Back to Journals » Research and Reports in Urology » Volume 15
Promising Experimental Treatments for Lupus Nephritis: Key Talking Points and Potential Opportunities
Authors Neves A , Viveiros L , Venturelli V , Isenberg DA
Received 1 May 2023
Accepted for publication 3 July 2023
Published 10 July 2023 Volume 2023:15 Pages 333—353
DOI https://doi.org/10.2147/RRU.S385836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Guglielmo Mantica
Ana Neves,1 Luísa Viveiros,2 Veronica Venturelli,3 David A Isenberg4
1Internal Medicine Department, Centro Hospitalar Universitário de São João, Oporto, Portugal; 2Internal Medicine Department, Centro Hospitalar Universitário de Santo António, Oporto, Portugal; 3Rheumatology Unit, Department of Medical Sciences, Università degli Studi di Ferrara, Azienda Ospedaliero-Universitaria S. Anna, Cona, Italy; 4Centre for Rheumatology, Department of Medicine, University College London, London, UK
Correspondence: David A Isenberg, The Department of Rheumatology, Division of Medicine, University College London, Room 423 the Rayne Building, London, WC1E 6JF, UK, Email [email protected]
Abstract: Lupus nephritis (LN) is a frequent and serious complication of systemic lupus erythematosus (SLE), impairing patients’ quality of life and significantly increasing mortality. Despite optimizing the use of conventional immunosuppressants and other biological drugs, its management remains unsatisfactory. This is mainly due to the heterogeneity of SLE, but also to insufficiently effective treatment regimens and clinical trial limitations (strict criteria, low number of patients included, and side effects). Most clinical trials of new biological therapies have failed to meet their primary endpoints in both general SLE and LN, with only two biological drugs (belimumab and anifrolumab) being approved by the Food and Drug Administration (FDA) for the treatment of SLE. Recently, several Phase II randomized controlled trials have evaluated the efficacy and safety of new biologics in LN, and some of them have demonstrated an improvement in clinical and laboratory measures. Multi-target therapies are also being successfully developed and encourage a belief that there will be an improvement in LN outcomes.
Keywords: biological therapies, systemic lupus erythematosus, lupus nephritis, trial
Introduction
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune rheumatic disease that can have severe and life-threatening presentations. This is explained mainly by its inflammatory activity, treatment-associated complications, concomitant diseases, and long-term sequelae. The main pathogenic feature of this disease is the presence of antibodies that bind autoantigens, forming immunocomplexes that precipitate in tissues, leading to organ damage. SLE is associated with significant morbidity and mortality rates reported to be 2- to 3-fold higher than the general population.1,2
In particular, lupus nephritis (LN) is one of the most serious complications in patients with SLE. However, definitions of LN have varied between studies. Some include all SLE patients with proteinuria, whilst others reported only biopsy-proven LN, but overall it seems to affect 30–40% of all SLE patients.3,4
Glomerular and interstitial inflammation mediated by immunocomplex precipitation lead to renal impairment, with further end-stage renal disease (ESRD), which impairs patients’ quality of life and overall survival.1,2 Therefore, prompt recognition and treatment are mandatory to minimize the impact of LN on these patients.
Over the past 60 years, the outlook for a patient with SLE has considerably improved, largely due to advances in diagnosis. For example, in the 1950s, the four-year survival for patients with SLE was 50%, while, currently, the fifteen-year survival is approximately 85%.5,6
However, focusing on LN, long-term dialysis became available only in the 1970s, concomitant to the introduction of better therapies and transplantation, which led to a 10% decrease in the ESRD risk at 10 and 15 years. Disappointingly, since 1990, the risk of LN patients progressing to ESRD has remained constant between 17% and 22% at 10 and 15 years, respectively.4,7–9 In addition, up to 25% of patients who achieve remission have a flare within 3 to 4 years.10 This reflects the need for an earlier diagnosis and better access to therapies and, in particular, trying to ensure patient adherence. However, the need for more efficacious drugs to improve patient outcomes and quality of life remains paramount.
The development of new biological drugs to treat patients with many autoimmune rheumatic diseases has dramatically improved the outcome and quality of life of patients, especially those with rheumatoid arthritis (RA), psoriatic arthritis, and ankylosing spondylitis.11 In contrast, in SLE, but especially in LN, very few therapies have been approved. B-cell targeting therapies, such as rituximab and belimumab, have proved to be effective in reducing glucocorticoid dosing, controlling disease activity, and inducing therapeutic response (especially in severe or refractory LN patients).12–14 Despite its approval, renal response after belimumab treatment was only observed in a third of LN patients, and rituximab did not meet its primary endpoints in either an LN or non-nephritis SLE trial.13,15 However, its utility is widely recognized and guidance on the treatment of LN from both the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) recommends its use.13,16–18 However, we still need to develop new directed therapies to become part of the standard therapy for LN patients, with improved efficacy, fewer side effects, and worldwide availability.
Here, we review the approved therapies for LN patients, the results of previous clinical trials, and future potential targets for LN.
Brief Overview of the Immunopathology of Lupus Nephritis
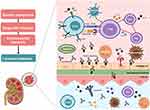
Understanding the immunopathology of SLE, with a special focus on LN, has led to a better understanding of this disease, and has also opened doors to the development of several new drugs, with different targets, that might potentially improve patient outcomes [Figure 1].
Autoantibodies are made by most healthy individuals and are present in patients for years before the first symptoms of SLE appear.19 The ability of autoantibodies to initiate clinical SLE depends on genetic background, epigenetic changes, and environmental exposure that interfere with normal innate and adaptive immunity paths, with consequent loss of tolerance, and increasing survival of autoreactive B cells. Several mechanisms are involved in this process, including defective clearance of apoptotic cells, the inability to degrade neutrophil extracellular traps (NETs), and abnormalities in B-cell receptor signaling.20
B lymphocytes from SLE patients play an important role as antigen-presenting-cells, inducing the activation of CD4+ auto-reactive T-cells, allowing the activation and differentiation of B-cells, and consequent production of high-affinity autoantibodies.21,22
As immune tolerance is lost, activation of tissue-resident phagocytic cells occurs, followed by the release of cytokines/chemokines from the fixed cells and activation of complement by the complement-binding immune complexes and autoantibodies fixed to glomeruli autoantigens. Infiltration of B, T (especially cytotoxic T cells and Th17 T cells), and dendritic cells, as well as monocytes from the peripheral blood, also occurs. Toll-like receptors (TLR) activation produces large amounts of inflammatory cytokines and IFN-alfa and IFN-beta, which also contribute to renal damage. The latter triggers the proliferation of parietal epithelial cells (and consequent glomerular crescent formation) and later production of extracellular matrix contributing to glomerulosclerosis.20 Ectopic clusters of follicular Th cells are also found in LN patients, which probably contribute to the pathogenesis of this disease.23
Measurement Tools
Different measurement tools have been in clinical trials used to ascertain SLE disease activity and response to therapies. The SLE disease activity index (SLEDAI) is a global activity index which captures disease activity in the previous 30 days. Two different versions have been used, the SLEDAI-2K and SELENA-SLEDAI.24 The Physician Global Assessment (PGA) is a visual analog scale, semi-quantitative, that accesses disease activity over the past two weeks.25 Although simple to use and calculate the score, it is a binary system and does not distinguish between patients who are better (but not completely recovered) from those who are worse or the same.
In contrast, the British Isles Lupus Assessment Group (BILAG) index evaluates, more comprehensively, disease activity over the past month in nine separate organ systems, assessing the items in each as new, same, worse, improving or never present. These data are converted into scores for each organ/system according to severity [A (severe), B (moderate), C (mild), D (no current activity), or E (no current or previous history)].26 Unlike the SLEDAI system, the BILAG can distinguish partial improvement from no change or deterioration.
The SLE responder index (SRI) combines the SELENA-SLEDAI, BILAG and PGA. SRI responses correspond to a ≥4-point reduction from baseline in SELENA-SLEDAI score and no new BILAG A score and no more than one new BILAG B organ domain score compared with baseline no worsening in PGA.25 Some clinical trials use SRI-3, SRI-5, or SRI-6 depending on the SELENA-SLEDAI score improvement.
The BILAG-based composite lupus assessment (BICLA) is a similar index to SRI, however with more weight on BILAG instead of SLEDAI. Requirements for BICLA response include BILAG improvement (all A scores at baseline improved to B, C or D, and all B scores improved to C or D); no deterioration of disease activity (no new BILAG A scores and ≥1 new B score); no worsening of the total SLEDAI-2K score from baseline; no significant deterioration (<10% worsening) on visual analog PGA; and no treatment failure (defined as non-protocol treatment).24
Conventional Lupus Nephritis Treatment
Renal biopsy is the gold standard for LN diagnosis, and therapeutic decisions are often based on the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of glomerular involvement.27
Classic immunosuppressants, such as steroids, cyclophosphamide, azathioprine, and mycophenolate mofetil, remain first-line therapy for LN treatment in most cases, with very few changes over the past 25 years.
Initial immunosuppressive treatment in patients with class I or II LN is rarely required unless needed for extrarenal manifestations of SLE. However, if the patient presents with a nephrotic syndrome with confirmed podocytopathy, low-dose glucocorticoid [with or without another immunosuppressive agent such as mycophenolic acid analogs (MPPA), azathioprine or a calcineurin inhibitor (CNI)] should be considered.28,29
For patients with class III (focal proliferative) or IV (diffuse proliferative) glomerulonephritis with or without a class V (membranous) component, initial treatment includes glucocorticoids plus either MPPA or low-dose intravenous cyclophosphamide or MPPA and a CNI.18,28
Once initial therapy is complete, patients should be placed on MPAA for maintenance. Azathioprine can be considered an alternative, notably if the patient wants to get pregnant, as well as belimumab if it has already been used as part of a triple therapy plan.18,28
Evidence for the optimal treatment of Class V LN is still limited, however, given the 10–30% risk of progression to ESRD of these patients who also present with concomitant nephrotic proteinuria, immunosuppressive therapy should be considered.28,29
In the presence of a non-responding or refractory disease, therapy with rituximab (anti-CD20 molecule) should be considered, as well as adding belimumab to the previous therapy.29,30
Hydroxychloroquine (or an equivalent antimalarial) should be administered to every patient with SLE (including those with lupus nephritis), given its beneficial effects on disease control, reduction in organ damage, and vascular complications.17,18,28,29,31
Voclosporin is a new calcineurin inhibitor, structurally similar to cyclosporine, but more potent and with decreased toxicity (without requiring drug-level monitoring). It has been approved by the FDA, and very recently by NICE, for the treatment of LN in combination with MPAA, and glucocorticoids.32 Exactly when to use it in LN patients has yet to be generally agreed.
