underlying its function using network pharmacology and molecular docking.
Methods: Forty mice were divided into five groups: Control, AGA-model, AGA-Positive, SEZW Low Dose, and SEZW High Dose. Hair regrowth in mice was evaluated by scoring hair on days 0, 14, and 28 post-treatment and weighing mouse hair on day 28 post-treatment. The targets of the active compounds of SEZW were obtained using the Traditional Chinese Medicine Database. AGA-related targets were downloaded from five databases. Then, the overlapping genes were identified. A protein-protein interaction network was constructed using the STRING database. Hub targets were determined through analysis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed. Finally, molecular docking of active compounds and hub targets was performed.
Results: Hair regrowth in mice in the SEZW treatment groups was significantly enhanced relative to that in the AGA-model mice. A total of 59 potential drug-disease targets were identified. Based on the GO/KEGG analysis results, oxidative stress and gland development were identified as potential mechanisms of action of SEZW in AGA treatment. The PI3K-Akt and AGE-RAGE signaling pathways and seven hub targets were identified as the potential underlying mechanism of SEZW function. Molecular docking results showed that the most active SEZW compounds bind stably to several of the candidate disease targets.
Conclusion: SEZW is effective in the treatment of AGA in a mouse model. Combined with network pharmacological analysis, the potential mechanisms, signaling pathways, and hub targets of SEZW in the treatment of AGA were identified, providing new ideas for further studies.
Keywords: Chinese herbal formulas, hair loss, hair regeneration, mechanism prediction
Introduction
Androgenetic alopecia (AGA), also known as male- or female-pattern alopecia, involves the miniaturization of hair follicles resulting in progressive hair thinning and shedding. Male-pattern alopecia primarily manifests as a receding frontal hairline with an M-shaped pattern, while female-pattern alopecia mainly manifests as diffuse hair thinning in a Christmas tree-like pattern on the top of the head. AGA can be accompanied by lighter hair color, higher oil production, and scalp inflammation, among other symptoms.1 The mechanism underlying AGA involves the shortening of the hair follicle anagen phase, premature entry into the telogen phase, and eventual miniaturization of hair follicles, resulting in slow hair growth and hair loss.2
AGA is a major type of alopecia affecting 60–70% of the population.3 In China, the prevalence of AGA is as high as 21.3% in men and 6.0% in women.4 Recently, there has been increasing evidence indicating that AGA, as a chronic progressive disease, not only affects the appearance of patients but also causes psychological disorders.5 Hence, early interventions and effective treatments of androgenetic baldness are vital.
To date, only two drugs have been approved by the United States Food and Drug Administration (FDA) and European Medicines Agency for the treatment of AGA: finasteride and minoxidil. However, finasteride is not recommended for women according to the FDA. Moreover, both drugs have side effects and are effective in <50% of patients.3 A variety of other treatments exist, such as dutasteride, low-level laser therapy, stem cell-based therapy, platelet-rich plasma, microneedles, and hair transplants. These treatments mainly focus on improving symptoms, and their effectiveness is intrinsically related to their maintenance.1 In addition, as these treatments are still under research and require further validation, we should consider their potential limitations regarding their effectiveness and side effects. Alternatively, various traditional Chinese herbs, including their monomers, have been reported to alleviate hair loss.6–8 Furthermore, since herbs are typically reported to have fewer side effects, they are promising treatments.9
In Traditional Chinese Medicine (TCM), hair loss is closely related to kidney and blood deficiencies. Kidney deficiency is a pathological change in the kidney with insufficient essence and impaired function. If the kidney essence and blood production are insufficient, the hair lacks nutrition, resulting in hair fall. Therefore, the TCM treatment of hair loss is based on tonifying the kidneys and nourishing the blood.
