Back to Journals » Journal of Pain Research » Volume 11
Prolonged-duration pulsed radiofrequency is associated with increased neuronal damage without further antiallodynic effects in neuropathic pain model rats
Authors Arakawa K, Kaku R, Kurita M, Matsuoka Y, Morimatsu H
Received 13 March 2018
Accepted for publication 20 August 2018
Published 30 October 2018 Volume 2018:11 Pages 2645—2651
DOI https://doi.org/10.2147/JPR.S168064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Erica Wegrzyn
Kyosuke Arakawa, Ryuji Kaku, Masako Kurita, Yoshikazu Matsuoka, Hiroshi Morimatsu
Department of Anesthesiology and Resuscitology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama City, Okayama, Japan
Aim of investigation: Pulsed radiofrequency (PRF) is a safe and effective approach for treating neuropathic pain. However, the optimal treatment conditions and analgesic mechanisms of PRF remain unclear. The aim of our study was to assess the beneficial and adverse effects of prolonged-duration PRF and the analgesic mechanisms of PRF treatment with neuropathic pain rats.
Methods: Male Sprague Dawley rats received L5 spinal nerve ligation (SNL) for developing neuropathic pain. Fourteen days after L5 SNL surgery, they were divided into three groups according to duration of PRF current for 6 minutes, 12 minutes, and none. PRF current was delivered via direct visualization adjacent to the L5 dorsal root ganglion (DRG). Pain behavior was evaluated every week after L5 SNL surgery, until day 28. Seven days after PRF treatment, L5 DRG tissue was harvested to detect levels of activating translation factor 3 (ATF3; a marker of neuronal damage) and hyperpolarization-activated cyclic nucleotide (HCN)-gated cation channels (key factors in neuropathic pain) using quantitative PCR.
Results: Before PRF application, withdrawal thresholds were significantly lower than at baseline and did not differ significantly between the three groups. After PRF application, withdrawal thresholds in the PRF6 and PRF12 groups were significantly increased compared to those in the sham group. However, those in the PRF6 and PRF12 groups did not differ significantly. The expression level of ATF3 mRNA in the PRF12 group was significantly higher than that in the sham group (P<0.01), but the expression of HCN1 and HCN2 channels did not differ significantly between the three groups.
Conclusion: Prolonged PRF exposure, from 6 to 12 minutes, was not only ineffective but also associated with increased neuronal damage. These findings do not support prolonged PRF exposure as a helpful treatment for neuropathic pain. In this study, the involvement of HCN channels in the antiallodynic effects of PRF was uncertain.
Keywords: pulsed radiofrequency, neuropathic pain, dorsal root ganglion, ATF3, HCN channels
Introduction
Neuropathic pain, caused by a lesion or dysfunction in the somatosensory nervous system, is challenging to treat.1 Although various pharmacologic treatments for neuropathic pain are available, conservative medication is often ineffective, causing adverse systemic effects. Nonpharmacologic treatments are, thus, favored.
Radiofrequency (RF), one of the nonpharmacologic treatments for neuropathic pain, is categorized into conventional RF (CRF) and pulsed RF (PRF). CRF has been used as a treatment for neuropathic pain via thermal lesioning of nervous tissue. On the contrary, PRF is a nondestructive RF technique2 in which electromagnetic currents (20 ms pulses of 500 kHz) are applied adjacent to the dorsal root ganglion (DRG) or sensory nerve (increasing but maintaining the local temperature below 42°C). PRF treatment is associated with long-lasting analgesic effects and few complications.
Several clinical reports have demonstrated the effects of PRF in the treatment of neuropathic pain.3–7 Moreover, animal studies have shown the analgesic mechanisms of PRF currents.8–11 Nevertheless, the antinociceptive mechanisms and optimal conditions for PRF treatment remain unclear.
The primary purpose of our study was to investigate the effects of prolonged PRF exposure in neuropathic pain model rats; we predicted that increasing PRF exposure times would be more effective for treating neuropathic pain. The secondary purpose of our study was to explore the antiallodynic, neurolytic, and modulating effects of PRF exposure.
