Back to Journals » Substance Abuse and Rehabilitation » Volume 13
Prolonged Codeine Administration Causes Degeneration of Myelinated Axons and Motor Dysfunction in Wistar Rats
Authors Archibong VB, Usman IM , Lemuel AM
Received 21 April 2022
Accepted for publication 1 November 2022
Published 11 November 2022 Volume 2022:13 Pages 73—81
DOI https://doi.org/10.2147/SAR.S365982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stephan Arndt
Victor Bassey Archibong,1– 3 Ibe Michael Usman,1 Ann Monima Lemuel1
1Department of Human Anatomy, Faculty of Basic Medical Sciences, Kampala International University, Bushenyi, Uganda; 2Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Cross River State, Nigeria; 3Department of Clinical Biology, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda
Correspondence: Ann Monima Lemuel, Tel +256 754031204, Email [email protected]
Purpose: Over-the-counter (OTC) anti-cough medications which contain codeine (an opioid) are extensively available in Nigeria, and hence prone to overuse or abuse. The study aimed to understand the effects of oral codeine administration on the integrity of neurons of the cerebral cortex and cerebellum and its behavioral implications in male Wistar rats.
Methods: Thirty adult male Wistar rats of comparable weights were obtained and randomly allocated into 5 groups: A, B, C, D, and E (n = 6). Drugs used for the study were ArchilinTM with codeine and dihydrocodeine 30mg. Group A served as control and was administered 0.5mL/kg of normal saline. Groups B and C were treated with 1mg/kg and 2mg/kg of dihydrocodeine, respectively; Group D and E received 2mL/kg and 4mL/kg of ArchilinTM codeine syrup, respectively. The ArchilinTM codeine syrup and dihydrocodeine solutions were administered to the animals based on their body weight, orally and daily with the aid of oropharyngeal tubes for 21 days. The experimental animals were subjected to neurobehavioral studies using beam walk and open field. At the end of the treatment period, the animals were anesthetized with ketamine-hydrochloride intraperitoneally. The brains were quickly dissected out, rinsed with normal saline, and tissue processed for myelin studies.
Results: The beam walking and open field result revealed that prolonged codeine administration interfered with motor function in the experimental animals. Sections of the prefrontal cortex and cerebellum of rats given normal saline showed normal myelin sheaths, whereas animals in the treatment group showed degenerating myelin compared to the control.
Conclusion: Prolonged consumption of prescription codeine causes degeneration of the myelin sheaths and this may affect the conduction of electrical impulses in myelinated axons thus resulting in motor function insufficiency.
Keywords: codeine, substance abuse, dihydrocodeine, cough syrup, Wistar rats
Introduction
Codeine is a common opioid used in the management of cough and mild pain.1 There has been a rapid increase in the use of prescription and non-prescription opioid drugs globally, resulting in thousands of recorded deaths.2 The high rate of use and patronage of opioids such as codeine is due to the euphoria it presents its consumers.3 The use of opioids has been associated with a high incidence of damaged relationships, suicide rates, road accidents, and sectorial violence in Northern Nigeria.3
Previous studies have documented myelin damage in alcoholism, liver damage, electrolyte imbalances, axonal degeneration, and toxic diseases.4 Damage to the myelin sheath and nerve fiber is often associated with increased functional insufficiency.4 However, limited studies have reported the specific effect of codeine use on myelin insufficiency despite the widespread use of this substance as a recreational drug. It is in light of this that the study was conceived.
Myelin sheaths play a very important role in the transport of action potential along nerve fibers in both the central and peripheral nervous systems.4 They serve as an insulator, with resultant saltatory conduction of electrical impulses from one Ranvier node to the other.5 Myelin is essential for normal motor, sensory, and cognitive function. Certain conditions can affect myelination, such as multiple sclerosis, leukodystrophies, and acquired inflammatory demyelinating disorder.6–8 Substance abuse may directly or indirectly affect myelination with behavioral consequence or manifestation,9 hence the need for the present study. Therefore, this study aimed to understand the effects of oral codeine administration on the integrity of neurons of the cerebral cortex and cerebellum, and its behavioral implications in male Wistar rats.