SEZW is a Chinese medicinal formula consisting of ErZhi Wan (EZW) plus Cornus officinalis (CO) and Rehmanniae Radix Praeparata (RRP), which have tonifying effects on the kidneys and blood. EZW is a commonly used formula in TCM composed of two Chinese herbs, Ecliptae Herba (EH) and Fructus Ligustri Lucidi (FLL), whose names originate from the specific seasons when the two herbs are harvested: winter and summer solstices, respectively. EH and FLL have similar efficacies in treating AGA and according to historical documents, they can also darken the hair when used in combination. Research has shown that the proliferation, differentiation, and death of follicular melanocytes occur along the hair cycle progresses.10 Furthermore, follicular melanogenesis includes the melanogenic activity of follicular melanocytes, migration of melanin granules, and pigmentation of the hair shaft.11 Therefore, we hypothesize that EZW not only can deepen hair color but also can promote hair growth. Even when CO, RRP, EH, and FLL are commonly used to treat AGA, the mechanism underlying the function of SEZW in alleviating hair loss remains unclear.
Since the current Western medicines for AGA have limited efficacy and applicability, it is worthwhile to consider TCM as an entry point for research and development of new treatments. In this study, we validated the efficacy of SEZW in the treatment of AGA by constructing an AGA mouse model. Network pharmacology was used to identify potential key targets and signaling pathways to explore the mechanism of action of SEZW in AGA treatment. Finally, the results were validated using molecular docking.
Materials and Methods
Materials
FLL granules (1 g, 10 g/bag), EH granules (1 g, 10 g/bag), RRP granules (4 g, 10 g/bag), and CO granules (1 g, 6 g/bag) were purchased from Zhangjiagang Traditional Chinese Medicine Hospital (Jiangyin Tianjiang Pharmaceutical Co., Ltd., Wuxi, China). Finasteride Tablets were obtained from Hangzhou MSD Pharmaceutical Co. Ltd. (1 mg/tablet, Hangzhou, China). Testosterone propionate injections were bought from Tianjin Jinyao Pharmaceutical Co. (1 mL × 25 mg, Tianjin, China).
One bag each of EH, FLL, RRP, and CO were added to pure water to obtain low (1 g/mL) and high concentration (2 g/mL) treatment solutions. Finasteride was powdered, added to a 0.5% sodium carboxymethylcellulose solution and mixed thoroughly to obtain a 0.1 mg/mL finasteride suspension. The testosterone propionate injection was diluted with injectable corn oil to a concentration of 5 mg/mL. Each solution was prepared weekly and stored at 4 °C. The solutions were warmed to room temperature before being administered.
Animals
Forty 6–8 weeks old males C57BL/6 mice, weighing 18–22 g, were purchased from the Suzhou Hengxingchen Biomedical Co. (Suzhou, China). The experiments were approved by the Animal Ethics Committee of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine according to guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892‐2018. The 40 mice were fed adaptively with free access to food and water for one week, after which a 2×3 cm area was marked on their dorsal side. The hair in this area was removed with an electric razor, and residual hair was removed with a depilatory cream. The dorsal skin of all mice was pink, suggesting that the hair follicles had entered the telogen phase. All mice except those in the control group were injected subcutaneously with 0.1 mL of 5 mg/mL testosterone propionate on the back every day for 28 days to obtain the AGA-model mice.12
Treatments
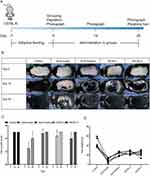
The 40 mice were randomly divided into five groups of eight mice each: Control, AGA-model, AGA-Positive, SEZW low dose (SEZW-L), and SEZW high dose (SEZW-H) groups. Mice in the AGA-Positive group were treated with 0.1 mL of 0.1 mg/mL finasteride suspension, while mice in the SEZW-L and SEZW-H groups were given 10 g/kg/d and 20 g/kg/d SEZW solution, respectively, via intragastric administration for 28 days. The mice were photographed on days 0, 14, and 28 during the dosing period to record hair growth and score the results (Figure 1A). Two trained technicians blindly measured hair regrowth ratios (%) according to the proportion of dorsal skin that turns black. As hairs enter the telogen phase the skin is pink, while in the anagen phase it is black. The evaluation criteria for hair regrowth were as follows: score 0=no growth observed; 1=up to 20% growth; 2=20–40% growth; 3=40–60% growth; 4=60–80% growth; and 5=80% to full growth.13 On day 28 of the experiment, all hair in the previously marked area on the dorsal side was removed using an electric razor. Subsequently, hair was weighed using a balance.
