Back to Journals » Drug Design, Development and Therapy » Volume 17
Prolonged Anesthesia Induction to Delivery Time Did Not Influence Plasma Remifentanil Concentration in Neonates
Authors Cai M, Liu H, Peng Y, Miao JK, Lei XF, Yu J
Received 7 February 2023
Accepted for publication 25 April 2023
Published 8 May 2023 Volume 2023:17 Pages 1395—1403
DOI https://doi.org/10.2147/DDDT.S407602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Meng Cai,1 Hao Liu,2 Yong Peng,2 Jing-Kun Miao,2 Xiao-Feng Lei,1 Jin Yu1
1Department of Anesthesiology, Chongqing Health Center for Women and Children, Women and Children’s Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Pediatrics, Chongqing Health Center for Women and Children, Women and Children’s Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Jin Yu, Department of Anesthesiology, Chongqing Health Center for Women and Children, Women and Children’s Hospital of Chongqing Medical University, No. 120, Longshan Road, Yubei District, Chongqing, 401147, People’s Republic of China, Tel +86-18623117820, Email [email protected]
Objective: Remifentanil, in combination with etomidate and sevoflurane, is commonly used in clinics for general anesthesia induction in cesarean section (CS). This study aimed to evaluate the correlation between the induction to delivery (I-D) time and neonatal plasma drug concentration and anesthesia, as well as its effects on neonates.
Methods: Fifty-two parturients in whom general anesthesia was induced for CS were divided into group A (I-D< 8 min) and group B (I-D≥ 8 min). Maternal arterial (MA), umbilical venous (UV), and umbilical arterial (UA) blood samples were collected at delivery to analyze the remifentanil and etomidate concentrations using liquid chromatography-tandem mass spectrometry.
Results: There were no statistically significant differences between the two groups in terms of plasma concentrations of remifentanil in the MA, UA, and UV blood (P > 0.05). The plasma concentration of etomidate in MA and UV was higher in group A than that in group B (P< 0.05), whereas the UA/UV ratio of etomidate was higher in group B than that in group A (P< 0.05). The Spearman rank correlation test showed no correlation between the I-D time and plasma remifentanil concentration in the MA, UA, and UV plasma (P> 0.05). The concentrations of etomidate in the MA and UV were negatively correlated with the I-D time (P < 0.05).
Conclusion: Prolonged I-D time did not significantly influence the maternal or neonatal plasma concentration of remifentanil. It is safe to administer remifentanil target-controlled infusion in combination with etomidate and sevoflurane for general anesthesia induction during CS.
Keywords: cesarean section, placental transport, general anesthesia, remifentanil, etomidate, drug concentration
Introduction
The greatest risk for parturients contraindicated for intraspinal anesthesia for cesarean section (CS) is the side effect of the anesthetics used in the induction of general anesthesia on the neonate.1,2 Although the placenta acts as a protective barrier, the degree of protection varies for different drugs and depends on multiple factors, such as lipid solubility, molecular weight, binding to maternal and fetal plasma proteins, and transporter-mediated efflux.3 Opioid analgesics, such as remifentanil, which have the pharmacokinetic features of rapid onset and offset durations, have been used at different doses with different application methods for anesthesia induction and maintenance in CS. The fentanyl fetus/placenta area under the curve (AUC) ratio has been reported as 39.1%.3 Remifentanil crosses the placenta and is quickly cleared from neonatal circulation. Pre-delivery remifentanil administration had no significant effect on the neonatal Apgar scores at 1 and 5 min or respiratory interventions required at birth.4 An early study5 reported that remifentanil alone can easily penetrate the placenta when used at low concentrations, and the maternal artery/umbilical arterial (MA/UA) ratio was 0.88. Another study6 on the placental transport rate of remifentanil reported that when combined with propofol, the umbilical venous (UV)/MA ratio was 63%. However, no significant serious adverse effects of the anesthetic drugs on neonatal outcomes at childbirth were reported in most of these studies. Another well-known and widely used drug for general anesthesia induction in CS is etomidate,7 which is cleared rapidly and does not cause myocardial or respiratory depression or hypotension. Notably, etomidate has been reported to have greater advantages for mothers with congenital heart disease previously.8,9 According to a previous study, the UV/MA ratio of etomidate was 86%.10
The potential adverse effects of general anesthetics on neonates depend on the plasma concentration of the drug and maybe the duration between anesthesia induction and delivery. However, there are concerns that a longer interval between anesthesia induction and fetal delivery will lead to higher fetal drug accumulation, thereby resulting in neonatal respiratory depression. Only a limited number of studies have focused on this dilemma. Therefore, this study aimed to analyze the drug concentration in the maternal, UA, and UV blood after the intravenous administration of remifentanil combined with etomidate for CS and investigate the correlation between plasma concentration and the induction to delivery (I-D) time.