Methods
Animals
This study was approved by the Animal Care and Use Committee of Okayama University Medical School (OKU-2014472). Animals were treated in accordance with the Ethical Guidelines for the Investigation of Experimental Pain in Conscious Animals, issued by the International Association for the Study of Pain.12
Adult male Sprague Dawley rats weighing 160–220 g were purchased from CLEA Japan Inc. (Tokyo, Japan). They were reared in transparent cages with paper bedding. Their housing environment was temperature controlled (25°C), with a 12-hour light–dark cycle. They were permitted free access to food and water.
Experimental design
In the first series of experiments, we investigated the influence of PRF on mechanical allodynia according to PRF exposure time. Rats were randomly assigned to one of the following three treatment groups 14 days after L5 spinal nerve ligation (SNL) surgery: 1) PRF6 group (n=11; rats received PRF currents to the ipsilateral L5 DRG for 6 minutes via direct visualization); 2) PRF12 group (n=11; rats received PRF currents to the ipsilateral L5 DRG for 12 minutes via direct visualization); and 3) sham group (n=11; rats did not receive PRF treatment).
In the second series of experiments, we examined the influence of PRF exposure time on the expression of activating transcription factor 3 (ATF3) mRNA (a marker of nerve injury)13 and hyperpolarization-activated cyclic nucleotide (HCN)-gated cation channels (HCN1 and HCN2; key factors in neuropathic pain)14 via PCR analysis on ipsilateral L5 DRGs. Rats were randomly assigned to one of the following four groups (Table 1): 1) PRF6 group (n=4), 2) PRF12 group (n=4), 3) sham group (n=4), and 4) naive group (n=4; nonoperated controls).
  | Table 1 Experimental assigned animal groups Abbreviations: DRG, dorsal root ganglion; PRF, pulsed radiofrequency; SNL, spinal nerve ligation. |
Neuropathic pain model
After determining the baseline thresholds, rats were anesthetized with isoflurane in O2 and nerve injury was produced by tight L5 SNL, as described by Kim and Chung.15 In brief, the animals were placed in a prone position to access the left L5 spinal nerve. The back fur was shaved, and the operative field was prepared with 70% ethanol (EtOH). Following a longitudinal incision, the left L4-S1 paraspinal muscles were separated from the spinous processes to visualize the L6 transverse process. Following removal of the left L6 transverse process, the left L5 spinal nerve was identified and ligated tightly with 6–0 silk sutures. After surgery, the incised muscles and skin were sutured in layers.
PRF application
The procedure for applying PRF adjacent to the exposed DRGs in neuropathic pain rat models was described by Perret et al.16 The procedure was modified as described later. For the L5 SNL procedures, anesthesia was induced and maintained with intraoperatively administered isoflurane in O2. The lumbosacral area was shaved, and the operative field was sterilized with 70% EtOH. The surgical incision was re-opened, and the paraspinal muscles were dissected from the L5–6 spinous process. The vertebral arch and ligated L5 spinal nerve were exposed. To expose the L5 DRG, L5–6 articular processes were partially removed with a small rongeur. The L5 DRG was identified by its proximal location to the L5 SNL ligature.
An RF electrode with a built-in thermocouple was placed adjacent to the L5 DRG under direct visualization (Figure 1). A 54 mm, 22 G guiding needle with a 4 mm active tip (Ac-4; Hakko, Tokyo, Japan) was modified as follows. The electrode was placed in a plastic tube (a 22 G catheter tip) allowing the 2 mm active distal end to be exposed for PRF stimulation. A RF generator with standard clinical specifications (model JK3; RDG Medical, Surrey, UK) was used. Before applying the PRF current, tissue impedance was measured and the presence of muscle contractions was checked using electrical stimulation at 3 Hz. If the impedance was above 1000 Ω, a few drops of normal saline were provided to the surgical field to decrease impedance. The electrode was adjusted to the right position until muscle contractions were observed with proper outputs between 0.3 and 0.7 V. After proper electrode placement, the PRF current was applied in 20 ms, 300 kHz RF pulses, delivered at a rate of 2 Hz. The maximum temperature was automatically controlled at 42°C.