Active Ingredients and Potential Targets Identification
Four Chinese herbs, EH, FLL, CO, and RRP, were searched for in the Traditional Chinese Medicine Database and Analysis Platform (TCMSP, https://tcmsp-e.com/). The active compounds in each herb were screened with the conditions of oral bioavailability (OB) ≥ 30% and drug-like properties (DL) ≥ 0.18. All potential targets corresponding to the ingredients in SEZW were collected. The names of the targets were normalized using the UniProt database (https://www.uniprot.org/). We selected targets that had been reviewed previously and removed duplicates and non-human targets. Finally, the compound-related targets were identified.
Identification of AGA-Related Targets
To obtain AGA-related targets, we used “Androgenetic alopecia” and “Alopecia seborrheic” as keywords to search in five gene databases: GeneCards (https://www.genecards.org/), OMIM (https://omim.org/), DrugBank (https://go.drugbank.com/), PharmGKB (https://www.pharmgkb.org/), and TTD (http://db.idrblab.net/ttd/). On the GeneCards database, genes with scores >0.5 were identified as those closely associated with AGA and were included in the study. Subsequently, we merged the AGA-related targets retrieved from the five databases.
Construction of Protein-Protein Interaction (PPI) Network for Drug-Disease Hub Targets
The Venn package in R was used to determine the overlap between the drug targets and disease-related genes, which were SEZW-AGA common targets. The PPI network was constructed by uploading candidate genes to the STRING database (https://string-db.org/). Subsequently, we imported the obtained results into the Cytoscape3.9.1 software for further analysis. CytoNCA, a Cytoscape plugin, was used to identify potential hub targets based on the analysis of six parameters: betweenness, closeness, degree, eigenvector, Local Average Connectivity-based method, and network. The top three active compounds in terms of the number of targets in the network and OB values were considered major active compounds.
GO and KEGG Enrichment Analyses
GO and KEGG enrichment analyses were performed to explore the potential functions of the candidate targets and crucial signaling pathways using the ClusterProfiler package in R4.2.1. Regarding the GO analysis, functions across the three categories, biological process (BP), cellular component (CC), and molecular function (MF), were considered statistically significant at P<0.05. Regarding the KEGG analysis, terms with P<0.05 were considered potential signaling pathways.
Molecular Docking Technology
The SEZW-AGA hub targets were considered receptor proteins, whereas the major active compounds were considered ligands. We obtained the core receptor protein and ligand structures from the Protein Data Bank (PDB) (http://www.rcsb.org/pdb/home/home.do) and PubChem databases (https://pubchem.ncbi.nlm.nih.gov/), respectively. The receptor proteins were pretreated via dehydration and hydrogenation using the PyMOL (https://pymol.org/2/) and AutoDockTools software (https://autodock.scripps.edu/download-autodock4/). Molecular ligands were converted into 3D structures using ChemOffice software (https://www.chemdraw.com.cn/). Subsequently, the receptor proteins and ligands were docked, and the docking fractions were calculated using AutoDockVina (https://vina.scripps.edu/). The lower the binding energy of the interactions, the better the binding ability between the receptors and ligands.14 Finally, the receptor-ligand complexes were visualized using PyMoL.
Results
Pharmacodynamic Study of SEZW on AGA-Model Mice
After 28 days of treatment, hair regrowth on the dorsal side of the mice in the AGA-model group was significantly slower than that of the mice in the other groups, indicating that the AGA-model mice had been constructed successfully (Figure 1B). After 2 weeks, hair regeneration reached more than 80% in the control group (Figure 1C), and there was a significant difference in hair regeneration scores between the control and the AGA-model group (P<0.01) (Figure 1C). The hair regeneration scores of mice in both the SEZW-L and SEZW-H groups were significantly higher than those of mice in the AGA-model group (P<0.01) (Figure 1C). Moreover, the mice in the AGA-Positive group showed faster hair growth, and their scores were significantly different from those of the mice in the AGA-model group (P<0.01) (Figure 1C). The hair regeneration scores of the mice in the Control, AGA-Positive, SEZW-L, and SEZW-H groups did not differ significantly (P>0.05) (Figure 1C), and mice in all these groups showed more than 80% hair regrowth after 28 days. The statistically significant difference between the hair scores of mice in the AGA-model and the other groups was maintained at 28 days (P<0.05) (Figure 1C). The results based on the hair weights of the mice supported these differences: The AGA-model mice had the lowest hair weight and the control group had the highest regenerated hair weight (Figure 1D). Compared to the AGA-model group, mice in the AGA-Positive, SEZW-L, and SEZW-H groups showed a significant increase in hair weight (P<0.01) (Figure 1D).