Materials and Methods
Animal Source and Handling
Thirty (30) adult male Wistar rats were obtained and kept in the animal house of the Faculty of Basic Medical Sciences, University of Calabar, in a well-ventilated cage and allowed free access to food and water before and during the experiment. Before the commencement of the experiment, the animals were acclimatized for two weeks. Ethical approval was sorted and obtained from the Faculty of Basic Medical Sciences Ethics Committee of the University of Calabar and registered as 008AN30418. The experimental animals were handled according to the international guidelines laid down by the National Institute of Health (NIH) for the regulation of the use of laboratory animals.10
Drug Preparation and Treatment
Dihydrocodeine tablet 30 mg (Dihydrocodeine BPTM, Bristol Laboratories Ltd, Berkhamsted, Herts, UK) and Codeine cough syrup (ArchilinTM with codeine, Archy Pharmaceuticals Ltd, Lagos, Nigeria) were obtained from a reputable drug store in Cross River State, Nigeria.
The stock solution for dihydrocodeine solution was prepared by dissolving the dihydrocodeine tablet (30 mg) in 10 mL of distilled water. Each reconstituted dihydrocodeine solution was prepared daily. The ArchilinTMcodeine syrup and dihydrocodeine solutions were administered to the animals according to their body weight via gastric intubation for 21 days (3 weeks).
Experimental Design
The experimental animals were randomly assigned to five groups (n = 6). Group A was administered normal saline (0.5 mL), group B received 1mg/kg of dihydrocodeine, group C received 2mg/kg of dihydrocodeine group D received 2mL/kg of ArchilinTMcodeine syrup, and group E received 4 mL/kg of ArchilinTMcodeine syrup. All administrations were done via gastric intubation. The various doses were as used in our previous study.11
The experimental animals were subjected to beam walk and open field tests during the experiment. At the end of the study period, animals were anesthetized with ketamine-hydrochloride intraperitoneally. The brains were quickly dissected out, rinsed with normal saline, and the tissue processed for myelin studies. Sections of the cerebral cortex and cerebellar cortex were stained with Luxol fast blue to demonstrate the integrity of the myelin sheath.
The Open Field Apparatus
The open field apparatus was locally improvised according to the design by Calvin Hall12 (It measures locomotion, exploration, and anxiety simultaneously).13
Procedure
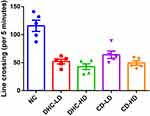
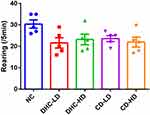
Rats were transferred to the test room, and they were left for 30 minutes to acclimatize before the commencement of the test. The rats were held by the tail and placed in one of the four corners of the open field, and each test rat was allowed to explore the open field for five minutes. The test animals were returned to their home cage at the end of each test. The test apparatus was cleaned with 70% ethyl alcohol, then allowed to dry between tests to eliminate olfactory cues. To assess the process of training to the novelty of the arena, rats were exposed to the apparatus for 5 minutes. The behaviors were scored according to the method of Brown et al,14 and it included line crossing and rearing. Each animal was then given a score for total locomotor activity that was calculated as the sum of line crosses and the number of rears. The line crossing is the frequency with which the rats crossed one of the grid lines with all four paws, while rearing is the frequency with which the rats stood on their hind legs in the maze.
Beam Walk Apparatus
The beam walking test apparatus was locally fabricated and used to assess balance and motor coordination.15–17
Procedure
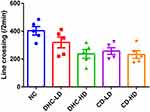
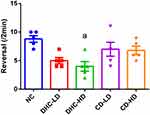
The rats were transferred to the test room and left for 30 minutes to acclimatize before the commencement of the test. The test animal was placed at one end of the beam. The maximum length of the trial is two minutes. The beam is cleaned with 70% ethanol and allowed to dry between trials. Behaviors scored were distance traveled, foot slips, number of turns, and latency to fall. An animal that fell during the trials was returned to its home cage and not to the beam.
Myelin Staining
The Luxol Fast Blue–modified Kluver’s myelin sheath method was created by Heinrich Kluver and Elizabeth Barrera in 1953.18 The tissues were deparaffinized and hydrated to 95% alcohol. Then kept in Luxol Fast Blue solution, overnight at 60°C. Sections were rinsed in 95% alcohol and distilled water. Then, sections were placed in lithium carbonate solution for 5 seconds, before being passed through 70% alcohol, with two changes of 10 seconds each. Then, sections were washed in distilled water. The sections were repeatedly passed through lithium carbonate solution, 70% alcohol, and washed in distilled water until there was a sharp contrast between the blue of the white matter and the colorless gray matter. Sections were later rinsed in 70% alcohol, stained in Eosin for 1 minute, and rinsed in distilled water. Furthermore, sections were stained in Cresyl violet for 1 minute, rinsed in distilled water, and dehydrated through 95% and 100% alcohol, before being cleared in xylene, and coverslipped.