Behavioral tests
Behavioral tests were performed between 08:00 and 10:00 before the L5 SNL surgery (day 0), 7 and 14 days after the L5 SNL surgery (days 7 and 14), and 7 and 14 days after the PRF procedure (days 21 and 28). After acclimatizing the rats in the site for 30 minutes, a mechanical stimulus was applied from underneath the mesh (openings 5×5 mm2) to the plantar aspect of the proximal part of the heel using the up/down method, with nine von Frey monofilaments (0.4, 0.6, 1, 1.4, 2, 4, 6, 8, and 15 g; Touch-Testi Sensory Evaluator; North Coast Medical, Morgan Hill, CA, USA). Each trial was initiated with a von Frey force of 2 g delivered to the left hind paw for approximately 1 second. If there was no withdrawal response, the next higher force was delivered. If there was a response, the next lower force was delivered. This procedure was performed four times after the first response. On the basis of the response pattern and the force of the final filament, the 50% response threshold (50% paw withdrawal threshold [PWT]) was calculated using the formula described by Chaplan et al.17
mRNA expression analysis
Animals were euthanized via beheading under anesthesia 1 week after PRF treatment. Left L5 DRG sections were removed, immediately stored in RNAlater (Qiagen NV, Venlo, the Netherlands), and kept in a refrigerator at 4°C to preserve RNA. Total RNA was extracted from tissues using the QIAzol Lysis Reagent (Qiagen NV) and RNeasy Lipid Tissue Mini Kit (Qiagen NV) according to the manufacturer’s instructions. The RNA concentration and purity of each sample were evaluated using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen NV) according to the manufacturer’s protocol. Thereafter, real-time reverse transcription-PCR (RT-PCR) was carried out using the StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific) and SYBR Premix Ex Taq II (Takara Bio, Shiga, Japan) with the primer pairs listed in Table 2. Original mRNA sequences are available in GenBank database (National Center for Biotechnology Information, Bethesda, MD, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels were used as internal controls. The cycle threshold value for each sample was used to calculate concentrations based on standard curves.
  | Table 2 Sequence of PCR primer pairs for the quantification of mRNA
|
Statistical methods
According to our preliminary experiments, we calculated that a sample size of 11 per group would provide a power of 80% to show a difference of 4.0 g in the PWTs using an SD of 3.2 and a two-sided type I error rate of 5%. Statistical tests were performed using the GraphPad Prism 5.0c software (GraphPad Software, Inc., La Jolla, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).18 With data from the behavioral tests, between-group (three groups) comparisons of PWTs were carried out using two-way repeated measures ANOVAs followed by Bonferroni’s post hoc tests. Between-group (four groups) comparisons of quantitative PCR results were carried out using one-way ANOVA followed by Bonferroni’s post hoc tests. Data from each group are presented as mean ± standard error of the mean (SEM). Probability values (P) less than 0.05 were considered statistically significant.
Results
Baseline data
The mean weight (g) of the rats did not differ significantly between the groups at baseline (day 0) or before DRG exposure surgery (day 14). The mean impedance and minimum voltage values of muscle constructions before PRF application were 801±21 Ω and 0.42±0.02 V, respectively, in the PRF6 group and 808±34 Ω and 0.43±0.03 V, respectively, in the PRF12 group; there were no significant differences between the groups.