Prediction of the Targets of SEZW
By setting thresholds of OB ≥30% and DL ≥0.18, we identified 10, 13, 20, and 2 active compounds for FLL, EH, CO, and RRP, respectively, from the TCMSP database. The corresponding targets for each active compound were also downloaded from the TCMSP database (Table 1). After deduplication and integration, we obtained 200 molecules targeted by the active compounds present in SEZW.
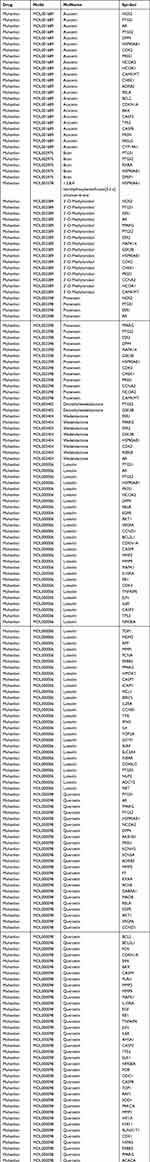 |
Table 1 Active compounds and targets selected for SEZW |
AGA-Related Target Prediction
We obtained 657, 12, 11, 745, and 0 candidate AGA-related genes from GeneCards, OMIM, DrugBank, PharmGKB, and TTD, respectively. Targets related to AGA in the GeneCards database were sorted and selected based on a relevance score greater than 0.5, which was considered a strong correlation. After removing duplicates, we obtained a final set of 1385 AGA-related genes (Figure 2A).
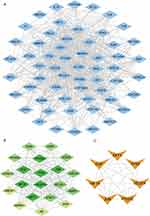
Construction of PPI Networks for Drug-Disease Targets
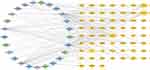
A total of 59 shared targets of drug compounds and diseases were found (Figure 2B). Based on these, a PPI network consisting of 59 nodes and 592 edges was constructed. Network edges were added based on evidence collected through text-mining, experiments, databases, co-expression, neighborhood, gene fusion, and co-occurrence, with the minimum required interaction score set as “medium confidence” (0.400). We imported the PPI into Cytoscape3.9.1 for further analysis and visualization (Figure 3A). After the first filtring through CytoNCA, 20 targets were identified (Figure 3B). After two filters, we identified numerous crucial targets within the network: AKT1, JUN, VEGFA, HIF1A, TP53, EGF, and PTGS2 (Figure 3C). According to the SEZW-AGA interaction network, quercetin (MOL000098), luteolin (MOL000006), and kaempferol (MOL000422) exhibited the highest degrees of interaction with 59 targets and acted on 45, 27, and 21 targets, respectively (Figure 4). Additionally, the OB values of quercetin, luteolin, and kaempferol were 46.43%, 36.16%, and 41.88%, respectively. Together, these findings suggest that quercetin, luteolin, and kaempferol are the primary active compounds in SEZW.