Development and Current Usage of Biological Drugs Approved for the Treatment of LN
Theoretically, drugs which block the antibody-producing B cell lineages ought to be effective in SLE. Belimumab is, currently, the only biological drug approved for LN by the Food and Drug Administration (FDA).33 The use of rituximab is approved in the United Kingdom by National Health System (NHS) England and, as mentioned, is recommended by EULAR, the ACR, and the Kidney Disease Improving Global Outcomes (KDIGO) group.17,18,28,34
Belimumab
Belimumab is a fully human IgG1λ monoclonal antibody that inhibits the soluble form of a B-cell survival factor, BAFF (also known as BLyS). It has been approved for LN treatment in combination with standard initial and subsequent therapy, either in intravenous or subcutaneous form, for patients 5 years or older with autoantibody-positive SLE.33 However, experience with this regimen is still limited, and its role in initial therapy has not been established.35
The addition of belimumab to standard initial and maintenance therapy appears to improve rates of renal response in patients with active LN. BLISS-LN, a Phase III trial, multinational, multicenter, randomized 448 class III to V LN patients to receive belimumab added to the initial standard therapy, or just standard therapy. In this trial, complete renal response was significantly higher in the belimumab group compared to those in the placebo (30 vs 20%, OR 1.7, 95% CI 1.1–2.7, p-value = 0.03). The risk of a kidney-related event or death was also lower in the belimumab group (there was one death in the belimumab group, compared to two in the placebo group).16 A post-hoc analysis was performed and demonstrated that higher rates of complete response with belimumab were seen in patients with a urine protein/creatinine ratio <3g/g. However, belimumab treatment still reduced the annual rate of estimated glomerular filtration rate (eGFR) decline, suggesting that the addition of belimumab to standard therapy could lower the risk of LN flare and eGFR decline in these patients.36 A 28-week open-label extension of BLISS-LN was undertaken, endorsing its safety and efficacy, consistent with the previous trial.37
Later, in the Italian BeRLiSS-LN cohort study, belimumab was used as an add-on therapy for LN patients with persistent disease despite initial therapy. Similar results were seen in the BLYSS-LN trial, confirming its efficacy, safety and glucocorticoid-sparing effect, reinforcing the view that belimumab can be used successfully in patients with an incomplete response or persistent disease after the initial therapy.38
Rituximab
Rituximab is a mouse/human chimeric type I anti-CD20 monoclonal antibody that induces B-cell lysis mediated by complement and Fc receptor bearing-cytotoxic cells, and by induced programmed cell death.39 B-cell depletion leads to lower production and concentration of several antibodies, for example, anti-dsDNA, anti-nucleosome, and anti-cardiolipin antibodies.40,41
Rituximab has long been successfully used in rheumatoid arthritis patients and vasculitis.42,43 However, the predicted B-cell depleting therapy benefit in SLE patients has been harder to demonstrate in clinical trials.
The LUNAR trial randomized 144 class III or IV LN patients to receive rituximab plus mycophenolate mofetil (MMF) compared to MMF alone. Rituximab therapy led to more responders, but no statistically significant differences in rates of complete or partial remission were seen after a year. Complete and partial renal response rates were 45.8% for patients receiving placebo and 56.9% among patients receiving rituximab (p-value = 0.18). Despite that, rituximab therapy led to significant reductions in anti-dsDNA antibody and C3/C4 levels, which were associated with reduced proteinuria.13 Many possible reasons to explain this failure have been discussed, including the heterogeneous nature of SLE patients marked by different B-cell phenotypes (especially some hematopoietic stem cells and plasma cells that do not express CD20), the higher levels of B-cell-activation factors (BAFF) following B-cell depletion, but its trial design, which allowed high levels of background medications, especially steroids for long periods, is a likely culprit.44–46
In spite of the lack of clinical trial success, many favorable results with rituximab have been reported in open-label/small-series studies and it has been increasingly used as an off-label drug, with useful efficacy and relative safety. A systematic analysis of 26 observational studies and case reports supported its use in LN patients refractory to cyclophosphamide or MMF-based therapies. In these patients, rituximab was revealed to be effective in inducing partial and, to a lesser extent, also complete remission of LN, with response rates between 67 and 87%, compared to high-dose cyclophosphamide in incident patients.14,47 Further case series support the use of rituximab as an inducer of remission among patients with LN with persisting or relapsing disease, especially in Class III LN.48–51
Rituximab use has also shown efficacy in reducing glucocorticoid dosing, especially if used at the time of diagnosis.12,52–55 In a prospective cohort study 50 class III to V LN patients were treated with 2 doses of rituximab and methylprednisolone on days 1 and 15, and maintenance treatment of MMF and hydroxychloroquine with no steroids. Ninety percent of the patients achieved complete or partial renal response at one year, with only two patients requiring 2 weeks of oral corticoids.55 Another group, in a study of 16 patients (each matched to 3 controls) followed for up to 6 years showed early treatment of patients with SLE with rituximab (followed by azathioprine and hydroxychloroquine) was safe, effective and enabled a striking reduction in steroid use.54 These results suggest that LN patients given rituximab treatment at/soon after diagnosis can safely avoid/minimize oral steroids.
However, rituximab infusion-related reactions can occur in up to 15% of patients.13 Nevertheless, its use has been already approved in the United Kingdom by NHS England, and, as above, it is recommended by EULAR, ACR and KDIGO.17,18,28
Novel Therapeutic Approaches
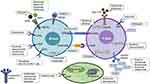
Despite the increasing development of several new biological drugs for autoimmune diseases in the past 20 years, little has changed in the prognosis of LN patients. However, several therapeutic targets and drugs are currently being investigated for LN and might eventually provide a better outcome for these patients [Figure 2].
Further Anti-CD20 Targeting Therapies
B-cell depletion therapy has already been proved to be effective, inducing B-cell lysis mediated by complement and Fc receptor bearing-cytotoxic cells, and by induced programmed cell death, leading to a lower production and concentration of several antibodies.40
Three fully humanized anti-CD20 monoclonal antibodies have been developed: obinutuzumab, ocrelizumab, and ofatumumab. They have increased affinity for FcγRIII, reducing redistribution and internalization, and enhancing its anti-CD20 therapeutic properties.46,56,57
Obinutuzumab, already approved for hematological malignancy treatment, has been shown to be better able to achieve B-cell depletion better in tissues in SLE patients and have a greater affinity for the FcγRIII on effector cells.58,59 A phase II randomized controlled trial (NOBILITY) in which 125 proliferative LN patients were treated with MPAA and glucocorticoids, the addition of obinutuzumab resulted in higher complete renal responses at week 76 (40 vs 18%, p = 0.007) and, at week 104 (54 vs 29%, p = 0.005), compared to placebo. Obinutuzumab treatment was also associated with a potent depletion of CD19+ B cells, without an increased incidence of adverse effects.56 Based on this study, obinutuzumab has already granted a breakthrough therapy designation by the FDA, while phase III trials are ongoing (NCT04702256).
Another anti-CD20 monoclonal antibody, ocrelizumab, has shown in vitro enhanced antibody-dependent cell-mediated cytotoxicity and reduced complement-dependent cytotoxicity versus rituximab. In rheumatoid arthritis (RA) trials, this therapy has significantly reduced progressive joint damage, as well as improved RA’s signs and symptoms. However, higher doses were associated with severe infectious events.60 The phase III BELONG study was conducted to determine the efficacy and safety of ocrelizumab in 381 patients with active LN. However, 28.3% of patients reported a serious adverse effect until the 48th week and 16.9% reported a serious infection. Thus, this study was interrupted due to the rate of serious infections associated with ocrelizumab treatment.57
Ofatumumab is also being studied as an alternative B cell-depleting agent in patients who are rituximab-intolerant and for whom B-cell depletion is deemed as an appropriate therapeutic strategy, since it binds to a distinct extracellular epitope of CD20, with a slower dissociation kinetics.61 Two case series with 12 and 4 active LN patients suggested ofatumumab as an alternative to rituximab in LN patients.62,63
B-Cell Signaling Targeted Therapies
B-cell-activating factors, such as BAFF, a member of the tumor necrosis factor family (also known as BLyS) are one of the most important factors associated with the survival of peripheral self-reactive B-cells. Higher levels of BAFF are also seen after rituximab B-cell depletion in some patients, and seem to be associated with higher levels of anti-dsDNA antibodies, and an increased relapse rate after rituximab therapy.44,64,65 Other B-cell activating factors, such as A proliferation-inducing ligand (APRIL), have been reported to be increased in LN patients.21,66 Fc fragment of IgG receptor IIb (FcγRIIB) can also increase the threshold for B-cell activation.67
Tabalumab is a fully human monoclonal antibody binding membrane and soluble BAFF. Two phase III trials studied its therapeutic effects on moderate-to-severe SLE patients, ILLUMINATE 1 and 2, which randomized 1138 and 1124 patients, respectively. However, the first trial did not meet the primary endpoint (proportion of patients achieving SRI-5 response at week 52) almost certainly because of a stipulation that any alteration in steroids meant the patient had failed and was withdrawn from the study. This was illogical as a reduction in steroids implies the patient was improving, so why prevent them from continuing with the study? The second trial did not have this stipulation and did meet its primary endpoint (SRI-4) but its secondary endpoints with a (time to severe flare, corticosteroid-sparing and fatigue) were not met. Eli Lilly® chose to halt further trials.68–70
Atacicept is a human recombinant fusion protein that binds to two B-cell activating factors BAFF and APRIL. ADRESS II, a phase IIb trial, assessed atacicept in moderate-to-severe SLE patients but excluded those with severe LN. Only patients on atacicept 150mg were associated with a lower SRI-6 score at week 26 [54.9% (adjusted OR 3.31; 95% CI 1.44–7.61), p = 0.005] versus placebo (28.8%). Atacicept also decreased the risk of flare in patients with more serologically active disease, although there was no difference in corticosteroid dosing.71 The higher dose arm of this trial was terminated prematurely as two patients died. In retrospect, this seems an unreasonable decision by the safety committee as, sadly in most clinical SLE trials, a small number of deaths are noted. APRIL-LN, a phase II/III trial of atacicept in combination with MMF in patients with LN, was terminated earlier due to an unexpected decline in serum immunoglobulin G on the first day in three patients. Two of them developed severe respiratory infections.72 Subsequently, it was recognized it was the use of MMF (before the atacicept was injected) that was linked to the fall in the immunoglobulin G levels, and integrated analysis of atacicept trials is reassuring about the safety of atacicept.73,74 Vera Therapeutics® recently acquired atacicept from Merck Serono® and are undertaking a new trial on LN (NCT05609812). Telitacicept, another fusion protein that binds both to BAFF and APRIL, has met its primary endpoint in a recent phase III trial for SLE patients, with a sustained SRI4 response at week 52 and a rapid and sustained increase of C3 and C4.75 Telitacicept is currently under phase II clinical trials for LN (NCT05680480).
