Back to Journals » Clinical Interventions in Aging » Volume 10
Proliferative effect of Hachimijiogan, a Japanese herbal medicine, in C2C12 skeletal muscle cells
Authors Takeda T , Tsuiji K, Li B, Tadakawa M, Yaegashi N
Received 16 October 2014
Accepted for publication 31 December 2014
Published 10 February 2015 Volume 2015:10 Pages 445—451
DOI https://doi.org/10.2147/CIA.S75945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Takashi Takeda,1,2 Kenji Tsuiji,2 Bin Li,2 Mari Tadakawa,2 Nobuo Yaegashi2
1Division of Women’s Health, Research Institute of Traditional Asian Medicine, Kinki University School of Medicine, Osaka, Japan; 2Department of Obstetrics and Gynecology, Tohoku University Graduate School of Medicine, Sendai, Japan
Background: Hachimijiogan (HJG), Ba-Wei-Di-Huang-Wan in Chinese, is one of the most popular herbal medicines in Japanese Kampo. HJG is often prescribed for the prevention and treatment of age-related diseases. Muscle atrophy plays an important role in aging-related disabilities such as sarcopenia. The purpose of this study was to investigate the possible beneficial effect of HJG on skeletal muscle.
Methods: Cells of murine skeletal muscle myoblast cell line C2C12 were used as an in vitro model of muscle cell proliferation and differentiation. The effect of HJG on C2C12 cell proliferation and differentiation was assessed. We counted the number of myotubes morphologically to assess the degree of differentiation.
Results: HJG treatment (200 µg/mL) for 3 days significantly increased C2C12 cell number by 1.23-fold compared with that of the control. HJG promoted the proliferation of C2C12 cells through activation of the ERK1/2 signaling pathway without affecting the Akt signaling pathway. HJG did not affect the differentiation of C2C12 cells.
Conclusion: HJG had beneficial effects on skeletal muscle myoblast proliferation. These findings may provide a useful intervention for the prevention and treatment of sarcopenia.
Keywords: ERK1/2 signaling pathway, herbal medicine, myoblast, proliferation, sarcopenia
Introduction
Muscle atrophy plays an important role in aging-related disabilities.1 Sarcopenia, a term originally used to describe the deficiency of muscle tissue observed in older age,2 is now used to describe low muscle mass as well as low muscle strength and function.3 It has been reported that the estimated direct health care cost due to sarcopenia was 18.5 billion dollars per year in the United States.4 Because the population is aging globally, the economic costs of sarcopenia are expected to escalate significantly. However, in spite of its clinical importance, no drug therapy for this condition is currently available. Protein nutrition and exercise are recommended for maintaining muscle function.5
Hachimijiogan (HJG), which is also called Ba-Wei-Di-Huang-Wan in Chinese, is one of the most popular herbal medicines in Japanese Kampo. HJG is prescribed for the treatment of “kidney deficiency”, which is a concept in traditional Chinese medicine. This concept refers to aging pathophysiology, such as lack of energy, eyesight, hearing, and muscle/skeletal function. HJG is often prescribed for the prevention and treatment of age-related diseases. It has been reported that HJG stimulates the production of testosterone in rats.6 HJG could also be effective in the treatment and prevention of osteoporosis in ovariectomized rats.7
The purpose of this study was to investigate the possible beneficial effect of HJG on skeletal muscle. Specifically, we studied the effect of HJG on the proliferation and the differentiation of C2C12 myoblasts.
Materials and methods
Chemicals and antibodies
HJG TJ-7 (Tsumura Co., Tokyo, Japan) is an extract from a mixture of Rehmanniae radix, Corni fructus, Dioscoreae rhizome, Alismatis rhizome, Hoelen, Moutan cortex, Cinnamomi cortex, and Aconiti tuber (27.3%, 13.6%, 13.6%, 13.6%, 13.6%, 11.4%, 4.5%, and 2.3% by weight). HJG was provided as a dried extract powder by Tsumura Co (Tokyo, Japan). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum, horse serum and other culture reagents were from Thermo Fisher Scientific (Waltham, MA, USA). The following antibodies were purchased: anti-phospho-ERK1/2, anti-ERK1/2, anti-Akt, and anti-phospho-Akt (Thr308) antibodies from Cell Signaling Technology (Danvers, MA, USA) and an anti-β-actin antibody from Sigma-Aldrich Co. (St Louis, MO, USA). PD98059 was purchased from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in dimethyl sulfoxide (DMSO). The resultant stock solutions (0.1% vol/vol) were added to the culture medium at the concentrations indicated in the results section. Control cells were treated with an equal amount of DMSO (0.1% vol/vol) to control for the cytotoxic effects of DMSO itself.