GO and KEGG Enrichment Analyses
GO and KEGG enrichment analyses were conducted, and 1298 BP, 28 CC, 91 MF, and 123 KEGG pathway terms were found to be enriched based on an adjusted P value <0.05. The top 10 terms in each category are listed in Table 2. From these enrichment results, the top five terms of each GO term and the top 10 KEGG pathways were chosen for visualization (Figure 5).
 |
Table 2 GO and KEGG enrichment analysis |
Molecular Docking Technology
We selected candidate hub targets (AKT1, JUN, VEGFA, HIF1A, TP53, EGF, and PTGS2) as protein receptors and the three major active compounds (quercetin, luteolin, and kaempferol) as ligands for validation using molecular docking. A binding energy of less than −5 kcal/mol was used as the threshold for stable binding. In our study, all binding values were less than −6 kcal/mol, indicating a strong receptor-ligand affinity (Table 3). We selected the top nine stable receptor-ligand complexes for visualization (Figure 6).
 |
Table 3 The molecular docking of the active compounds to the core targets |
Discussion
Network pharmacology is a new drug design strategy based on rapid systems biology and multidirectional pharmacology developments. Nogales15 argued that diseases are currently diagnosed by phenotypes aimed at alleviating symptoms rather than by mechanisms and treatments, which often only have minimal transient effects. The approach to drug development has changed with the rise of network pharmacological research. Instead of focusing on a single symptom and target, we used a disease-multitarget-multidrug model. This involves the use of two or more drugs to synergistically target key network proteins, thereby addressing the underlying disease at the root cause. The “treatment based on syndrome differentiation” approach in TCM involves combining various drugs to target the underlying nature of pathological changes; therefore, aligns with the drug-multitarget-multidrug model in network pharmacological research.
Thus, network pharmacology is a valid method to analyze the mechanism of action of SEZW in the treatment of AGA. This treatment strategy is characteristic of the TCM “treatment based on syndrome differentiation” approach, which uses TCM theory to analyze relevant clinical data, clarify the essentiality of diseases, and identify the ‘syndrome”, which can reflect the combination of the cause, location, nature of the disease, and its tendency to develop and change. From this, treatment rules, methods, and prescriptions are formulated.
In this study, we obtained 200 SEZW targets from the TCMSP database and 1385 AGA-associated genes from the GeneCards, OMIM, DrugBank, PharmGKB, and TTD databases. Then, 59 candidate SEZW-AGA targets were identified at the intersection of these lists. The above results were imported into the STRING tool, and a PPI network was constructed with 59 nodes and 592 edges. In addition, we identified seven potential hub targets: AKT1, JUN, VEGFA, HIF1A, TP53, EGF, and PTGS2. Some of these genes have been investigated previously for their relevance in hair growth. For example, VEGFA (vascularization endothelial growth factor A) has been shown to regulate vascularization and vessel size around the hair follicle, thus altering the size of follicles as blood vessels are required to provide nutrients.13 In addition, Gaofeng Wang et al16 found that activation of HIF1A signaling by inducing tissue hypoxia promoted wound-induced hair follicle neogenesis.
Quercetin, luteolin, and kaempferol were identified as the major active compounds in SEZW. They are flavonoids that can be identified in EZW through mass spectrometry.17 Quercetin has previously been shown to stimulate melanogenesis in mice hair follicle melanocytes and promote hair shaft growth in cultured human hair follicles.18,19
Subsequently, GO and KEGG enrichment analyses were performed on the 59 candidate targets. These targets were mainly enriched for processes involving the response to oxidative stress, reactive oxygen species, gland development, cellular response to chemical stress, and cellular response to oxidative stress. Among these, oxidative stress, defined as the excess of reactive oxygen species relative to antioxidants, has been linked to neurodegenerative diseases, cardiovascular diseases, diabetes mellitus, and many other pathologies.20 Studies have shown a close relationship between oxidative stress and AGA. For example, Bahta et al21 found that the premature senescence of balding dermal papilla cells in vitro is associated with the expression of oxidative stress and DNA damage markers. Upton et al22 further investigated the role of oxidative stress in the pathogenesis of AGA in relation to cell senescence, migration, and the secretion of hair follicle inhibitory factors. Moreover, AGA patients have shown increased oxidative stress in vivo.23,24 However, few drugs used to treat AGA target the pathological mechanisms of oxidative stress, and those that do require further validation. EZW has been demonstrated to inhibit oxidative stress and regulate metabolism in a diabetic cardiomyopathy-related study.25 Therefore, we hypothesized that the mechanism of action of SEZW in treating AGA may be related to oxidative stress. Experimental research is needed to investigate this further.