Blisibimod is a molecule with features peptide and antibody, inhibiting both membrane and soluble BAFF. The CHABLIS-SC1 study, a phase III trial, enrolled 442 SLE patients to receive blisibimod versus placebo. It excluded patients with severe active LN, but 29.9% of patients had renal involvement. This study did not meet its primary endpoint, but blisibimod treatment has demonstrated a corticosteroid tapering effect, and, also, in patients with renal disease, a reduction of over 50% in urinary protein/creatinine ratio between the 24 to the 52 weeks, suggesting that this drug could have benefits in LN patients.76 Another phase III trial – CHABLIS7.5 to address the efficacy and safety of blisibimod in patients with and without LN is ongoing (NCT02514967).
Briobacept is another BAFF-binding protein. Studies in SLE induced-mice demonstrated its effectiveness in inhibiting anti-dsDNA antibody development, reducing proteinuria, and improving glomerular changes.77 Despite these promising results, its efficacy and safety in human SLE have not been studied yet.
Combining a B-cell activation inhibitor to rituximab could prevent the expansion of autoreactive B cells by high levels of a BAFF resulting from B-cell depletion.78 Thus, the CALIBRATE trial, a phase II trial, studied the outcome of 43 patients with recurrent or refractory LN at 24–48 weeks of a regimen that included cyclophosphamide, prednisone, and rituximab, followed by maintenance therapy with belimumab, compared with the same regimen, but without belimumab. This was a safety trial, not powered to look critically at clinical benefit, but reduced maturation of transitional to naïve B cells and enhanced censoring of autoreactive B-cells were noted.79
BEAT-Lupus, a phase II 52-week clinical trial with 52 patients demonstrated that belimumab following rituximab in SLE patients (including 38% with LN) significantly reduced anti-dsDNA antibody levels by 70% and reduced the risk of severe flare by week 52.80
BLISS-BELIEVE trial, a phase III 104-week trial, compared belimumab therapy with placebo, belimumab plus rituximab and belimumab added to the standard of care therapy in patients with non-renal SLE. However, at week 52, the association between rituximab and belimumab did not improve disease control or remission. Despite the fact that severe active LN patients were excluded from this trial, greater reductions in proteinuria levels were observed in the belimumab plus rituximab group.81
SynBioSe – a phase II single-arm study with 16 patients with refractory disease and 12 with LN at the baseline treated with belimumab plus rituximab as induction therapy and with belimumab as maintenance showed a long-lasting reduction of anti-dsDNA and anti-C1q autoantibodies and prevention of B-cell repopulation for the 2-year follow-up, as well as a 60% complete renal response in this period.82
These findings provide some support for further investigation on the simultaneous use of B-cell targeted therapies with different mechanisms. SynBioSe-2, a phase III trial, comparing standard of care LN treatment versus rituximab and belimumab association, is currently recruiting patients (NCT03747159).83
TNF-Like-Molecule Targeting Therapies
BIIBB023, a monoclonal antibody targeting a TNF-like inducer of apoptosis (TWEAK) that promotes renal inflammation, mesangial proliferation, tubular cell death and fibrosis, was studied in a phase II trial in 276 class III and IV LN patients after induction treatment with MMF and steroids. However, no difference was found in renal response rates, or in the renal response duration. In addition, treatment-emergent adverse effects were reported in 85% of treated patients (versus 76% of patients on placebo), which led to this trial being stopped at week 44.84
Co-Stimulatory Blockade
B-cell depletion therapy is also associated with a reduced expression of CD40L, and other T-cell activation markers. Interactions between CD40L on T cells and CD40 on B cells in the renal interstitium are critical for the local expansion of autoantibody-producing B-cells in LN. Therapies that target this CD40-CD40L and its inducible co-stimulatory molecule (ICOS-ICOSL) ligation pathways may also improve LN management.85,86
Dapirolizumab is a polyethylene glycol-conjugated antigen-binding fragment targeting CD40L. A 24-week phase II study randomized 182 SLE patients to receive dapirolizumab. Patients with stable LN were permitted entry, whereas patients with class III or IV LN, proteinuria >2g/day, estimated glomerular filtration rate <45 mL/min/1.73 m2, and serum creatinine >2.5 mg/dl were excluded. Although the primary endpoint of this study BICLA response rates were not met, several clinical measures of disease activity generally improved from baseline in dapirolizumab-treated patients. Furthermore, greater reductions in anti-dsDNA levels and increases in C3 and C4 levels were observed in these patients, with an acceptable safety profile and tolerance. No renal endpoints were evaluated.87
Abatacept is CTA-4 agonist that interrupts CD80/CD86 interaction with CD28, modulating T-cell co-stimulation, and inhibiting T-cell proliferation and cytokine production. It has been approved for RA, and its co-stimulation inhibition has been found to be effective in LN mice.88,89 The ACCESS trial – a 52-week phase II trial randomized 134 patients with class III or IV LN (with or without class V) and urine protein-to-creatinine ratio >1 to receive either abatacept or placebo added to low-dose cyclophosphamide followed by azathioprine. This trial showed no differences with respect to primary outcomes (complete response at week 24), secondary outcomes or safety issues.90
Another phase II/III trial randomized 298 class III or IV LN patients to receive either abatacept (10mg/kg or 30mg/kg) or placebo but this time in association with MMF. The primary endpoint (maintenance of glomerular filtration rate, minimal proteinuria, and inactive urinary sediment over the 52-week treatment time) was not achieved. However, renal improvement response rates at week 51 were higher in both of the abatacept groups (22.2% in the abatacept 30mg/kg group, 27.3% in the abatacept 10mg/kg group versus 20% in the placebo group). In a subgroup analysis, patients with nephrotic-range proteinuria had a 20–30% greater reduction in urine protein-to-creatinine ratio versus placebo, from week 24 until the end of this study. Abatacept also showed greater improvements in serological activity.91 However, the complete renal response criteria were more strict than in other LN trials, which may have led to the failure to achieve its primary endpoint.88
A phase III trial with abatacept versus placebo with similar primary outcomes as the previously described also showed no differences with abatacept treatment. However, abatacept-treated patients demonstrated a more rapid improvement in proteinuria and earlier sustained complete response.92 Despite these discouraging results, abatacept may still have a place in LN treatment but also in articular and cutaneous involvement of SLE.93
Some phase II clinical trials are ongoing evaluating the safety and efficacy of molecules interfering with B- and T-cell interaction, for example with CD40 antagonists such as iscalimab (NCT03610516), BI 655064 (NCT03385564, NCT02770170 and VIB4920 (NCT05201469)).
Anti-CD19 Targeted Therapies
CD19, as a B-cell surface marker, can also be a therapeutic target in order to increase B-cell depletion in patients with SLE, especially LN. However, obexelimab (XmAb5871) is a humanized monoclonal antibody with increased affinity to FcγRIIb, a reversible B-cell inhibitor. One hundred and four moderately active SLE patients were randomized to receive obexelimab vs placebo. However, the primary endpoint, the proportion of patients with no loss of improvement (defined by improvement of at least 4 points in SLEDAI score or more than one-grade decrease in one or more BILAG index A or B scores) by day 225, was not met in the obexelimab cohort, not even in the subpopulation with seropositive disease. These results lead the manufacturer to decide not to proceed to phase III trials.94
Anti-CD22 Targeted Therapies
CD22 is expressed on the surface of B-cells and modulates B-cell activation and migration. Epratuzumab is a humanized anti—CD22 monoclonal antibody that inhibits B-cell activation. The first phase II/III trials were interrupted due to an interruption in drug supply, however exploratory pooled analyses described a 44.1% (15 out of 34 patients) response with epratuzumab 360/m2 versus placebo (30% - 9 out of 30 patients).95 EMBLEM – another phase IIb trial was conducted with 227 patients. Responder rates between epratuzumab and placebo groups were not significant. However, in a post-hoc analysis, epratuzumab 600mg weekly showed reductions in musculoskeletal, mucocutaneous, cardio-respiratory, neuropsychiatric, constitutional and renal BILAG systems compared to placebo.96 EMBODY 1 and 2, two phase III trials, enrolled 786 and 788 patients to receive epratuzumab vs standard of care therapy. Unfortunately, the primary endpoint, defined as improvement at week 48 based on the BICLA, was not achieved. 265 and 258 patients, respectively, prematurely discontinued due to lack of efficacy. All trials demonstrated a B-cell reduction of 30–50%; however, complement and autoantibody levels remained constant.97 LN patients were excluded from these studies. Later, a post-hoc analysis suggested that SLE accompanied by Sjögren syndrome seems to benefit from epratuzumab.98 Despite this, UCB® decided not to proceed with further studies.
Anti-CD38 Targeted Therapies
Daratumumab is a human monoclonal antibody that targets CD38, a glycoprotein that is highly expressed in plasma cells, already approved for multiple myeloma treatment. Two cases were reported in 2020 where it was used to treat two very active SLE patients, one of them with class III and V LN, already treated with MPAA, cyclophosphamide, and bortezomib. Treatment with daratumumab (followed by maintenance with belimumab) was associated with a significant reduction in pathogenic anti-dsDNA antibodies, as well as overall improvement, with proteinuria lowering and LN improvement.99 A phase II trial evaluating the efficacy and safety of daratumumab in 12 patients with LN for 24 months is currently recruiting (NCT04868838).