Methods
Study Design
This study was approved by the Research Ethics Committee of the Chongqing Health Center for Women and Children (approval number: 2021-011) and was conducted in accordance with the Declaration of Helsinki. The study was registered at the Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org) (registration ID: ChiCTR2100046547). Informed consent was obtained from all parturients who participated in the study.
Parturients aged 23–42 years with a single birth, at 37–40+4 weeks of gestation, and requiring elective or emergency CS but contraindicated for intraspinal anesthesia (ASA classification II), who could only be operated under general anesthesia were enrolled in the study. The exclusion criteria were as follows: 1) mental illness, 2) cognitive impairment, 3) high risk of difficult airway, and 4) full stomach requiring conscious tracheal intubation.
Remifentanil target-controlled infusion (TCI) was administered in combination with a single bolus of etomidate and rocuronium and simultaneous sevoflurane inhalation to induce general anesthesia during CS. The maternal and neonatal blood concentrations of remifentanil and etomidate immediately after umbilical cord ligation were analyzed to estimate placental transport efficiency. The clinical side effects on the mothers and neonates were recorded. The correlation between plasma anesthetic concentration and I-D time was also evaluated.
Anesthetic Procedure
Electrocardiography (ECG), non-invasive cuff blood pressure, respiration, and pulse oxygen saturation were applied for monitoring the parturient, who was laid supine in a left-leaning position. Before initiating anesthesia induction, oxygen was administered at 5 L/min for spontaneous breathing. TCI of remifentanil (lot number 10A06191, Yichang Renfu Pharmaceutical Co, Ltd, Hubei) at 5 ng/mL and 5% sevoflurane (lot number: 21052131, Shanghai Hengrui Pharmaceutical Co., Ltd) inhalation was performed simultaneously to induce anesthesia. Etomidate (0.25 mg/kg, lot number: YT210910, Nhwa Pharma. corporation) and rocuronium bromide (0.6 mg/kg, lot number: SE210801, Guangdong Jiabo Pharmaceutical Co., Ltd, Hubei) were administered intravenously with maternal loss of consciousness. Endotracheal intubation was performed, and mechanical ventilation (tidal volume at 8 mL/kg; frequency between 12–15 beats/min) and the end-tidal carbon dioxide partial pressure level (30–40 mmHg) were maintained. The sevoflurane concentration was decreased to 1.5% after administering the muscle relaxant until the fetus was delivered. After umbilical cord ligation, propofol TCI (lot number: RT425, Aspen Pharma Trading Limited) at 3.5 ug/mL and remifentanil at 5 ng/mL were used for anesthesia maintenance. The Beijing Silugao I-type TCI-infusion pump with built-in Minto pharmacokinetic parameters was used. A neonatal pediatrician was in attendance to meet the neonatal resuscitation needs.
Neonatal Assessment
A stopwatch was used to record three time intervals: induction to skin incision (I-S), uterine incision to delivery (U-D), and I-D time. A midwife and a pediatrician evaluated the Apgar scores of the neonates at three time points: 1 min, 5 min, and 10 min after delivery. The umbilical artery blood gas analysis results, neonatal weight, and resuscitation measures for neonates, including tactile stimulation, bag-mask ventilation, and tracheal intubation, were recorded.
Sampling and Analytical Method
MA, UV, and UA blood samples were obtained at the same time immediately after umbilical cord ligation to analyze the drug concentrations of remifentanil and etomidate. The samples were collected in anticoagulant tubes with sodium citrate and centrifuged for 10 min at 3000 rpm at 4°C. The centrifuged plasma was isolated and stored at −80°C for further analysis. The concentrations of remifentanil and etomidate were determined using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The data were processed and quantified using Mass Lynx Mass Spectrometry Software (Waters, Milford, Massachusetts, USA).