The KEGG enrichment analysis identified 123 significantly enriched pathways, including the PI3K-Akt signaling pathway. Akt, which was previously identified as a hub target, is also a key downstream molecule in the PI3k/Akt signaling pathway. This pathway has a significant impact on cancer and neurological, metabolic, and cardiovascular diseases, among others.26–29 Through activation or inhibition of downstream proteins, PI3K/Akt regulates signal transduction and is involved in numerous biological processes, such as cell proliferation, migration, differentiation, apoptosis, and metabolism.30 Research by Yu Chen31 has shown that the PI3K-Akt signaling pathway plays a crucial role in de novo hair follicle regeneration. This provides further theoretical support for exploring the possible involvement of the PI3K/AKT signaling pathway in the pathological process of AGA.
Our study has some limitations. We have demonstrated the promotion of hair growth by SEZW in AGA-model mice; however, large-scale clinical studies need to be conducted to confirm these results.
Conclusion
SEZW promoted hair growth in mice with AGA. Based on network pharmacology, we identified the possible mechanisms of action of SEZW in the treatment of AGA, finding that the response to oxidative stress, gland development, and chemical stress are critical targets. Further, the PI3K/Akt and AGE-RAGE signaling pathways were identified as potential signaling pathways through which SEZW exerts its therapeutic effects. AKT1, JUN, VEGFA, HIF1A, TP53, EGF, and PTGS2 were identified as hub molecules for the efficacy of SEZW in AGA treatment.
Abbreviations
AGA, androgenetic alopecia; BP, biological process; CC, cellular component; CO, Cornus officinalis; DL, drug-like properties; EH, Ecliptae Herba; FDA, the United States Food and Drug Administration; FLL, Fructus Ligustri Lucidi; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; MF, molecular function; OB, oral bioavailability; PDB, Protein Data Bank; PPI, Protein-Protein Interaction; RRP, Rehmanniae Radix Praeparata; SEZW, Supplemented Erzhi Wan; SEZW-H, SEZW high dose; SEZW-L, SEZW low dose; TCM, Traditional Chinese Medicine; TCMSP, Traditional Chinese Medicine Database and Analysis Platform.
Data Sharing Statement
All relevant data are contained within the article.
Ethics Approval
The network pharmacological analysis was approved by the Ethics Committee of the Affiliated Zhangjiagang Hospital of Soochow University. The experiments were approved by the Animal Ethics Committee of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine according to guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892‐2018.
Acknowledgments
The authors thank Muyao Wu1 and Liping Chen2 for their careful help in the experiments. The authors gratefully acknowledge contributions from the TCMSP, Uniprot, GeneCards, OMIM, PharmGKB, DrugBank, and TTD databases.
1Department of Rehabilitation, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, China; 2Department of Gastroenterology, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation for Young Scientists of China (No. 82205113); the Youth Science and Technology Project of Zhangjiagang (No. ZJGQNKJ202035); and the Natural Science Foundation for Young Scientists of Zhangjiagang TCM Hospital (No. zzyq1902).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Katzer T, Leite Junior A, Beck R, da Silva C. Physiopathology and current treatments of androgenetic alopecia: going beyond androgens and anti-androgens. Dermatol Ther. 2019;32(5):e13059. doi:10.1111/dth.13059
2. Ryu YC, Park J, Kim YR, et al. CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD(2). Cells. 2023;12(4):555. doi:10.3390/cells12040555
3. J R, W D-E. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014;18(7):787–806. doi:10.1517/14728222.2014.922956
4. Wang TL, Zhou C, Shen YW, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol. 2010;162(4):843–847. doi:10.1111/j.1365-2133.2010.09640.x
5. Han SH, Byun JW, Lee WS, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012;24(3):311–318. doi:10.5021/ad.2012.24.3.311