Anti-CD6 Targeted Therapies
CD6 is a costimulatory receptor expressed on T-cells, and, together with activated leukocyte cell adhesion molecule (ALCAM), regulates T-cell activation and function. The presence of soluble ALCAM in SLE patients is associated with active renal involvement, suggesting CD6/ALCAM pathway as a disease biomarker and a therapeutic target.100
The EQUALISE study is a phase Ib multiple ascending-dose study of Itolizumab (a humanized monoclonal antibody that binds CD6 and blocks ALCAM interaction) in 55 patients with SLE with or without active class III or IV LN. Results are still awaited (NCT04128579).
Bruton’s Tyrosine Kinase-Targeted Therapies
Another potential therapy target is Bruton’s tyrosine kinase (BTK), which is a cytoplasmic tyrosine kinase present in cells of hematopoietic origin, essential to intracellular signaling in the development, survival, and activation of B-cells. BTK+ cells in SLE patient’s blood seem to correlate with disease activity.101,102 BTK inhibitors have been shown to limit the development of nephritis in SLE-prone mice; however, no benefit was yet identified in human SLE patients.102
Fenebrutinib, an oral highly selective BTK inhibitor, was studied in 260 SLE patients in the ATHOS phase II trial. Patients with proliferative LN were excluded. In this trial, clinical efficacy was not achieved, although a sustained strong BTK inhibition was demonstrated. However, fenebrutinib treatment significantly reduced CD19+ B-cells, immunoglobulins, and increased complement C3 and C4, with an acceptable safety profile.103 As well as fenebrutinib studies, phase II trial with evobrutinib (with no LN patients) demonstrated no clinical differences versus placebo.104 Other BTK inhibitors, such as ibrutinib, branebrutinib, elsubrutinib (alone or in combination with upadacitinib), orelabrutinib, TAS 5315, have already shown efficacy in SLE animal models, and are currently in clinical trials.105
Janus-Kinase Inhibitors
Janus-kinase (JAK) and Signal Transducers and Activators of Transcription (STAT) cascade signaling lead to the activation of different proinflammatory cytokines (interleukins, interferons, as well as endocrine factors), enhancing T- and B-cell interaction and antibody production. Thus, inhibiting this pathway could also be a target for SLE patients, and tofacitinib (a JAK/STAT inhibitor) has been used in mice successfully reducing proteinuria and improving renal function.106 One case report described a patient with RA and SLE with class III LN resistant to azathioprine, hydroxychloroquine, MMF, rituximab and cyclosporin A treatments, who started tofacitinib 5mg bd with persistent complete renal response.107
Deucravacitinib is a highly selective tyrosine kinase 2 (TYK2), a JAK family member, studied in SLE patients in the phase II trial PAISLEY, where active LN patients were excluded. However, deucravacitinib treatment led to higher response rates for SRI-4, with an acceptable safety profile.108 A phase II clinical trial to evaluate the safety and effectiveness of deucravacitinib in LN patients, PAISLEY LN, failed to enroll a sufficient number of patients.
Baricitinib is an oral selective inhibitor of JAK 1 and 2, approved for AR, and was implicated in phase II and III trials for SLE patients (without severe LN). In a phase II trial, baricitinib 4mg significantly improved disease activity compared to placebo.109 SLE-BRAVE-I and II were phase III trials randomly assigned 760 and 775 patients, respectively, to receive baricitinib 4 mg, baricitinib 2 mg, or placebo once daily for 52 weeks. The SLE-BRAVE I met its primary endpoint (SRI-4 response at week 52) in the 4mg baricitinib group; however, this positive result was not replicated in SLE-BRAVE II.110,111 None of the secondary endpoints were met in either study, including glucocorticoid tapering. Additional analyses are being performed to understand this discordance.
A 6-month clinical trial with 60 patients to evaluate the efficacy of baricitinib (a JAK1 and 2 inhibitor) to induce remission of active LN versus cyclophosphamide is ongoing (NCT05432531).
Proteasome Inhibitors
Proteasomes are one of the main protein degradation systems. Its inhibition leads to the accumulation of defective immunoglobulin chains with subsequent apoptosis of plasma cells with a decrease in immunoglobulin production. This mechanism could lead to a better control of SLE severity and damage. Bortezomib approved for multiple myeloma treatment, decreases anti-dsDNA antibody levels, proteinuria and kidney damage, as well as a type-I IFN activity inhibition, leading to a prolonged survival in SLE mice.112 Case series with class III to V LN patients suggested that bortezomib could be a safe and effective treatment for resistant LN patients, with significantly reduced proteinuria, lower levels of anti-dsDNA levels, and, mostly partial, renal responses.113–115
However, bortezomib carried a 30% risk of peripheral neuropathy, as well as thrombocytopenia, and indications for its use need to be better defined. As was noted with bortezomib, next-generation proteasome inhibitors delanzomib and carfilzomib also have shown a decrease in anti-dsDNA levels, proteinuria, and kidney damage in SLE mice, and could be a good alternative as they are associated with less adverse effect rates.116,117
MISSION, a phase Ib/II study of zetomipzomib (KZR-616 – a selective inhibitor of the immunoproteasome) in patients with SLE (with or without LN) was completed recently. However, the results are yet to be published (NCT03393013). A phase II trial comparing the efficacy and safety of two different doses of zetomipzomib with placebo in patients with active LN is ongoing (NCT05781750).
Interleukin-Targeting Therapies
IL-23 is an important contributor to the expansion and maintenance of Th17 cells, enhancing the CD4+ production of IL-17, which induces the production of several inflammatory cytokines. IL-17 has been found to be a good biomarker for SLE activity and a predictor of LN remission.118
Two case reports suggest that secukinumab (an antibody targeting IL-17) may bring some improvement for LN patients.119,120 One described a 29-year-old woman with SLE previously treated with prednisolone, thalidomide, azathioprine, methotrexate, and rituximab, who developed a class IV lupus nephritis that relapsed despite the addition of immunoglobulin and belimumab to the previous therapy. Leflunomide was started with no renal response. After that, secukinumab was started (300mg id subcutaneously) with a complete renal response 8 months after the treatment.120 The other case described a 62-year-old woman with psoriasis vulgaris and SLE with LN previously treated with steroids and cyclosporin A, who presented with a nephrotic syndrome along with exacerbation of psoriasis and alopecia. The kidney biopsy showed a Class IV and V LN. She was first started on high-dose steroids and MMF, then changed to cyclophosphamide without any renal response. Peripheral blood mononuclear cells were analyzed using flow cytometry, suggesting a substantial proliferation of Th17 cells. Secukinumab was started (300mg weekly) with a marked reduction in SLE activity and reduced levels of creatinine, proteinuria and serological markers.119
A two-year phase III trial is ongoing to evaluate the efficacy and safety of subcutaneous secukinumab 300 mg compared to placebo, in combination with standard of care therapy, in patients with active class III or IV LN (NCT04181762).
Ustekinumab is a monoclonal antibody that inhibits both IL-12 and IL-23, interfering with Th17 activity. This anti-IL12/23 drug met its endpoints in a phase II trial of non-LN but failed to do so in a phase II trial. Further studies have been abandoned.121,122
Guselkumab is a monoclonal antibody that binds to IL-23. A phase II randomized trial to access the efficacy and safety of this drug in LN patients has already finished. Results are yet to be published (NCT04376827).
IL-1 is a proinflammatory cytokine associated with mesangioproliferative nephritis in mice. Treatment with an IL-1 antagonist (such as anakinra) has demonstrated a reduction in proteinuria and lower renal damage in LN mice.123 Measurement of an anti-IL-1 antagonist could help to predict future renal involvement in LN patients.124 Some case reports suggest that anakinra may be an effective and safe treatment for SLE patients; however, no randomized trials have been done so far.125,126
IL-2 is a main regulator of Treg cells, and its deficiency can lead to B- and T-cell hyperactivity. Aldesleukin (a recombinant human IL-2) was used in a single center in Germany in 10 SLE patients with active and refractory disease. Its use was associated with an increase in Treg-cells, as well as an increase in levels of complement. All of the patients showed a reduction in disease activity, with 8 patients achieving a complete response after 9 weeks of treatment.127 More studies are needed in order to address its efficacy in SLE patients. A prospective, single-center study is comparing the efficacy and safety of human umbilical cord mesenchymal stem cells and low-dose IL-2 in the treatment of LN (NCT05631717).
Another interleukin strongly implicated in LN pathogenesis is IL-6, and like IL-17, is also considered to be a biomarker of SLE activity and a good predictor of LN remission.118 IL-6 inhibition delays LN onset in mice and reduces proteinuria and serum creatinine levels helping to preserve glomerular function and structure.128 A phase II study randomized 25 class III or IV LN patients with persistent disease, despite standard-of-care therapy, to receive either sirukumab (an anti-IL6 monoclonal antibody) or placebo. However, treatment with sirukumab did not result in an overall change in proteinuria from baseline to week 24, despite binding to IL-6 with high affinity and specificity. There was also no significant improvement in disease activity scores, and patients treated with sirukumab experienced significantly more adverse effects, mostly infection-related ones, during this trial.129 No further trials have been undertaken with this drug.
Anti-IFNα
Type I interferon plays an important role in SLE pathology and is correlated with disease activity, especially in LN patients.130 Anifrolumab is a human monoclonal antibody that binds to type I interferon receptor subunit 1, was approved by the FDA in 2021 for SLE patients with moderate-to-severe manifestations (but without severe active renal or neuropsychiatric involvement) following MUSE, TULIP-1 and 2 trials.131 Evidence that it is effective in LN patients is lacking. TULIP-LN is a 52-week phase II trial, aiming to randomize 147 class III and IV LN patients to receive anifrolumab 300mg monthly, anifrolumab 900mg three times and then 300mg monthly, or placebo along with standard therapy. The primary endpoint, a change in baseline 24-hour urine protein–creatinine ratio at week 52 combined anifrolumab versus placebo groups, was not met. However, a more intensive treatment with anifrolumab was associated with more complete renal responses (45.5% with anifrolumab vs 31.1% placebo) and also with sustained corticoid reductions. The incidence of herpes zoster and influenza infections was higher among patients on anifrolumab treatment (16.7% vs 8.2% placebo).132 Overall, anifrolumab in higher doses could be a good alternative to LN patients, but more studies are needed to support this hypothesis. IRIS, a 116-week phase III randomized controlled trial, is recruiting LN patients to evaluate the efficacy and safety of anifrolumab plus standard of care (mycophenolate mofetil and corticosteroids) in active class III or IV LN patients (NCT05138133).