Grouping and Statistical Analysis
The enrolled participants were divided into group A (I-D<8 min) and group B (I-D≥8 min) according to the I-D time. Since I-D time in most situations is 6.9±1.2 minutes,6 the cut-off value was selected as 8 min. Generally, the I-D time in most cases will not exceed 8 min,11 which is close to the unpublished data in our center.
SPSS version 21.0 was used for statistical analyses. Normally distributed data are expressed as mean ± standard deviation (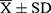 ). The correlation between the interval time and the plasma concentrations of anesthetic drugs was evaluated using the Spearman rank correlation test. The concentrations of remifentanil or etomidate and other measurement data were compared between the two groups using the Student’s t-test. Counting data, such as the Apgar scores, were compared using the chi-square test. P-values were set at 0.05, indicating statistical significance.
). The correlation between the interval time and the plasma concentrations of anesthetic drugs was evaluated using the Spearman rank correlation test. The concentrations of remifentanil or etomidate and other measurement data were compared between the two groups using the Student’s t-test. Counting data, such as the Apgar scores, were compared using the chi-square test. P-values were set at 0.05, indicating statistical significance.
Results
General Information
Fifty-two parturients were enrolled in this study. The differences in age, height, body weight, body mass index (BMI), gestational week, preoperative complications, preoperative laboratory examination, and I-S interval between the groups showed no statistical significance (P > 0.05). The I-D and U-D intervals in group B were significantly longer than those in group A (P < 0.05; Table 1).
 |
Table 1 General Information and Preoperative Laboratory Test Results of Two Groups |
Indications for Selecting General Anesthesia
General anesthesia was induced due to a history of lumbar disc herniation or lumbar surgery in 44.23% and thrombocytopenia in 30.77% of cases. Table 2 presents the proportion of other causes.
 |
Table 2 Reasons for Choosing General Anesthesia |
Neonatal Information
No statistically significant differences were observed between the Apgar scores at 1, 5, and 10 min; resuscitation measures; and umbilical artery blood analysis results of the two groups (P > 0.05) (Table 3).
 |
Table 3 Neonatal Scores, Umbilical Artery Blood Gas Analysis and Resuscitation Measures of Two Groups |
Plasma Concentrations of Remifentanil and Etomidate
No statistically significant differences were observed between the two groups in terms of the concentrations of remifentanil in the MA, UA, and UV blood or the UA/UV and UV/MA ratios of remifentanil immediately after umbilical cord ligation (P>0.05) (Table 4). The plasma concentrations of etomidate in the MA and UV blood of group A at umbilical cord ligation were higher than those of group B, whereas the UA/UV ratios of etomidate concentration at umbilical cord ligation were higher in group B than those in group A (P<0.05). No significant differences were observed between the two groups in terms of the concentrations of etomidate in UA blood and UV/MA ratio (P>0.05) (Table 5).
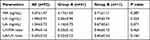 |
Table 4 Blood Concentrations of Remifentanil of the Totality and Two Groups |
 |
Table 5 Blood Concentrations of Etomidate of the Totality and Two Groups |
Correlation analysis did not reveal a significant correlation between the I-D time and plasma remifentanil concentrations in MA, UA, and UV. However, the mean concentrations of etomidate in the MA and UV blood were negatively correlated with the I-D time (P < 0.05; Figures 1 and 2).
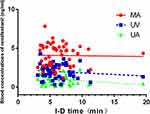 |
Figure 1 Correlation analysis of remifentanil concentrations from maternal and neonatal blood (UV, UA) with I-D time. r(MA) =−0.019, P=0.897; r(UV) =−0.128, P =0.386; r(UA) =−0.224, P= 0.125. |
 |
Figure 2 Correlation analysis of etomidate concentrations from maternal and neonatal blood (UV, UA) with I-D time. r(MA) =−0.444, P=0.002; r(UV) =−0.451, P =0.001; r(UA)=−0.283, P= 0.051. |
Discussion
The negative impact of general anesthetics used to induce general anesthesia during CS on the fetus is a major source of concern. The fetal side effects are thought to be more severe due to the prolonged use of anesthetics for various reasons. The negative impact of anesthetic drugs on the fetus is related to the rate of placental drug transport and the metabolism rate, which influence the concentration of drugs reaching the fetus.