6. Zhu H, Gao Y, Yang J, Li J, Gao J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF-β and mitochondrial signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):4000–4008. doi:10.26355/eurrev_201806_15285
7. Zhang N, Park D, Park H. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement Altern Med. 2013;13(1):9. doi:10.1186/1472-6882-13-9
8. Roh S, Kim C, Lee M, Hwang S, Rang M, Yoon Y. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2002;30(1):43–49. doi:10.1016/S0923-1811(02)00060-9
9. Dhariwala MY, Ravikumar P. An overview of herbal alternatives in androgenetic alopecia. J Cosmet Dermatol. 2019;18(4):966–975. doi:10.1111/jocd.12930
10. Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24(3):401–410. doi:10.1111/j.1755-148X.2011.00855.x
11. Slominski A, Wortsman J, Plonka P, Schallreuter K, Paus R, Tobin D. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi:10.1111/j.0022-202X.2004.23528.x
12. Wang ZD, Feng Y, Ma LY, Li X, Ding WF, Chen XM. Hair growth promoting effect of white wax and policosanol from white wax on the mouse model of testosterone-induced hair loss. Biomed Pharmacother. 2017;89:438–446. doi:10.1016/j.biopha.2017.02.036
13. Zhang T, Cao S, Yuan H, Park S. Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway. Pharmaceuticals. 2021;14(11):1128. doi:10.3390/ph14111128
14. Tan X, He Y, Ou Y, Xiong X, Deng Y. Exploring the Mechanisms and Molecular Targets of Taohong Siwu Decoction for the Treatment of Androgenetic Alopecia Based on Network Analysis and Molecular Docking. Clin Cosmet Investig Dermatol. 2022;15:1225–1236. doi:10.2147/CCID.S361820
15. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
16. Wang G, Sweren E, Andrews W, et al. Commensal microbiome promotes hair follicle regeneration by inducing keratinocyte HIF-1α signaling and glutamine metabolism. Science Advances. 2023;9(1):eabo7555. doi:10.1126/sciadv.abo7555
17. Fu L, Ding H, Han L, et al. Simultaneously targeted and untargeted multicomponent characterization of Erzhi Pill by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry. J Chromatogr A. 2019;1584:87–96. doi:10.1016/j.chroma.2018.11.024
18. Huang S, Mu F, Li F, et al. Systematic Elucidation of the Potential Mechanism of Erzhi Pill against Drug-Induced Liver Injury via Network Pharmacology Approach. Evid Based Complement Alternat Med. 2020;2020:6219432. doi:10.1155/2020/6219432
19. Kim J, Kim S, Choi Y, et al. Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules. 2020;25(17):56.
20. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. 2020;38(2):167–197. doi:10.1016/j.ccell.2020.06.001
21. Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J Invest Dermatol. 2008;128(5):1088–1094. doi:10.1038/sj.jid.5701147
22. Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol. 2015;135(5):1244–1252. doi:10.1038/jid.2015.28
23. Kaya Erdogan H. The role of oxidative stress in early-onset androgenetic alopecia. J Cosmet Dermatol. 2016;1: 1–4.
24. Prie IL, Tivig I, Stoian I, Giurcaneanu C. Oxidative stress in androgenetic alopecia. J Med Life. 2016;9(1):79–83.
25. Peng M, Xia T, Zhong Y, et al. Integrative pharmacology reveals the mechanisms of Erzhi Pill, a traditional Chinese formulation, against diabetic cardiomyopathy. J Ethnopharmacol. 2022;296:115474. doi:10.1016/j.jep.2022.115474
26. Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi:10.1016/j.cellsig.2014.08.019
27. Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–1496. doi:10.7150/ijbs.27173
28. Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10(1):54. doi:10.1186/s13578-020-00416-0
29. Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. doi:10.1242/dev.137075
30. Xie Y, Yan B, Hou M, et al. Erzhi pills ameliorate cognitive dysfunction and alter proteomic hippocampus profiles induced by d-galactose and Abeta(1-)(40) injection in ovariectomized Alzheimer’s disease model rats. Pharm Biol. 2021;59(1):1402–1414. doi:10.1080/13880209.2021.1990353
31. Chen Y, Fan Z, Wang X, et al. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res Ther. 2020;11(1):144. doi:10.1186/s13287-020-01650-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.