Sifalimumab is another INFα inhibitor. A phase II trial that randomized 431 patients given different doses of sifalimumab versus placebo demonstrated clinical efficacy of sifalimumab in the treatment of moderate-to-severe SLE patients with an inadequate response to standard-of-care treatments.133 However, active LN patients were not included in this study, and its efficacy in this group of patients needs to be better addressed.
Complement Inhibition
Complement is a component of the innate immune system with significant implications for modulating adaptive immune response, T-cell activation and B-cell regulation. Its inhibition has already been demonstrated to be implicated in lupus pathogenesis.
Eculizumab is a recombinant humanized monoclonal antibody that binds to C5 complement, blocking the activation pathway. Its use in mice has demonstrated a significant reduction in proteinuria and renal dysfunction.134 However, eculizumab has not been studied for LN. However, phase II clinical trials to study other complement inhibitors such as ravulizumab (an anti-C5 monoclonal antibody – NCT04564339), narsoplimab [an anti- mannose-binding protein-associated serine protease 2 (MASP-2) antibody targeting the lectin pathway of the complement system – NCT02682407], APL-2 (an anti-C5 monoclonal antibody – NCT03453619), iptacoban (a factor B inhibitor of the complement pathway – NCT05268289), ALXN2050 (a factor D inhibitor of the complement pathway – NCT05097989) in LN patients are ongoing.
Other Immunosuppressants
Following the inflammatory cascade, renal-tissue macrophages are activated, contributing to tissue damage. Laquinimod (5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide) is an immunomodulatory drug that increases the proportion of anti-inflammatory type II monocytes/macrophages, decreasing proinflammatory ones, reducing antigen presentation, thus leading to a decrease in INF-γ, IL-12, IL-17, IL-23 and TNFα. Studies with LN mouse models suggested that Laquinimod could contribute to improved survival, proteinuria and glomerular changes.135 In a phase II trial 46 patients were randomized to receive laquinimod 0,5mg/day, laquinimod 1mg/day, or placebo, together with standard-of-care therapy, for 24 weeks. Patients receiving laquinimod had a greater eGFR and proteinuria change vs placebo. However, this trial was not powered to establish definitely any difference between these groups.136 No further studies have been done in LN.
Another immunomodulating agent – iguratimod, whose mechanisms of action are not fully understood, seems to have an inhibitory effect on inflammatory cytokines and immunoglobulin production. In preclinical studies with lupus-prone mice, this drug prevented LN and decreased the amount of proteinuria as well as decreased immunocomplex deposition.137 A Chinese study cohort with 14 refractory or relapsing LN patients (with no SLE extra-renal involvement) substituted iguratimod for previous immunosuppressant agents, with no changes in steroid dosing. Of the thirteen eligible patients, 38.5% achieved complete response and 53.8% achieved partial response at week 24, with the maintenance of eGFR in all patients. However, 25% of the patients experienced a relapse during the 144-week follow-up.138 A Chinese multicenter randomized 52-week parallel positive drug-controlled trial is ongoing in order to demonstrate whether iguratimod can be used as an alternative induction or maintenance therapy for class III to V LN patients (NCT02936375).139
Mizoribin is an immunosuppressant similar to MMF that inhibits inosine monophosphate dehydrogenase, by inhibiting both humoral and cellular immune responses. It has been approved for LN only in Japan (where the experience with this drug is largely confined), invariably co-prescribed with steroids in LN maintenance therapy, and seems to be well tolerated.140 However, some reports had shown inconsistent results regarding its effects on proteinuria and on the timing to flare versus steroids.141–144 The optimal dose of this drug is yet to be found.145 Some other reports suggest that mizoribin could have increased efficacy when associated with other immunosuppressants, such as tacrolimus, as an induction therapy.146,147 A post-marketing surveillance study that included 559 patients on mizoribin plus steroids (with or without tacrolimus) showed renal remission in 63.3% of patients, with 26.5% achieving complete renal remission at week 24, and a good safety profile.148 More studies are needed in order to establish mizoribin efficacy versus placebo or other drugs in LN patients.
Sirolimus is an mTOR inhibitor (also called rapamycin). A recent meta-analysis included 49 patients with SLE treated with sirolimus and demonstrated that 41.2% of LN patients achieved renal remission; however, no significant changes in proteinuria were found.149 A study to investigate the efficacy of sirolimus for mild proteinuric flares in patients with Class III/IV (with or without class V) LN is currently ongoing (NCT04892212).
Cell-Based Therapies
Hematopoietic stem cell transplantation (HSCT), by eliminating self-reactive memory T and B cells, has been reported as a potential target for refractory SLE patients, already reported in some small trials, with a good and long-term clinical and serological response, without evidence of relapse or progression in 5 years achieving 50% in two cohort studies with 85 and 50 SLE patients. However, no randomized trials have been done so far. In addition, the invasive nature of this procedure, its toxicity and the high infectious risk associated with it should be weighed up for each patient.150–152 Mesenchymal stem cells (MSCs) can also exert immunomodulatory effects, downregulating active immune responses. The clinical efficacy of MSC in treating patients with refractory autoimmune disorders, including SLE, is encouraging. An open-label clinical trial with 81 active LN patients refractory to standard-of-care treatment was submitted to allogeneic bone marrow MSC transplantation. A total of 60.5% of patients experienced complete or partial remission during the 12 months, and a significant difference was observed in the average renal BILAG score, proteinuria and creatinine values.153 However, MSC treatment has been associated with relapsing rates of 12.5% and 16.7% at 6 and 12 months, respectively, which could reflect the need to repeat MSC infusions after 6 months to a more prolonged efficacy.154
A Chinese randomized trial with 18 LN patients showed that MSC had no additional effect over standard immunosuppression, and the trial was abandoned.155
Chimeric antigen receptor T (CAR-T) cells targeting the surface antigen CD19, depleting B-cells, have emerged recently from cancer immunotherapy development, as a potential target for SLE patients, especially LN.156 A small series of 5 resistant LN patients were treated with anti-CD19 CAR-T cells. Clinical remission was observed in all of the 5 patients, with marked reductions in proteinuria and creatinine levels. Serological markers normalized, including anti-dsDNA antibody levels, and, despite B-cell reconstitution, no SLE relapse was seen during the follow-up (5 to 17 months) and no immunosuppressive medication was required since the initiation of CAR T cell treatment.157 A clinical trial with 9 refractory LN patients to access safety and effectiveness of CD19/BCMA CAR-T cells is currently ongoing (NCT05085418).
Discussion
In this article, we have summarized the new drugs that have been and are being developed for LN patients. LN treatment is still based on conventional immunosuppressants, and there has been little evidence of improved outcomes in the past thirty years.
The development of new biological drugs is an expensive and laborious process. SLE is a very heterogeneous disease, with a wide interindividual variation in molecule expression, which could explain why some LN patients respond well to one drug, while others do not. A precision approach is badly needed.
Belimumab is the only FDA-approved drug for LN patients. However, complete renal response was only observed in 30% of LN patients included in the BLYSS-LN trial, and this response was more clear-cut in patients with a urine protein/creatinine ratio <3g/g. It is only approved for patients with an incomplete response or persistent disease after the standard therapy, and with positive serology for anti-dsDNA antibodies, which excludes almost a third of all SLE patients.16,36,37
Rituximab has only been approved by NHS England but is also recommended as an alternative treatment for non-responding or refractory LN by ACR and EULAR outlines.28,29 Despite its failure to achieve its primary endpoint in the LUNAR trial, rituximab has been associated with several clinical and serological improvements. Since it is a widely available and reasonably safe drug, it has been widely used off-label, with good clinical outcomes.13,47 Rituximab failure has been associated with increased levels of BAFF.44 Thus, combined therapies with a B-cell depleting therapy, rituximab plus a BAFF inhibitor, have been undertaken, notably the CALIBRATE and BLISS-BELIEVE trials. However, the potential benefits of combining both drugs have given some conflicting results. CALIBRATE was a reassuring study on LN patients and not powered to look at benefit.79,81 BEAT-Lupus and SynBioSe trials suggested that belimumab added to rituximab in induction therapy, with belimumab added to standard-of-care therapy for maintenance, could reduce the risk of a severe disease flare, but also could improve renal outcomes inducing remission.80,83 However, the BLISS-BELIEVE trial (of non-renal SLE patients) in which every patient received belimumab and half also received rituximab showed no benefit for those treated with combination of monoclonals.81 It may be the sequence of giving biologics is important, with rituximab to be given first, followed by belimumab, being more likely to succeed.
Encouragingly, new drugs are showing some promising results in phase II and III trials regarding LN [Table 1]. Obinutuzumab, blisibimod, abatacept, anifrolumab and baricitinib added to standard-of-care therapy for LN have been shown to improve outcome for LN patients.
 |
Table 1 Phase II and III Trials for Lupus Nephritis |
In the NOBILITY phase II trial, obinutuzumab showed significantly higher complete renal responses at week 76 and week 104 together with a potent depletion of CD19+ B-cells. If phase III trials confirm these results, obinutuzumab – a fully humanized antibody, with a lower risk of allergic reactions – could potentially replace rituximab in the treatment of refractory LN.56
Blisibimod, a BAFF-inhibitor, has also demonstrated in CHABLIS-SC1 trial, a reduction of urinary protein/creatinine ratio between the 24 to the 52 weeks, suggesting that this drug could have benefits in LN patients.76
Abatacept trials showed little difference in clinical endpoints (although criteria for response were more strict than other LN trials), but improvements in proteinuria and earlier sustained complete response have been associated with this treatment, leaving some hope for future trials with abatacept in LN patients.88,90,91,93
Anifrolumab, already approved for SLE patients, has been associated with more complete renal responses compared to a placebo.132 Phase III trials are ongoing, and LN patients might benefit from this drug.
Baricitinib, a JAK 1 and 2 inhibitor, demonstrated promising results in phase II trials on SLE patients.109 However, phase III trials, SLE-BRAVE I and II, were discordant on the results since the primary endpoint reached in SLE-BRAVE I with baricitinib 4mg was not replicated in SLE-BRAVE II.110,111 Additional studies are needed to better clarify these results. A trial to evaluate the efficacy of baricitinib on LN is ongoing.
More recently, CAR-T-cells have emerged with some impressive results in 5 LN patients in terms of proteinuria and creatinine levels, as well as in serological markers, with no relapses seen during their follow-up and with no other immunosuppressive medication required.157 Results on rather more LN patients are awaited to determine whether this costly and potentially hazardous approach may have a role for hard-to-treat LN patients.