Cell and cell culture
Cells of the murine skeletal muscle myoblast cell line C2C12 were obtained from RIKEN Cell Bank (Ibaraki, Japan). C2C12 cells were cultured in growth medium (GM) consisting of DMEM with 10% fetal bovine serum, at 5% CO2 and 37°C. To induce differentiation, the cells were grown to 80% confluence in GM and then the medium was replaced with differentiation medium (DM) consisting of DMEM with 2% heat-inactivated horse serum. HJG was resuspended and diluted with double-distilled water to obtain final concentrations of 1–200 μg/mL.
Cell counting
C2C12 cells were seeded at 3,000 cells/well in 24-well plates for 24 hours. Subsequently, HJG was added at the concentrations indicated in the Results section and the cells were further cultured for 3 days. For morphology analysis, cells were fixed with methanol/acetone as described previously.8 The cells were counted using a hemocytometer by light microscopy. Three wells were counted for each condition. All experiments were repeated at least three times with essentially similar results. Data are shown as averages of triplicate samples of a representative experiment, with standard deviation indicated by bars.
Cell proliferation assay
Cell proliferation was evaluated using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay system 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS assay) (Promega Corporation, Fitchburg, WI, USA). C2C12 cells were seeded at 300 cells/well in 96-well plates with GM for 24 hours. Subsequently, HJG was added at the concentrations indicated in the Results section and the cells were cultured for a further 3 days. Then, one fifth of the volume of CellTiter 96 Aqueous One Solution was added to each well and incubated for an additional 3 hours. Absorbance at 490 nm was determined using a microplate reader as recommended by the manufacturer. All experiments were performed in triplicate and repeated at least three times with essentially similar results. Results are expressed as the light absorbance at 490 nm.
Protein extraction and Western blotting analysis
Cells at 70%–80% confluence cultured for 24 hours in a 100 mm dish containing GM were exposed to different doses of HJG as indicated. The cells were harvested and lysed in buffer containing 20 mM HEPES (pH 7.9), 400 mM sodium chloride, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM ethylene glycol tetraacetic acid, 1.5 mM magnesium chloride, 1 mM dithiothreitol, 0.1% protease inhibitor cocktail, and 0.5% Nonidet P-40. Cell lysates (40 μg per well) were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories Inc., Hercules, CA, USA). Immunoreactive proteins were detected using a Western blotting kit in accordance with the manufacturer’s protocol (EMD Millipore, Billerica, MA, USA). The relative density of the specific bands was quantified using the image detection software, Image Lab 5.0 (Bio-Rad Laboratories Inc.). To calculate normalized density, the density of phospho-ERK1/2 or phospho-Akt was dived by the value of total ERK or total Akt, respectively.
Myotube counting
C2C12 cells were seeded at 1×104 cells/well in 24-well plates in GM and were grown to 80% confluence. The medium was then switched to DM with the indicated doses of HJG for 3 days. By growing with DM, C2C12 cells differentiate into multinucleated myotubes. For morphology analysis, cells were fixed with methanol/acetone as described previously.8 A myotube was defined as containing more than three nuclei within cellular structures, so as not to count the cells undergoing mitosis. The numbers of multinucleated myotubes present in a field (1 μm2 ×100) were counted using a phase-contrast microscope 3 days after switching to DM. Data are shown as averages of five fields, with standard deviation indicated by bars.
Statistical analysis
Statistical analysis was performed using Excel 2007 (Microsoft Corporation, Redmond, WA, USA) with add-in software Excel Statistic 2006 (SSRI, Tokyo, Japan). The significance of differences between means was evaluated by analysis of variance (ANOVA), followed by Dunnett’s test. Data are expressed as means ± standard deviation. Statistical significance was set at P<0.05.
Results
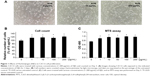
HJG promotes C2C12 cell proliferation
We analyzed the effect of HJG on C2C12 cell proliferation. C2C12 cells were incubated with various concentrations of HJG (1–200 μg/mL) for 3 days, and the cell number was counted (Figure 1A and B). We found that C2C12 cell proliferation was significantly promoted by 1.16-, 1.18-, 1.15-, and 1.23-fold compared with that of the control by HJG treatment at 1, 10, 100, and 200 μg/mL, respectively (CON 1±0.056 versus [vs] HJG 1 μg/mL 1.16±0.085, P=0.041; 10 μg/mL 1.18±0.062, P=0.024; 100 μg/mL 1.15±0.063, P=0.030; 200 μg/mL 1.23±0.060, P=0.022; Figure 1B). We further studied the stimulatory effect of HJG on C2C12 cell growth using an MTS assay. We confirmed that HJG treatment resulted in a statistically significant promotion of C2C12 cell proliferation (CON 0.764±0.026 vs HJG 1 μg/mL 0.912±0.049, P=0.034; 10 μg/mL 0.967±0.023, P=0.012; 100 μg/mL 0.996±0.023, P=0.012; 200 μg/mL 0.980±0.021, P=0.021; Figure 1C).