Behavioral testing
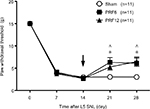
The results of behavioral testing in the sham, PRF6, and PRF12 groups are shown in Figure 2. At baseline (day 0), no between-group differences were observed in PWTs. Thereafter, neuropathic pain states were induced by L5 SNL. Tactile allodynia was observed starting 7 days after L5 SNL, and PWTs were significantly decreased in all groups compared to baseline values. Induction of tactile allodynia was determined to be PWTs <5 g on day 14. There were no between-group differences in PWTs from baseline to day 14, indicating that L5 SNL was associated with the same level of neuropathic pain in all groups. None of the rats showed difficulty moving their legs.
PRF procedures were performed on the rats in neuropathic pain states 14 days after L5 SNL. PRF treatment partially reversed L5 SNL-induced tactile allodynia. When the PRF6 and PRF12 groups were compared with the sham group, statistically significant attenuations in pain responses were noted on days 21 and 28. However, no significant differences were observed between the PRF6 and PRF12 groups throughout the study period.
ATF3 mRNA expression in ipsilateral L5 DRGs
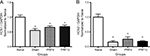
The expression levels of ATF3 mRNA are shown in Figure 3. ATF3 mRNA expression in the naive group was very low and significantly lower than ATF3 mRNA expression levels in the sham, PRF6, and PRF12 groups. ATF3 mRNA expression in the PRF12 group was significantly higher than in the sham group (P<0.01).
HCN1 and HCN2 mRNA expressions in ipsilateral L5 DRG
The expression levels of HCN1 and HCN2 mRNAs are shown in Figure 4. The expression levels of HCN1 and HCN2 in the sham, PRF6, and PRF12 groups were significantly decreased compared with those in the naive group. However, there were no significant differences between the sham, PRF6, and PRF12 groups. HCN1 and HCN2 mRNA levels decreased following L5 SNL, but PRF treatment was not associated with the altered expression of HCN1 and HCN2 mRNA.
Discussion
The present study aimed to investigate effects of extended PRF exposure times on mechanical allodynia following L5 SNL in rats. PRF application to DRGs was associated with significant antiallodynic effects. The antiallodynic effect of 12 minutes’ PRF treatment was not significantly different from that of 6 minutes’ treatment, suggesting that prolonging PRF exposure does not increase the antiallodynic effects of PRF. In contrast, expression of ATF3 mRNA, a key marker of neurological damage,19 significantly increased following treatment with PRF for 12 minutes compared to no PRF treatment. The main finding of the present study was that increased PRF exposure time is not associated with increased antiallodynic effects but may be associated with neurological damage. However, the present study could not elucidate the relationship between PRF application and expression of HCN channels.
To explore the effects of PRF in neuropathic pain, L5 SNL and direct PRF models were selected. SNL models may lead to long-lasting and reproducible mechanical hypersensitivity that can be assessed using the von Frey test.15 Moreover, the nerve injury responsible for pain in this model was in the L5 DRG, allowing PRF application via visualization of the site. Thus, we employed the direct visualization model, originally described by Perret et al,16 which applies PRF adjacent to the exposed L5 DRG. This invasive approach is different from clinical practice. However, our results are indicative of the effects of PRF in comparison with a sham operation group.
The time point of tissue harvest was only 7 days after PRF procedure (day 21) in this study, though we performed behavioral tests 7 and 14 days after the PRF procedure (days 21 and 28). Because ATF3 is a transcription factor followed by subsequent gene expression,19 we consider that the decrease in ATF3 after the transient increase by the nerve injury does not necessarily mean the recovery of the nerve. Tsujino et al19 reported that ATF3 expression reached a peak from 3 to 7 days after the peripheral nerve transection and then declined. Therefore, day 28 was too late to evaluate the nerve damage and the samples were harvested at 7 days after the PRF procedure.