Conclusions
The development of new drugs to improve outcome in patients with LN has been a challenging process compared to other rheumatological diseases. This could be explained by the big heterogenicity between SLE patients in terms of clinical features, serological and molecular markers, as well as histological features. However, there is an urgent need to improve clinical trial design, with an appropriate population, and adjusted-outcome measures, in order to prove proper benefits of these new drugs and change the outcome of LN patients.
Disclosure
Dr Veronica Venturelli reports non-financial support from Boehringer Ingelheim, outside the submitted work. D.A.I. has received honoraria from Amgen, AstraZeneca, UCB Pharma, Vera Therapeutics, Merck Serono; personal fees from Eli Lilly. These honoraria are passed onto a local arthritis charity. The authors report no other conflicts of interest in this work.
References
1. Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706–1713. doi:10.1136/annrheumdis-2013-205171
2. Moghaddam B, Marozoff S, Li L, Sayre EC, Zubieta JAA. All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatol. 2022;61(1):367–376. doi:10.1093/rheumatology/keab362
3. Rovin BH. Systemic Lupus Erythematosus.
4. Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatol. 2015;55(2):252–262. doi:10.1093/rheumatology/kev311
5. Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev. 2004;3(6):423–453. doi:10.1016/j.autrev.2004.04.002
6. Merrell M. Determination of prognosis in chronic disease illustrated by Systemic Lupus Erythematosus. J Chronic Dis. 1853;1:12–32. doi:10.1136/bmj.s3-1.6.117
7. Ward MM. Changes in the incidence of End-Stage Renal Disease Due To Lupus Nephritis in the United States, 1996 – 2004. J Rheumatol. 2010;36(1):63–67. doi:10.3899/jrheum.080625
8. Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients with Lupus Nephritis, 1971-2015: a Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol. 2016;68(6):1432–1441. doi:10.1002/art.39594
9. Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatol. 2011;1424–1430. doi:10.1093/rheumatology/ker101
10. Houssiau FA, D’Cruz D, Sangle S, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi:10.1136/ard.2010.131995
11. Burmester GR, Bijlsma JWJ, Cutolo M, McInnes IB. Managing rheumatic and musculoskeletal diseases-past, present and future. Nat Rev Rheumatol. 2017;13(7):443–448. doi:10.1038/nrrheum.2017.95
12. Pepper R, Griffith M, Kirwan C, et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant. 2009;24(12):3717–3723. doi:10.1093/ndt/gfp336
13. Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi:10.1002/art.34359
14. Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, et al. Clinical and immunological effects of Rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8(3):1–9. doi:10.1186/ar1954
15. Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi:10.1002/art.27233
16. Furie R, Rovin BH, Houssiau F, et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med. 2020;383(12):1117–1128. doi:10.1056/nejmoa2001180
17. Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64(6):797–808. doi:10.1002/acr.21664
18. Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):S713–S723. doi:10.1136/annrheumdis-2020-216924
19. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of Autoantibodies before the Clinical Onset of Systemic Lupus Erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi:10.1056/NEJMoa021933
20. Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24(9):1357–1366. doi:10.1681/ASN.2013010026
21. Canny SP, Jackson SW. B Cells in Systemic Lupus Erythematosus: from Disease Mechanisms to Targeted Therapies. Rheum Dis Clin North Am. 2021;47(3):395–413. doi:10.1016/j.rdc.2021.04.006
22. Fillatreau S, Manfroi B, Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(2):98–108. doi:10.1038/s41584-020-00544-4
23. Liarski VM, Kaverina N, Chang A, et al. Cell Distance Mapping Identifies Functional T Follicular Helper Cells in Inflamed Human Renal Tissue. Sci Transl Med. 2014;6(230). doi:10.1126/scitranslmed.3008146
24. Ohmura K. Which is the best SLE activity index for clinical trials? Mod Rheumatol. 2021;31(1):20–28. doi:10.1080/14397595.2020.1775928
25. Luijten KMAC, Tekstra J, Bijlsma JWJ, Bijl M. The Systemic Lupus Erythematosus Responder Index (SRI); A new SLE disease activity assessment. Autoimmun Rev. 2012;11(5):326–329. doi:10.1016/j.autrev.2011.06.011
26. Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology. 2005;44(7):902–906. doi:10.1093/rheumatology/keh624
27. Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–796. doi:10.1016/j.kint.2017.11.023
28. Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4):S1–S276. doi:10.1016/j.kint.2021.05.021
29. Rojas-Rivera JE, García-Carro C, Ávila AI, et al. Documento de consenso del Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) para el diagnóstico y tratamiento de la nefritis lúpica. Nefrología. 2023;43(1):6–47. doi:10.1016/j.nefro.2022.10.005
30. Draft PR. Kdigo 2023 Clinical Practice Guideline for the Management of Lupus Nephritis Confidential. Do Not Distribute Public Review Draft. 2023:56.
31. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi:10.1136/ard.2008.101766
32. Heo Y-A. Voclosporin: first Approval. Drugs. 2021;81(5):605–610. doi:10.1007/s40265-021-01488-z
33. GlaxoSmithKline. Highlights of prescribing information: BENLYSTA (belimumab). Available from: https://gskpro.com/content/dam/global/%0Dhcpportal/en_US/Prescribing_Information/Benlysta/pdf/BENLYSTA-PIMG-%0DIFU.PDF.
34. NHS England. Clinical Commissioning Policy Rituximab for refractory Systemic Lupus Erythematosus (SLE) in adults and post-pubescent children [200402P] Commissioning Position; 2020. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/07/Rituximab-for-refractory-Systemic-Lupus-Erythematosus-in-adults-and-post-pubescent-children-2.pdf.
35. Guerreiro Castro S, Isenberg DA. Belimumab in systemic lupus erythematosus (SLE): evidence-to-date and clinical usefulness. Ther Adv Musculoskelet Dis. 2017;9(3):75–85. doi:10.1177/1759720X17690474
36. Rovin BH, Furie R, Teng YKO, et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022;101(2):403–413. doi:10.1016/j.kint.2021.08.027
37. Furie R, Rovin BH, Houssiau F, et al. Safety and Efficacy of Belimumab in Patients with Lupus Nephritis. Clin J Am Soc Nephrol. 2022;17(11):1620–1630. doi:10.2215/cjn.02520322
38. Gatto M, Saccon F, Andreoli L, et al. Durable renal response and safety with add-on belimumab in patients with lupus nephritis in real-life setting (BeRLiSS-LN). Results from a large, nationwide, multicentric cohort. J Autoimmun. 2021;124(September):102729. doi:10.1016/j.jaut.2021.102729
39. Cerny T, Borisch B, Introna M, Johnson P, Rose AL. Mechanism of action of rituximab. Anticancer Drugs. 2002;13(1):S3–S10. doi:10.1097/00001813-200211002-00002
40. Cambridge G, Leandro MJ, Teodorescu M, et al. B Cell Depletion Therapy in Systemic Lupus Erythematosus Effect on Autoantibody and Antimicrobial Antibody Profiles. J Am College Rheumatol. 2006;54(11):3612–3622. doi:10.1002/art.22211
41. Sciascia S, Rubini E, Radin M, Cecchi I, Rossi D, Roccatello D. Anticardiolipin and anti-beta 2 glycoprotein-I antibodies disappearance in patients with systemic lupus erythematosus and antiphospholipid syndrome while on belimumab. Ann Rheumatic Dis. 2018;1(3):1–2. doi:10.1136/annrheumdis-2018-213496
42. Edwards JCW, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology. 2001;40(2):205–211. doi:10.1093/rheumatology/40.2.205
43. Habibi MA, Alesaeidi S, Zahedi M, Hakimi Rahmani S, Piri SM, Tavakolpour S. The Efficacy and Safety of Rituximab in ANCA-Associated Vasculitis: A Systematic Review. Biology. 2022;11:1767. doi:10.3390/biology11121767
44. Ehrenstein MR, Wing C. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol. 2016;12(6):367–372. doi:10.1038/nrrheum.2016.18
45. Lazarus MN, Turner-Stokes T, Chavele KM, Isenberg DA, Ehrenstein MR. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatol. 2012;51(7):1208–1215. doi:10.1093/rheumatology/ker526
46. Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther. 2013;15(SUPPL.1):1–8. doi:10.1186/ar3908
47. Weidenbusch M, Römmele C, Schröttle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28(1):106–111. doi:10.1093/ndt/gfs285
48. Li K, Yu Y, Gao Y, Zhao F, Liang Z, Gao J. Comparative Effectiveness of Rituximab and Common Induction Therapies for Lupus Nephritis: a Systematic Review and Network Meta-Analysis. Front Immunol. 2022;13(April):1–9. doi:10.3389/fimmu.2022.859380
49. Díaz-Lagares C, Croca S, Sangle S, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev. 2012;11(5):357–364. doi:10.1016/j.autrev.2011.10.009
50. Kronbichler A, Brezina B, Gauckler P, Quintana LF, Jayne DRW. Refractory lupus nephritis: when, why and how to treat. Autoimmun Rev. 2019;18(5):510–518. doi:10.1016/j.autrev.2019.03.004
51. Iwata S, Saito K, Hirata S, et al. Efficacy and safety of anti-CD20 antibody rituximab for patients with refractory systemic lupus erythematosus. Lupus. 2018;27(5):802–811. doi:10.1177/0961203317749047
52. Melander C, Sallée M, Trolliet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol. 2009;4(3):579–587. doi:10.2215/CJN.04030808
53. Ezeonyeji AN, Isenberg DA. Early treatment with rituximab in newly diagnosed systemic lupus erythematosus patients: a steroid-sparing regimen. Rheumatology. 2012;51(3):476–481. doi:10.1093/rheumatology/ker337
54. Gracia-Tello B, Ezeonyeji A, Isenberg D. The use of rituximab in newly diagnosed patients with systemic lupus erythematosus: long-term steroid saving capacity and clinical effectiveness. Lupus Sci Med. 2017;4(1):1–9. doi:10.1136/lupus-2016-000182
55. Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–1286. doi:10.1136/annrheumdis-2012-202844
56. Furie RA, Aroca G, Cascino MD, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81(1):100–107. doi:10.1136/annrheumdis-2021-220920
57. Mysler EF, Spindler AJ, Guzman R, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 2013;65(9):2368–2379. doi:10.1002/art.38037
58. Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell - mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi:10.1182/blood-2009-06-225979
59. Reddy V, Klein C, Isenberg DA, et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatol. 2017;56(7):1227–1237. doi:10.1093/rheumatology/kex067
60. Rigby W, Tony HP, Oelke K, et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. 2012;64(2):350–359. doi:10.1002/art.33317