Statistical Analysis
The data obtained from the open field test and beam walking test were analyzed using GraphPad Prism (version 5.01, San Diego California, USA). Kruskal–Wallis ANOVA was used to compare the means from the different treatment groups since some of the data obtained were not normally distributed. Observed differences were considered significant at p < 0.05, and the result was presented as mean ± standard error of mean.
Results
Open Field Test
The results of the open field test revealed lowered line crossing and rearing frequency in the groups administered both high and low doses of dihydrocodeine and codeine when compared to the control group (Figures 1 and 2).
Beam Walking Test
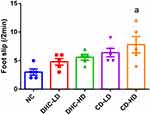
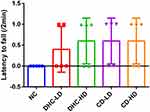
The result of the beam walking test revealed lowered mean line crossing and reversal frequency in the groups administered both high and low doses of dihydrocodeine and codeine when compared to the control group (Figures 3 and 4). On the other hand, the frequency of foot slip and latency of fall were observed to be higher following the administrations of both high and low doses of dihydrocodeine and codeine when compared to the control group (Figures 5 and 6).
Histo-Morphological Studies
The photomicrograph of sections from the prefrontal cortex of rats given 1mg/kg of normal saline showed normal myelin sheaths (Figure 7A). The photomicrograph of sections from the prefrontal cortex of rats administered low and high doses of dihydrocodeine (1 and 2 mg/kg respectively), and low and high doses of codeine (2 and 4 mL/kg respectively) showed degenerating myelin compared to the control (Figure 7B–E respectively).
The photomicrograph of sections from the cerebellum of rats administered normal saline showed normal myelin sheaths. The photomicrograph of sections from the cerebellum of rats administered low and high doses of dihydrocodeine (1 and 2 mg/kg respectively), and low and high doses of codeine (2 and 4 mL/kg respectively) showed degenerating myelin (Figure 8B–E respectively) compared to the control (Figure 8A).
Discussion
This study was centered on the assessment of the effects of codeine-containing cough (CCC) medications on the myelin integrity of neurons of the cerebral cortex and cerebellum of Wistar rats and its behavioral consequence. The result of the present study revealed that the rats administered both high and low doses of dihydrocodeine and codeine were associated with decreased locomotion and general ambulatory activity in the open field test, evident by lowered line crossing and rearing frequency. Evidence of motor dysfunction manifesting in the form of foot slips and imbalance on the beam walk apparatus was also observed in treatment groups. Results from myelin-stained sections of the cerebral cortex of rats revealed evidence of degenerating myelin in animals treated with 1mg/kg of dihydrocodeine, 2mg/kg of dihydrocodeine, 2mL/kg of ArchilinTM codeine syrup and 4mL/kg of ArchilinTM codeine syrup compared to the control group. Myelin-stained sections of the cerebellum of rats revealed evidence of degenerating myelin in animals treated with 1mg/kg of dihydrocodeine, 2mg/kg of dihydrocodeine, 2mL/kg of ArchilinTM codeine syrup, and 4mL/kg of ArchilinTM codeine syrup compared to the control group.
The observed alteration in locomotion, general ambulatory activity, and motor dysfunction following the administration of codeine cough-containing medications was in line with the report of Jaymin,19 who concluded that dependency on prescription opioids is associated with structural changes and consequent functional manifestation. The functional changes associated with dependency on prescription opioids may include altered impulse control, as well as motivational and reward function.19
The findings from the study suggest that prolonged use of codeine cough medications could affect myelin integrity causing motor dysfunction. This supports other studies, which have reported that prolonged codeine uses cause neuroinflammatory reactions in the brain resulting in the upregulation of GFAP, and this neuroinflammatory response is mediated by oxidative stress.20 The abnormal absence of myelin can indicate a region afflicted by a demyelinating disease, such as multiple sclerosis or peripheral neuropathy. Myelin is formed from tightly wrapped layers of the surface membranes of neuroglial cells that surround the axon; these are Schwann cells in peripheral nerves and oligodendrocytes in the central nervous system. Normal myelin is a compact spiral of phospho, and sphingolipid bilayers, with various protein molecules within and between these layers. Myelin sheath degeneration commonly occurs when a neuron dies or if its axons have been severed, the myelin sheath surrounding the degenerating axon breaks up and is phagocytosed.21,22 Myelin damage disrupts the normal conduction of impulses in affected neurons, with resultant disruption in motor, sensory, and cognitive function.23 Degradation of myelin in neurons of the cerebral cortex as seen in the study may have affected the conduction of electrical signals in neurons, this could in turn impair normal functions of the cerebral and cerebellar cortex, as such possibly explain the observed alteration in locomotion, general ambulatory activity and motor dysfunction in the present study.