The type of anesthesia used during CS is an important determinant of maternal and fetal outcomes.12 All drugs administered to the mother during the induction of anesthesia cross the placenta in time and affect neonatal outcomes, such as respiratory and heart rate and muscular tension. Unstable maternal blood pressure, heart rate, uterine tone, and uteroplacental perfusion induced by any medication, including anesthetics, indirectly influence fetal performance. The I–D and U–D times are reliable prognostic factors for neonatal outcomes. The I-D and U-D times should not exceed 10 min and 3 min, respectively.1
In an effort to determine the effect of I–D time on the fetal plasma concentration of remifentanil at delivery, this study aimed to determine the extent of placental transport of remifentanil and etomidate during CS under general anesthesia. Liquid chromatography-tandem mass spectrometry analysis revealed no correlation between the I-D time and fetal plasma concentrations of remifentanil and etomidate.
Based on previous literature6 and our practice data, the participants were divided into relatively shorter and longer groups according to I-D time. The I-D time was 5.43 and 10.23 minutes in the shorter and longer groups, respectively. This grouping method is sufficient to explain the impact of the prolonged I-D time and is consistent with clinical practice.
According to the 2019 obstetric anesthesia and analgesia Practice Guide of the American College of Obstetricians and Gynecologists,13 the choice of anesthesia should fully consider the urgency of the operation, contraindications of intraspinal puncture, and other factors. The proportion of CS performed under general anesthesia is 5.8% generally, which increases to 14.6% in the case of urgent CS.14 The data from our institute showed that general anesthesia was induced in 11.54% of cases due to emergent factors, of which 86.54% was due to contraindications for intraspinal puncture.
Remifentanil is the optimal option if CS must be performed under general anesthesia, as it is an ultra-short-acting potent μ-receptor agonist with a context-sensitive half-time of 3–5 min. Placental transport occurs quickly, and the drug is metabolized and redistributed rapidly in the fetus when administered via intravenous infusion.5 Continuous intravenous infusion of low-dose remifentanil at 0.05 μg.kg−1min−1 could significantly improve the experience of parturients undergoing repeated CS under epidural anesthesia, without obvious maternal or neonatal adverse effects.15 Pre-delivery infusion of remifentanil (0.06–0.46 mcg.kg−1min−1) has no significant influence on airway interventions at birth or the neonatal Apgar scores at 1 and 5 min.4 Another study also reported that remifentanil bolus (1 μg.kg−1) administered immediately before general anesthesia induction, followed by infusion at 0.15 μg.kg−1.min−1 for elective CS significantly attenuated the maternal hemodynamic response and lipid peroxidation during the entire duration of I-D without compromising the neonatal outcomes.16 A meta-analysis, which included 17 studies (n=987), indicated the safety of remifentanil (0.5–1 ug/kg or 2–3 ug/kg/h), with no significant side effects on the Apgar scores or neonatal respiratory intervention.17
In our study, Apgar scores of less than 8 accounted for 7.7% of the 52 neonates, and the prolonged I-D time had no effect on neonatal performance. Another study reported that the continuous intravenous infusion of propofol in combination with remifentanil after the bolus dose for the induction of anesthesia during CS and prolongation of the I-D time (18 min) within a certain limit, had no adverse influence on the fetus.6 However, higher neonatal resuscitation needs (tactile stimulation 31.5% and bag-mask ventilation 50%) at 1 min were reported in a study when remifentanil was administered at a loading dose of 2 μg/kg over 10 min followed by a continuous infusion of 2 μg/kg/h,18 which is a different administering regimen from that used in our study. The differences between the enrolled participants and the remifentanil administration protocol also influenced the outcomes, indicating that closed monitoring and resuscitation preparation are important in clinical practice.