61. Zhang B. Ofatumumab. InMAbs. 2009;1(4):326–331.
62. Masoud S, McAdoo SP, Bedi R, Cairns TD, Lightstone L. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatol. 2018;57(7):1156–1161. doi:10.1093/rheumatology/key042
63. Haarhaus ML, Svenungsson E, Gunnarsson I. Ofatumumab treatment in lupus nephritis patients. Clin Kidney J. 2016;9(4):552–555. doi:10.1093/ckj/sfw022
64. Carter LM, Isenberg DA, Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 2013;65(10):2672–2679. doi:10.1002/art.38074
65. Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119(5):1066–1073. doi:10.1172/JCI38010
66. Susianti H, Hanggara DS, Lestari KD, Purnamasari P, Aprilia A. Analysis of TNF-like weak inducer of apoptosis for detecting lupus nephritis. Comp Clin Path. 2022;31(2):313–316. doi:10.1007/s00580-022-03334-4
67. Smith KGC, Clatworthy MR. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10(5):328–343. doi:10.1038/nri2762
68. Isenberg DA, Petri M, Kalunian K, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, Phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(2):323–331. doi:10.1136/annrheumdis-2015-207653
69. Merrill JT, Van Vollenhoven RF, Buyon JP, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled stu. Ann Rheum Dis. 2016;75(2):332–340. doi:10.1136/annrheumdis-2015-207654
70. Rovin BH, Dooley MA, Radhakrishnan J, Ginzler EM, Forrester TD, Anderson PW. The impact of tabalumab on the kidney in systemic lupus erythematosus: results from two Phase 3 randomized, clinical trials. Lupus. 2016;25(14):1597–1601. doi:10.1177/0961203316650734
71. Stohl W, Merrill JT, Looney RJ, et al. Treatment of systemic lupus erythematosus patients with the BAFF antagonist “peptibody” blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials. Arthritis Res Ther. 2015;17(1):215. doi:10.1186/s13075-015-0741-z
72. Ginzler EM, Wax S, Rajeswaran A, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14(1):1–7. doi:10.1186/ar3738
73. Gordon C, Bassi R, Chang P, et al. Integrated safety profile of atacicept: an analysis of pooled data from the atacicept clinical trial programme. Rheumatol Adv Pract. 2019;3(2):1–12. doi:10.1093/rap/rkz021
74. Isenberg D, Cogollo E, Amaral-Silva M. Profile of atacicept and its potential in the treatment of systemic lupus erythematosus. Drug Des Devel Ther. 2015;8:1331. doi:10.2147/DDDT.S71276
75. Wang L, Li J, Xu D, Fang J, Van Vollenhoven R, Zhang F. Efficacy and safety of Telitacicept, a novel BLyS/April Dual Inhibitor, In Patients With Systemic Lupus Erythematosus: a Phase 3, Randomized, Placebo-Controlled 52-week Study. Ann Rheum Dis. 2023;82(Suppl 1):90.2–91. doi:10.1136/annrheumdis-2023-eular.1727
76. Merrill JT, Shanahan WR, Scheinberg M. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(6):883–889. doi:10.1136/annrheumdis-2018-213032
77. Kayagaki N, Yan M, Seshasayee D, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity. 2002;17(4):515–524. doi:10.1016/S1074-7613(02)00425-9
78. Kraaij T, Kamerling SWA, de Rooij ENM, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. 2018;91:45–54. doi:10.1016/j.jaut.2018.03.003
79. Atisha-Fregoso Y, Malkiel S, Harris KM, et al. Phase II Randomized Trial of Rituximab Plus Cyclophosphamide Followed by Belimumab for the Treatment of Lupus Nephritis. Arthritis Rheumatol. 2021;73(1):121–131. doi:10.1002/art.41466
80. Shipa M, Embleton-Thirsk A, Parvaz M, et al. Effectiveness of Belimumab After Rituximab in Systemic Lupus Erythematosus A Randomized Controlled Trial. Ann Intern Med. 2021;174(12):1647–1657. doi:10.7326/M21-2078
81. Aranow C, Allaart C, Amoura Z, et al. Efficacy and Safety of Subcutaneous Belimumab (BEL) and Rituximab (RTX) Sequential Therapy in Patients with Systemic Lupus Erythematosus: the Phase 3, Randomized, Placebo-Controlled BLISS-BELIEVE Study [Abstract]. Arthritis Rheumatol. 2021;73(suppl 10):456.
82. Kraaij T, Arends EJ, Van Dam LS, et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant. 2021;36(8):1474–1483. doi:10.1093/ndt/gfaa117
83. van Schaik M, Arends EJ, Soonawala D, et al. Efficacy of belimumab combined with rituximab in severe systemic lupus erythematosus: study protocol for the phase 3, multicenter, randomized, open-label Synbiose 2 trial. Trials. 2022;23(1). doi:10.1186/s13063-022-06874-w
84. Furie R, Malvar A, Navarra SV, Smirnakis K, Kong J, Franchimont SF. Evaluation of the Efficacy, Safety, and Tolerability of BIIB023 As an Adjunct to Standard of Care in Subjects with Lupus Nephritis [abstract]. Arthritis Rheumatol. 2016;68(suppl):75.
85. Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–513. doi:10.1002/art.20858
86. Liu Z, Liu S, Zhang Y, et al. Distinct roles of ICOS and CD40L in human T-B cell adhesion and antibody production. Cell Immunol. 2021;368:104420. doi:10.1016/j.cellimm.2021.104420
87. Furie RA, Bruce IN, Dörner T, et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatol. 2021;60(11):5397–5407. doi:10.1093/rheumatology/keab381
88. Pimentel-Quiroz VR, Ugarte-Gil MF, Alarcón GS. Abatacept for the treatment of systemic lupus erythematosus. Expert Opin Investig Drugs. 2016;25(4):493–499. doi:10.1517/13543784.2016.1154943
89. Schiffer L, Sinha J, Wang X, et al. Short Term Administration of Costimulatory Blockade and Cyclophosphamide Induces Remission of Systemic Lupus Erythematosus Nephritis in NZB/W F1 Mice by a Mechanism Downstream of Renal Immune Complex Deposition. J Immunol. 2003;171(1):489–497. doi:10.4049/jimmunol.171.1.489
90. Meyers EC, Solorzano BR, James J, Ganzer PD, Elaine S. Treatment of Lupus Nephritis With Abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014;66(11):3096–3104. doi:10.1002/art.38790
91. Furie R, Nicholls K, Cheng TT, et al. Efficacy and safety of Abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 2014;66(2):379–389. doi:10.1002/art.38260
92. Dooley MA, Appel GB, Furie R, et al. A Phase III Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Abatacept or Placebo on Standard of Care in Patients with Active Class III or IV Lupus Nephritis [abstract]. Arthritis Rheumatol. 2018;J70(suppl 9):3306. doi:10.1136/annrheumdis-2018-eular.3306
93. Danion F, Rosine N, Belkhir R, et al. Efficacy of Abatacept in systemic lupus erythematosus: a retrospective analysis of 11 patients with refractory disease. Lupus. 2016;25(13):1440–1447. doi:10.1177/0961203316640911
94. Merrill JT, June J, Koumpouras F, et al. Top-Line Results of a Phase 2, Double-Blind, Randomized, Placebo-Controlled Study of a Reversible B Cell Inhibitor, XmAb®5871, in Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2018;70(suppl 10):L14.