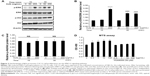
HJG-induced C2C12 cell proliferation via the ERK1/2 signaling pathway
The ERK1/2 signaling pathway is known to be involved in myoblast proliferation.9,10 On the other hand, myoblast differentiation is promoted by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.11 Next, we analyzed whether these signaling pathways play a role in HJG-induced C2C12 cell proliferation. First, we measured ERK1/2 activity and Akt activity by Western blotting analysis. ERK1/2 activity was significantly increased at 15 minutes by 2.89-fold compared with that of the control by 200 μg/mL HJG treatment (HJG 0 μg/mL vs 200 μg/mL 2.89±0.22, P<0.0001; Figure 2A and B) and at 30 minutes by 2.19- and 2.14-fold compared with that of the control by 50 μg/mL and 200 μg/mL HJG treatment, respectively (HJG 0 μg/mL vs 50 μg/mL 2.19±0.133, P<0.0001; 200 μg/mL 2.14±0.061, P<0.0001; Figure 2A and B). HJG did not affect the activity of Akt (15 minutes HJG 0 μg/mL vs 50 μg/mL 0.886±0.066, P=0.636; 200 μg/mL 1.01±0.127, P=0.999; 30 minutes HJG 0 μg/mL vs 50 μg/mL 0.988±0.135, P=0.997; 200 μg/mL 1.025±0.122, P=0.987; Figure 2A and C). In order to evaluate the necessity of ERK1/2 in HJG-induced cell proliferation, we used an MEK1 inhibitor, PD98059. We found that pretreatment with PD98059 abolished the effect of HJG on C2C12 cell proliferation (CON 0.864±0.026 vs HJG 0.5 μg/mL 1.025±0.049, P=0.028; 1 μg/mL 1.084±0.054, P=0.024; 10 μg/mL 1.146±0.036, P=0.012; 100 μg/mL 1.154±0.023, P=0.011; HJG 0.5 μg/mL + PD98059 0.846±0.037, P=0.994; 1 μg/mL 0.905±0.025, P=0.863; 10 μg/mL 0.915±0.035, P=0.888; 100 μg/mL 0.936±0.046, P=0.765; Figure 2D).
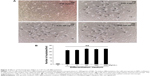
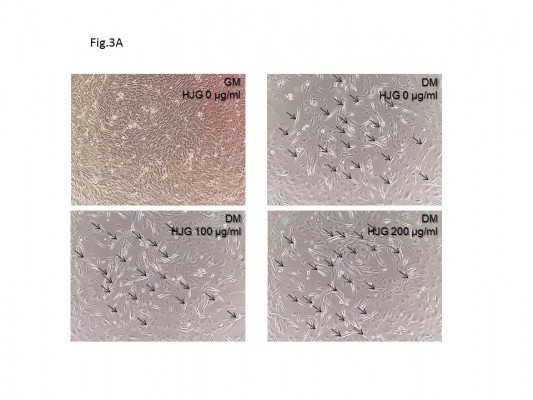
HJG did not change C2C12 cell differentiation
The C2C12 cell line is a good in vitro model system for the differentiation of myoblasts to myotubes. Next, we analyzed the effect of HJG on C2C12 cell differentiation. After switching to DM, we could detect myotubes having more than three nuclei (Figure 3A). The addition of HJG to the DM did not change the numbers of differentiated myotubes (DM HJG 0 μg/mL 22.3±0.875 cells/field vs HJG 1 μg/mL 22.7±1.146 cells/field, P=0.999; 10 μg/mL 24.6±1.25 cells/field, P=0.926; 100 μg/mL 24.0±1.27 cells/field, P=0.983; 200 μg/mL 24.6±1.06 cells/field, P=0.925; Figure 3B).
Discussion
This is the first report about the effect of herbal medicine on skeletal muscle cells. In this study, we showed that HJG stimulated the proliferation of C2C12 cells by activating ERK1/2 signaling.
Skeletal muscle formation/myogenesis involves a complex process of myogenic satellite cell proliferation and differentiation. After muscle tissue damage, quiescent myogenic satellite cells become activated and proliferate.12 After that, these cells express myogenic markers and fuse to form myofibers. Our data show that HJG stimulated undifferentiated myoblast cell proliferation and did not affect the differentiation process. On the whole, HJG may help to induce the repair of damage to muscle tissue.