The optimal PRF exposure time is currently unknown. Tanaka et al11 compared 2, 4, and 6 minutes of percutaneous PRF in resiniferatoxin-treated rats, reporting that increased PRF exposure durations led to significant increases in antiallodynic effects. In clinical practice, PRF exposure times are arbitrarily selected at the surgeon’s discretion. Wan et al20 reported that 15 minutes of PRF applied to DRGs significantly affected postherpetic neuralgia in humans. However, Ozsoylar et al21 compared 2 and 6 minutes of percutaneous PRF in SNL rats, reporting that the antiallodynic effects of PRF currents were not significantly different between 2 and 6 minutes. To explore the influence of prolonged PRF exposure times, we compared 6 and 12 minutes of PRF and found that the effects of PRF currents administered for 12 minutes were not significantly different from those of PRF administered for 6 minutes. Tanaka et al11 and Ozsoylar et al21 employed percutaneous PRF on the sciatic nerve, distant from DRGs. Perret et al22 showed that 2 minutes of PRF on L5 DRGs via direct visualization reduced tactile allodynia in L5 SNL rats. To our knowledge, this study is the first to compare the effectiveness of different durations of PRF applied on DRGs in rats.
PRF application is a nondestructive technique. However, Erdine et al23,24 reported that PRF caused ultrastructural changes in DRG cell morphology and sensory nociceptive axons. Hamann et al25 showed that PRF application to DRGs caused the upregulation of ATF3. Therefore, ATF3 mRNA expression levels were examined to investigate the degree of nerve damage induced by prolonged PRF exposure times on L5 DRGs. We found that expression of ATF3 mRNA was associated with PRF exposure time; ATF3 expression in the naive group was very low, indicative of intact neurons, whereas higher ATF3 mRNA expression levels were recorded in the sham, PRF6, and PRF12 groups, indicative of serious nerve damage. Thus, prolonged PRF exposure times may be associated with increased nerve injury. However, further investigations are needed to understand the extent to which nerve injury by PRF is related to adverse effects in clinical practice.
Treatment via PRF is thought to act by altering synaptic transmission and inducing neuromodulatory effects.26 Higuchi et al27 found that PRF application to DRGs resulted in significant increases in c-fos immunoreactive neurons in the dorsal horn, suggesting the activation of dorsal horn neurons. Vallejo et al9 showed that PRF currents directed toward the sciatic nerve attenuated several pain-related genes in peripheral injury rat models. Recently, Liu et al28 showed that PRF application was associated with the recovery of HCN1 and HCN2 channel expressions in DRGs in sciatic nerve chronic constriction injury rats. HCN channels play a key role in the development of neuropathic pain, as they generate hyperpolarization-activated currents (Ih) that are considered major causes of ectopic neuronal activation in neuropathic pain states.13,29 Interestingly, Chaplan et al30 reported that HCN1 and HCN2 channels in DRGs decreased in SNL rats, although Ih expression was dramatically increased. It remains unclear how the discrepancy between Ih current density and HCN expression can be explained. Moreover, it is unclear how upregulation of HCN channels contributes to the observed antiallodynic effects, as previously shown by Liu et al.28 In the present study, the effect of PRF application on the expression of HCN1 and HCN2 mRNA was not significant. Therefore, further investigation is required to explore the mechanisms involved in the antiallodynic effects of PRF.
Our study has some limitations. First, only male rats were used. Sex differences in pain behavior and therapeutic response are well known. The male rats were chose in order to avoid heterogeneous character in female rats by the hormonal cycle. Second, an invasive approach was selected to apply a PRF probe to the DRG. In clinical practice, PRF application to DRGs is performed percutaneously under fluoroscopic guidance; however, this is not possible in rats. A direct approach to the DRG was reasonable to clarify the effect of PRF currents to DRGs. Third, investigation of ATF3 and HCN channel was only expression levels of mRNA. An opportunity for future research is to examine the protein expression or distribution, as the size of the samples collected in this study did not allow for the detection of multiple protein expression at the same time. This could allow for greater clarity for the effects of PRF currents in neurological damage.
Conclusion
The results of the present study indicate that the extension of PRF exposure times may have a limited effect on neuropathic pain in rats, and it may actually be associated with neurolytic effects. Although we could not determine the involvement of HCN channels in the antiallodynic effects of PRF, further exploration of the mechanisms of PRF treatment is required to elucidate the optimum conditions for PRF treatment.