95. Wallace DJ, Gordon C, Strand V, et al. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatol. 2013;52(7):1313–1322. doi:10.1093/rheumatology/ket129
96. Wallace DJ, Kalunian K, Petri MA, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73(1):183–190. doi:10.1136/annrheumdis-2012-202760
97. Clowse MEB, Wallace DJ, Furie RA, et al. Efficacy and Safety of Epratuzumab in Moderately to Severely Active Systemic Lupus Erythematosus: results From Two Phase III Randomized, Double-Blind, Placebo-Controlled Trials. Arthritis Rheumatol. 2017;69(2):362–375. doi:10.1002/art.39856
98. Gottenberg JE, Dörner T, Bootsma H, et al. Efficacy of Epratuzumab, an Anti-CD22 Monoclonal IgG Antibody, in Systemic Lupus Erythematosus Patients With Associated Sjögren’s Syndrome: post Hoc Analyses From the EMBODY Trials. Arthritis Rheumatol. 2018;70(5):763–773. doi:10.1002/art.40425
99. Ostendorf L, Burns M, Durek P, et al. Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2020;383(12):1149–1155. doi:10.1056/nejmoa2023325
100. Chalmers SA, Ramachandran RA, Garcia SJ, et al. The CD6/ALCAM pathway promotes lupus nephritis via T cell–mediated responses. J Clin Invest. 2022;132(1):1–17. doi:10.1172/JCI147334
101. Kong W, Deng W, Sun Y, et al. Increased expression of bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin Rheumatol. 2018;37(1):43–49. doi:10.1007/s10067-017-3717-3
102. Lorenzo-Vizcaya A, Fasano S, Isenberg DA. Bruton’s Tyrosine Kinase Inhibitors: a New Therapeutic Target for the Treatment of SLE? ImmunoTargets and Therapy. 2020;9:105–110. doi:10.2147/ITT.S240874
103. Isenberg D, Furie R, Jones NS, et al. Efficacy, Safety, and Pharmacodynamic Effects of the Bruton’s Tyrosine Kinase Inhibitor Fenebrutinib (GDC-0853) in Systemic Lupus Erythematosus: Results of a Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2021;73(10):1835–1846. doi:10.1002/art.41811
104. Wallace DJ, Dörner T, Pisetsky DS, et al. Efficacy and Safety of the Bruton’s Tyrosine Kinase Inhibitor Evobrutinib in Systemic Lupus Erythematosus: results of a Phase II, Randomized, Double-Blind, Placebo-Controlled Dose-Ranging Trial. ACR Open Rheumatol. 2023;5(1):38–48. doi:10.1002/acr2.11511
105. Neys SFH, Rip J, Hendriks RW, Corneth OBJ. Bruton’s Tyrosine Kinase Inhibition as an Emerging Therapy in Systemic Autoimmune Disease. Drugs. 2021;81(14):1605–1626. doi:10.1007/s40265-021-01592-0
106. Zhou M, Guo C, Li X, et al. JAK/STAT signaling controls the fate of CD8+CD103+ tissue-resident memory T cell in lupus nephritis. J Autoimmun. 2020;109. doi:10.1016/j.jaut.2020.102424
107. Garufi C, Mancuso S, Spinelli FR, et al. Janus kinases inhibitors for treating patients with rhupus. Jt Bone Spine. 2020;87(6):673–674. doi:10.1016/j.jbspin.2020.05.010
108. Morand E, Pike M, Merrill JT, et al. Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: a Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2023;75(2):242–252. doi:10.1002/art.42391
109. Dörner T, van Vollenhoven RF, Doria A, et al. Baricitinib decreases anti-dsDNA in patients with systemic lupus erythematosus: results from a phase II double-blind, randomized, placebo-controlled trial. Arthritis Res Ther. 2022;24(1):112. doi:10.1186/s13075-022-02794-x
110. Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet. 2023;401(10381):1011–1019. doi:10.1016/S0140-6736(22)02546-6
111. Morand EF, Vital EM, Petri M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet. 2023;401(10381):1001–1010. doi:10.1016/S0140-6736(22)02607-1
112. Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–755. doi:10.1038/nm1763
113. Segarra A, Arredondo KV, Jaramillo J, et al. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-center experience. Lupus. 2020;29(2):118–125. doi:10.1177/0961203319896018
114. Sjöwall C, Hjorth M, Eriksson P. Successful treatment of refractory systemic lupus erythematosus using proteasome inhibitor bortezomib followed by belimumab: description of two cases. Lupus. 2017;26(12):1333–1338. doi:10.1177/0961203317691371
115. Hiepe F, Alexander T, Peukert R, et al. Refractory SLE patients respond to the proteasome inhibitor bortezomib. Ann Rheum Dis. 2012;71(Suppl 1):A15.3–A16. doi:10.1136/annrheumdis-2011-201230.34
116. Seavey MM, Lu LD, Stump KL, Wallace NH, Ruggeri BA. Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int Immunopharmacol. 2012;12(1):257–270. doi:10.1016/j.intimp.2011.11.019
117. Ichikawa HT, Conley T, Muchamuel T, et al. Novel Proteasome Inhibitors Have a Beneficial Effect in Murine Lupus via the dual inhibition of Type I Interferon and autoantibody secreting cells. Arthritis Rheum. 2012;64(2):493–503. doi:10.1002/art.33333
118. Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015;76(2):280–287. doi:10.1016/j.cyto.2015.05.007
119. Satoh Y, Nakano K, Yoshinari H, et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus. 2018;27(7):1202–1206. doi:10.1177/0961203318762598
120. Costa R, Antunes P, Salvador P, Oliveira P, Marinho A. Secukinumab on Refractory Lupus Nephritis. Cureus. 2021;13(8):15–19. doi:10.7759/cureus.17198
121. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and Safety of Ustekinumab in Patients With Active Systemic Lupus Erythematosus: results of a Phase II Open-label Extension Study. J Rheumatol. 2022;49(4):380–387. doi:10.3899/jrheum.210805
122. Van Vollenhoven RF, Kalunian KC, Dörner T, et al. Phase 3, multicentre, randomised, placebo-controlled study evaluating the efficacy and safety of ustekinumab in patients with systemic lupus erythematosus. Ann Rheum Dis. 2022;81(11):1556–1563. doi:10.1136/ard-2022-222858
123. Sun H, Liu W, Shao J, Xu H, Xiao K, Sheng G. Study on immunoregulation by interleukin-1 receptor antagonist in NZB/W F mice. J Tongji Med Univ. 1997;17(1):18–20. doi:10.1007/bf02887995
124. Brugos B, Kiss E, Dul C, et al. Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol. 2010;71(9):874–877. doi:10.1016/j.humimm.2010.06.004
125. Dein E, Ingolia A, Connolly C, Manno R, Timlin H. Anakinra for Recurrent Fevers in Systemic Lupus Erythematosus. Cureus. 2018;10(12):2010–2012. doi:10.7759/cureus.3782
126. Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64(4):630–633. doi:10.1136/ard.2004.025858
127. Humrich J, von Spee-Mayer C, Siegert E, et al. Low-Dose IL-2 Therapy in Refractory SLE: results from Single Center Phase I/IIa Clinical Trial [abstract]. Arthritis Rheumatol. 2016:68.
128. Cash H, Relle M, Menke J, et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Fas lpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37(1):60–70. doi:10.3899/jrheum.090194
129. Rovin BH, van Vollenhoven RF, Aranow C, et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment With Sirukumab (CNTO 136) in Patients With Active Lupus Nephritis. Arthritis Rheumatol. 2016;68(9):2174–2183. doi:10.1002/art.39722
130. Feng X, Wu H, Grossman JM, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2951–2962. doi:10.1002/art.22044
131. Tanaka Y, Tummala R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod Rheumatol. 2021;31(1):1–12. doi:10.1080/14397595.2020.1812201
132. Jayne D, Rovin B, Mysler EF, et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann Rheum Dis. 2022:496–506. doi:10.1136/annrheumdis-2021-221478
133. Khamashta M, Merrill JT, Werth VP, et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(11):1909–1916. doi:10.1136/annrheumdis-2015-208562
134. Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/W F1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A. 1996;93(16):8563–8568. doi:10.1073/pnas.93.16.8563
135. Lourenço V, Wong M, Hahn BH, Palma-Diaz MF, Skaggs BJ. Laquinimod delays and suppresses nephritis in lupus-prone mice and affects both myeloid and lymphoid immune cells. Arthritis Rheumatol. 2014;66(3):674–685. doi:10.1002/art.38259
136. Jayne D, Appel G, Chan TM, Barkay H, Weiss R, Wofsy D. LB0003 A Randomized Controlled Study of Laquinimod in Active Lupus Nephritis Patients in Combination with Standard of Care. Ann Rheum Dis. 2013;72(Suppl 3):A164.2–A164. doi:10.1136/annrheumdis-2013-eular.528
137. Xia Y, Fang X, Dai X, et al. Iguratimod ameliorates nephritis by modulating the Th17/Treg paradigm in pristane-induced lupus. Int Immunopharmacol. 2021;96(March). doi:10.1016/j.intimp.2021.107563
138. Kang Y, Yan Q, Fu Q, et al. Iguratimod as an alternative induction therapy for refractory lupus nephritis: a preliminary investigational study. Arthritis Res Ther. 2020;22(1):1–8. doi:10.1186/s13075-020-02154-7
139. Yan Q, Du F, Kang Y, et al. Comparison of iguratimod and conventional cyclophosphamide with sequential azathioprine as treatment of active lupus nephritis: study protocol for a multi-center, randomized, controlled clinical trial (iGeLU study). Trials. 2021;22(1):1–13. doi:10.1186/s13063-021-05475-3
140. Kawasaki Y. Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol. 2009;2009. doi:10.1155/2009/681482
141. Tanaka Y, Yoshikawa N, Hattori S, et al. Combination therapy with steroids and mizoribine in juvenile SLE: a randomized controlled trial. Pediatr Nephrol. 2010;25(5):877–882. doi:10.1007/s00467-009-1341-4
142. Nishi E, Kameda H, Ogawa H, et al. Efficacy of weekly mizoribine pulse therapy in refractory lupus nephritis. Mod Rheumatol. 2013;23(1):97–103. doi:10.1007/s10165-012-0645-6
143. Zhang M, Xing CY, Liu J. Study of the efficacy of mizoribine in lupus nephritis in Chinese patients. Rheumatol Int. 2013;33(11):2737–2742. doi:10.1007/s00296-013-2804-2
144. Kagawa H, Yamanaka R, Hiromasa T. First-line Combination Strategy Provides Favorable 5-year Outcomes for Patients with Lupus Nephritis: a Single-center Observational Study. Acta Med Okayama. 2022;76(5):547–555. doi:10.18926/AMO/64036
145. Fuke T, Abe Y, Hibino S, et al. Mizoribine requires individual dosing due to variation of bioavailability. Pediatr Int. 2012;54(6):885–891. doi:10.1111/j.1442-200X.2012.03733.x
146. Tanaka H, Aizawa T, Watanabe S, Oki E, Tsuruga K, Imaizumi T. Efficacy of mizoribine-tacrolimus-based induction therapy for pediatric lupus nephritis. Lupus. 2014;23(8):813–818. doi:10.1177/0961203314528553
147. Kagawa H, Hiromasa T, Yamanaka R, et al. The first year results of mizoribine/tacrolimus-based multitarget treatment for consecutive patients with lupus nephritis. Clin Exp Nephrol. 2018;22(6):1371–1378. doi:10.1007/s10157-018-1597-8
148. Takeuchi T, Okada K, Yoshida H, Yagi N. Post-marketing surveillance study of the long-term use of mizoribine for the treatment of lupus nephritis: 2-Year results. Mod Rheumatol. 2018;28(1):85–94. doi:10.1080/14397595.2017.1349573
149. Peng L, Wu C, Hong R, et al. Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: a real-world study and meta-analysis. Ther Adv Musculoskelet Dis. 2020;12(6):1759720X2095333. doi:10.1177/1759720X20953336
150. Burt RK, Han X, Gozdziak P, et al. Five year follow-up after autologous peripheral blood hematopoietic stem cell transplantation for refractory, chronic, corticosteroid- dependent systemic lupus erythematosus: effect of conditioning regimen on outcome. Bone Marrow Transplant. 2018. doi:10.1038/s41409-018-0173-x
151. Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95(2):284–292. doi:10.3324/haematol.2009.013458
152. Burt RK. Nonmyeloablative Hematopoietic Stem Cell Transplantation for Systemic Lupus Erythematosus. JAMA. 2006;295(5):527. doi:10.1001/jama.295.5.527
153. Gu F, Wang D, Zhang H, et al. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol. 2014;33(11):1611–1619. doi:10.1007/s10067-014-2754-4
154. Wang D, Li J, Zhang Y, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16(2). doi:10.1186/ar4520
155. Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76(8):1436–1439. doi:10.1136/annrheumdis-2017-211073
156. Mougiakakos D, Krönke G, Völkl S, et al. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2021;385(6):567–569. doi:10.1056/NEJMc2107725
157. Mackensen A, Müller F, Mougiakakos D, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28(10):2124–2132. doi:10.1038/s41591-022-02017-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.