The two groups showed no differences in terms of the remifentanil concentrations in the MA, UA, and UV plasma or the UA/UV and UV/MA ratios of remifentanil concentration immediately after umbilical cord ligation, indicating that the I-D time did not affect the amount of remifentanil transported to the fetus. No correlation was observed between the blood remifentanil concentrations (MA, UV, and UA blood) and I-D time. A previous study reported that the mean concentrations of remifentanil in the MA, UA, and UV blood at delivery in the shorter time (I-D) group were 2.25, 1.43, and 0.65 ng/mL, respectively, whereas those in the longer time (I-D) group were 1.96, 1.25, and 0.75 ng/mL respectively.6 However, the I-D interval grouping cut-off was 16 min in this previous study (depending on the time of disinfection and surgical towel placement), whereas it was 8 min in our study. In addition, the remifentanil administration methods (1 µg/kg for induction and 7 µg/kg/h for maintenance) also differed from those used in our study (TCI of remifentanil 5 ng/mL, TCI). Kan et al5 reported that the mean UV/MA and UA/UV ratios of remifentanil were 0.88±0.78 and 0.29±0.07 when remifentanil was infused intravenously at 0.1 μg.kg−1. min−1. The interaction between the combination of drugs and different detection methods (compared with gas chromatography with high-resolution mass spectrometry-selected ion monitoring, liquid chromatography-tandem mass spectrometry analysis has a strong separation ability and lower detection limit) may have caused the deviation between the studies.
The placental transport rate of etomidate is higher than that of remifentanil. The plasma etomidate concentrations in the MA and UV blood were reduced due to single administration when the I-D time was prolonged. The etomidate concentrations in the MA and UV plasma were negatively correlated with the I-D interval. This kind of negative correlation was not observed in UA. This may be because UA blood is the end of drug metabolism and etomidate is given as a single dose in the study.
A combination of intravenous and inhalation anesthesia was selected to achieve sufficient anesthesia depth in a short time and avoid intraoperative awareness, and sevoflurane was used in the induction phase but was stopped immediately after intubation to avoid its effects on uterine contraction. Pre-exposure to low concentrations of sevoflurane reduced the bispectral index values in the interval before delivery, indicating that this method may reduce the risk of maternal intraoperative awareness.19
Our study has some limitations. Since collecting neonatal blood was difficult, the drug metabolism in the neonatal blood could not be measured continuously. Moreover, drug transport during general anesthesia in preterm births and twins requires further study.
In conclusion, remifentanil, in combination with etomidate and sevoflurane by TCI, can be used safely for the induction of general anesthesia during CS. A prolonged I-D time (8 min) did not influence the plasma remifentanil concentration in neonates or result in any neonatal side effects.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author (Jin Yu; [email protected]). The data are not publicly available because this is a clinical trial containing information that could compromise the privacy of research participants.
Ethics Approval and Consent to Participate
This trial was approved by the Medical Ethics Committee of Chongqing Health Center for Women and Children (reference number: 2021-011) and registered with Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn, registration number: ChiCTR2100046547). The trial was performed in accordance with International Conference on Harmonization - Guidelines for Good Clinical Practice (ICH-GCP) and Declaration of Helsinki. Informed consents were obtained from all participants.
Acknowledgments
We are grateful to Prof. Yongchun Su at Chongqing Youyoubaobei Women and Children’s Hospital for his manuscript preparation assistance.
Author Contributions
JY designed the study. XL and JM coordinated the clinic procedures and laboratory tests. MC, HL and YP conducted the drug concentration tests. JY and MC performed the statistical analyses and drafted the paper. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Natural Science Foundation of Chongqing of China (No. cstc2021jcyj-msxmX0763) and Chongqing Maternal and Child Health Scientific Research and Cultivation Project (No. 2021FY106), National Key Clinical Speciality Construction Project (Obstetrics and Gynecology).
Disclosure
The authors declare that they have no competing interests.