Acknowledgment
We would like to thank Editage (www.editage.jp) for English language editing.
Author contributions
KA helped with study design, data analysis, and animal experiments. RK helped with study design. MK helped with animal experiments. YM designed molecular constructs and analyzed the data. HM helped with conducting the study and coordinated the construction of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. | ||
Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41. | ||
van Zundert J, Patijn J, Kessels A, Lamé I, van Suijlekom H, van Kleef M. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain. 2007;127(1-2):173–182. | ||
Ke M, Yinghui F, Yi J, et al. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician. 2013;16(1):15–25. | ||
Liliang PC, Lu K, Liang CL, Tsai YD, Hsieh CH, Chen HJ. Pulsed Radiofrequency Lesioning of the Suprascapular Nerve for Chronic Shoulder Pain: A Preliminary Report. 2009;10(1):70–75. | ||
Manolitsis N, Elahi F. Pulsed radiofrequency for occipital neuralgia. Pain Physician. 2014;17(6):709–717. | ||
Rohof O. Intradiscal pulsed radiofrequency application following provocative discography for the management of degenerative disc disease and concordant pain: a pilot study. Pain Pract. 2012;12(5):342–349. | ||
Hagiwara S, Iwasaka H, Takeshima N, Noguchi T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain. 2009;13(3):249–252. | ||
Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–E613. | ||
Lee JB, Byun JH, Choi IS, Kim Y, Lee JS. The Effect of Pulsed Radiofrequency Applied to the Peripheral Nerve in Chronic Constriction Injury Rat Model. Ann Rehabil Med. 2015;39(5):667. | ||
Tanaka N, Yamaga M, Tateyama S, Uno T, Tsuneyoshi I, Takasaki M. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. Anesth Analg. 2010;111(3):784–790. | ||
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. | ||
Hunt D, Raivich G, Anderson PN. Activating Transcription Factor 3 and the Nervous System. Front Mol Neurosci. 2012;5(February):1–17. | ||
Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89(3):847–885. | ||
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. | ||
Perret D, Kim D, Li K, Luo ZD. Exposure of the Dorsal Root Ganglion to Pulsed Radiofrequency Current in a Neuropathic Pain Model of Peripheral Nerve Injury. Pain Res. 2012;851:275–284. | ||
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. | ||
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. | ||
Tsujino H, Kondo E, Fukuoka T, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15(2):170–182. | ||
Wan CF, Liu Y, Dong DS, et al. Bipolar High-Voltage, Long-Duration Pulsed Radiofrequency Improves Pain Relief in Postherpetic Neuralgia. Pain Physician. 2016;19(5):E721–E728. | ||
Ozsoylar O, Akçali D, Cizmeci P, Babacan A, Cahana A, Bolay H. Percutaneous pulsed radiofrequency reduces mechanical allodynia in a neuropathic pain model. Anesth Analg. 2008;107(4):1406–1411. | ||
Perret DM, Kim DS, Li KW, et al. Application of pulsed radiofrequency currents to rat dorsal root ganglia modulates nerve injury-induced tactile allodynia. Anesth Analg. 2011;113(3):1–616. | ||
Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9(3):251–256. | ||
Erdine S, Bilir A, Cosman ER, Cosman ER. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–417. | ||
Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10(2):171–176. | ||
Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir. 2011;153(4):763–771. | ||
Higuchi Y, Nashold BS, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002;50(4):850–856. | ||
Liu Y, Feng Y, Zhang T. Pulsed Radiofrequency Treatment Enhances Dorsal Root Ganglion Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels in a Rat Model of Neuropathic Pain. J Mol Neurosci. 2015;57(1):97–105. | ||
Brown SM, Dubin AE, Chaplan SR, Diego S. The role of pacemaker currents in neuropathic pain. Pain Pract. 2004;4(3):182–193. | ||
Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23(4):1169–1178. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.