Conclusion
In conclusion, prolonged use of prescription codeine causes degeneration of the myelin sheaths and this may affect the conduction of electrical impulses in myelinated axons, thus resulting in motor function insufficiency.
Acknowledgments
The authors wish to thank everyone that participated in the process of conducting this research work and up to the production of this manuscript for publication.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Peechakara BV, Gupta M. Codeine. xPharm Compr Pharmacol Ref. 2022;1–4. doi:10.1016/B978-008055232-3.61504-1
2. NIDA. Overdose death rates National Institute on Drug Abuse (NIDA). Available from: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
3. Mohamadi A, Chan JJ, Lian J, et al. Risk factors and pooled rate of prolonged opioid use following trauma or surgery: a systematic review and meta-(regression) analysis. J Bone Jt Surg Am Vol. 2018;100(15):1332–1340. doi:10.2106/JBJS.17.01239
4. Kiernan JA. Histochemistry of staining methods for normal and degenerating myelin in the central and peripheral nervous systems. J Histotechnol. 2013;30(2):87–106. doi:10.1179/HIS.2007.30.2.87
5. Keizer J, Smith GD, Ponce-Dawson S, Pearson JE. Saltatory propagation of Ca2+ waves by Ca2+ Sparks. Biophys J. 1998;75(2):595–600. doi:10.1016/S0006-3495(98)77550-2
6. van der Knaap MS, Bugiani M. Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 2017;134(3):351–382. doi:10.1007/S00401-017-1739-1
7. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi:10.1016/S0140-6736(08)61620-7
8. Lewis RA. Chronic inflammatory demyelinating polyneuropathy. Curr Opin Neurol. 2017;30(5):508–512. doi:10.1097/WCO.0000000000000481
9. Rice J, Gu C. Function and mechanism of myelin regulation in alcohol abuse and alcoholism. Bioessays. 2019;41(7):e1800255. doi:10.1002/BIES.201800255
10. IACUC. Institutional animal care & use committee animal care. Available from: https://animalcare.umich.edu/institutional-animal-care-use-committee.
11. Archibong VB, Ekanem TB, Igiri AO, et al. Immunohistochemical studies of codeine medication on the prefrontal cortex and cerebellum of adult Wistar rats. Cogent Med. 2020;7(1). doi:10.1080/2331205X.2020.1824390
12. Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18(3):385–403. doi:10.1037/H0071444
13. Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:52434. doi:10.3791/52434
14. Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet. 1999;29(4):263–271. doi:10.1023/A:1021694307672
15. Goldstein LB. Rapid reliable measurement of lesion parameters for studies of motor recovery after sensorimotor cortex injury in the rat. J Neurosci Methods. 1993;48(1–2):35–42. doi:10.1016/S0165-0270(05)80005-6
16. Cummings BJ, Engesser-Cesar C, Cadena G, Anderson AJ. Adaptation of a ladder beam walking task to assess locomotor recovery in mice following spinal cord injury. Behav Brain Res. 2007;177(2):232–241. doi:10.1016/J.BBR.2006.11.042
17. Usman IM, Adebisi SS, Musa SA, et al. Tamarindus indica ameliorates behavioral and cytoarchitectural changes in the cerebellar cortex following prenatal aluminum chloride exposure in Wistar rats. Anat Cell Biol. 2022;55(3):320–329. doi:10.5115/acb.22.033
18. Klüver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12(4):400–403. doi:10.1097/00005072-195312040-00008
19. Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(7):2098–2114. doi:10.1093/BRAIN/AWQ138
20. Archibong VB, Ekanem T, Igiri A, Ofutet EO, Ifie JE. The effect of codeine administration on oxidative stress biomarkers and the expression of the neuron-specific enolase in the brain of Wistar rats. Naunyn Schmiedeberg’s Arch Pharmacol. 2021;394(8):1665–1673. doi:10.1007/S00210-021-02094-2
21. Perry VH, Brown MC. Role of macrophages in peripheral nerve degeneration and repair. Bioessays. 1992;14(6):401–406. doi:10.1002/BIES.950140610
22. Lassmann H, Ammerer HP, Kulnig W. Ultrastructural sequence of myelin degradation. I. Wallerian degeneration in the rat optic nerve. Acta Neuropathol. 1978;44(2):91–102. doi:10.1007/BF00691474
23. Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 2016;15(9):967–981. doi:10.1016/S1474-4422(16)30043-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.