References
1. Robins K, Lyons G. Intraoperative awareness during general anesthesia for cesarean delivery. Anesth Analg. 2009;109(3):886–890. doi:10.1213/ane.0b013e3181af83c1
2. Rollins M, Lucero J. Overview of anesthetic considerations for cesarean delivery. Br Med Bull. 2012;101105–101125. doi:10.1093/bmb/ldr050
3. Mehta D, Li M, Nakamura N, et al. In vivo pharmacokinetic analyses of placental transfer of three drugs of different physicochemical properties in pregnant rats. Reprod Toxicol. 2022:111194–111203. doi:10.1016/j.reprotox.2022.06.007
4. Shaylor R, Ginosar Y, Avidan A, Eventov-Friedman S, Amison N, Weiniger CF. Pre-delivery remifentanil infusion for placenta accreta cesarean delivery under general anesthesia: an observational study. J Matern Fetal Neonatal Med. 2016;29(17):2793–2797. doi:10.3109/14767058.2015.1104297
5. Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88(6):1467–1474. doi:10.1097/00000542-199806000-00008
6. Hu L, Pan J, Zhang S, et al. Propofol in combination with remifentanil for cesarean section: placental transfer and effect on mothers and newborns at different induction to delivery intervals. Taiwan J Obstet Gynecol. 2017;56(4):521–526. doi:10.1016/j.tjog.2016.09.010
7. Downing JW, Buley RJ, Brock-Utne JG, Houlton PC. Etomidate for induction of anaesthesia at caesarean section: comparison with thiopentone. Br J Anaesth. 1979;51(2):135–140. doi:10.1093/bja/51.2.135
8. Clivatti J, Smith RL, Sermer M, Silversides C, Carvalho JC. Cardiac output monitoring during cesarean delivery in a patient with palliated tetralogy of Fallot. Can J Anaesth. 2012;59(12):1119–1124. doi:10.1007/s12630-012-9793-6
9. Ho YC, Boey SK, Varughese Mathews AM, See HG, Hwang NC. An unusual case of a parturient with uncorrected pentalogy of Fallot presenting for elective cesarean section delivery of twins. Anesth Essays Res. 2018;12(1):267–270. doi:10.4103/aer.AER_126_17
10. Esener Z, Sarihasan B, Guven H, Ustun E. Thiopentone and etomidate concentrations in maternal and umbilical plasma, and in colostrum. Br J Anaesth. 1992;69(6):586–588. doi:10.1093/bja/69.6.586
11. Rossouw JN, Hall D, Harvey J. Time between skin incision and delivery during cesarean. Int J Gynaecol Obstet. 2013;121(1):82–85. doi:10.1016/j.ijgo.2012.11.008
12. Andersen HF, Auster GH, Marx GF, Merkatz IR. Neonatal status in relation to incision intervals, obstetric factors, and anesthesia at cesarean delivery. Am J Perinatol. 1987;4(4):279–283. doi:10.1055/s-2007-999791
13. American College of, O. and Gynecologists’ Committee on Practice, B.-O. Acog practice bulletin no. 209: obstetric analgesia and anesthesia. Obstet Gynecol. 2019;133(3):e208–e225. doi:10.1097/AOG.0000000000003132
14. Juang J, Gabriel RA, Dutton RP, Palanisamy A, Urman RD. Choice of anesthesia for cesarean delivery: an analysis of the national anesthesia clinical outcomes registry. Anesth Analg. 2017;124(6):1914–1917. doi:10.1213/ANE.0000000000001677
15. Yan W, Xiong Y, Yao Y, Zhang FJ, Yu LN, Yan M. Continuous intravenous infusion of remifentanil improves the experience of parturient undergoing repeated cesarean section under epidural anesthesia, a prospective, randomized study. BMC Anesthesiol. 2019;19(1):243. doi:10.1186/s12871-019-0900-x
16. Kutlesic MS, Kocic G, Kutlesic RM. Os efeitos do remifentanil sobre os marcadores do estresse oxidativo durante a cesariana, em correlação com a hemodinâmica materna e o desfecho neonatal: um estudo randômico controlado [The effects of remifentanil used during cesarean section on oxidative stress markers in correlation with maternal hemodynamics and neonatal outcome: a randomized controlled trial]. Braz J Anesthesiol. 2019;69(6):537–545. Portuguese. doi:10.1016/j.bjan.2019.05.005
17. White LD, Hodsdon A, An GH, Thang C, Melhuish TM, Vlok R. Induction opioids for caesarean section under general anaesthesia: a systematic review and meta-analysis of randomised controlled trials. Int J Obstet Anesth. 2019;404–413. doi:10.1016/j.ijoa.2019.04.007
18. Li C, Li Y, Wang K, Kong X. Comparative evaluation of remifentanil and dexmedetomidine in general anesthesia for cesarean delivery. Med Sci Monit. 2015;213806–213813. doi:10.12659/msm.895209
19. Choi WJ, Kim SH, Koh WU, et al. Effect of pre-exposure to sevoflurane on the bispectral index in women undergoing caesarean delivery under general anaesthesia. Br J Anaesth. 2012;108(6):990–997. doi:10.1093/bja/aes036
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