A prospective, population-based study showed that lower vitamin D and higher parathyroid hormone (PTH) levels increased the risk of sarcopenia.13 It has been reported previously that HJG suppresses age-related PTH elevation in aged rats.14 In this study, we showed that HJG has a direct favorable effect on muscle cells and it is also possible that the suppressive effect of HJG on PTH elevation has a role in preventing sarcopenia indirectly.
Japanese herbal medicine (Kampo) is derived from traditional Chinese medicine starting from the 5th to 6th century, followed by major modifications over a long period in Japan. Kampo formulas are available as powdered extracts produced by drug manufacturers, so they can be prescribed conveniently just like Western-style powders. These drugs are authorized by the Japanese government and their quality is regulated, similarly to that of Western medicines.15 Because Kampo formulas are of high quality and contain standardized ingredients, recently, their pharmacologic actions have begun to be studied at the molecular level.16–20
The underlying mechanisms of the proliferative effect of HJG on C2C12 cells and the active ingredient(s) for this effect are currently unknown. Because HJG consists of eight herbs and contains many ingredients, further studies are necessary to clarify the active components at the molecular level. Among them, Rehmanniae radix could activate ERK1/2 signaling in vascular endothelial cells.21 HJG and Cinnamomi cortex could activate peroxisome proliferator-activated receptor alpha (PPARα) in kidney cells.22 Considering that PPARα agonist WY-14,643 could activate ERK1/2 signaling in C2C12 cells,23 it may be possible that such a PPARα and ERK signaling pathway may be involved in the proliferative effect of HJG in C2C12 cells.
Recently, an aging muscle model using C2C12 cells following multiple population doublings was reported.8,24,25 These cells showed impaired differentiation compared with parental control cells. Moreover, these cells had similar morphology and signaling properties to those observed in aged human muscle cells. Analysis of the anti-aging effect of HJG using this aging muscle model will give new insights into sarcopenia research.
HJG has a history of centuries of use for the treatment for age-related degenerative disease. This long history of the drug supports its safety. However, it is important to show its efficacy by evidence from clinical trials.
Conclusion
Our results indicate that the Japanese herbal medicine HJG promotes the proliferation of C2C12 cells via the ERK1/2 signaling pathway. This may explain one of the anti-aging effects of HJG at the molecular and cellular levels. We propose HJG as a candidate drug for the prevention and treatment of sarcopenia.
Disclosure
Takashi Takeda has received a research grant from Tsumura Co. Kenji Tsuiji, Bin Li, Mari Tadakawa, and Nobuo Yaegashi declare that they have no conflicts of interest.
References
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. | ||
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5 Suppl):990S–991S. | ||
Bijlsma AY, Meskers CG, Westendorp RG, Maier AB. Chronology of age-related disease definitions: osteoporosis and sarcopenia. Ageing Res Rev. 2012;11(2):320–324. | ||
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–85. | ||
Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. | ||
Usuki S. Hachimijiogan produces testosterone in adult rat testes. Am J Chin Med. 1988;16(3–4):93–105. | ||
Chen H, Wu M, Kubo KY. Combined treatment with a traditional Chinese medicine, Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) and alendronate improves bone microstructure in ovariectomized rats. J Ethnopharmacol. 2012;142(1):80–85. | ||
Deane CS, Hughes DC, Sculthorpe N, Lewis MP, Stewart CE, Sharples AP. Impaired hypertrophy in myoblasts is improved with testosterone administration. J Steroid Biochem Mol Biol. 2013;138:152–161. | ||
Czifra G, Toth IB, Marincsak R, et al. Insulin-like growth factor-I-coupled mitogenic signaling in primary cultured human skeletal muscle cells and in C2C12 myoblasts. A central role of protein kinase Cdelta. Cell Signal. 2006;18(9):1461–1472. | ||
Sarbassov DD, Jones LG, Peterson CA. Extracellular signal-regulated kinase-1 and -2 respond differently to mitogenic and differentiative signaling pathways in myoblasts. Mol Endocrinol. 1997;11(13):2038–2047. | ||
Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. 2000;275(47):36750–36757. | ||
Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985). 2001;91(2):534–551. | ||
Visser M, Deeg DJ, Lips P; Longitudinal Aging Study A. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. | ||
Ikeda R, Mizoguchi K. Hachimijiogan (Ba-Wei-Di-Huang-Wan), a herbal medicine, improves unbalance of calcium metabolism in aged rats. J Ethnopharmacol. 2009;124(2):176–181. | ||
Imanishi J, Watanabe S, Satoh M, Ozasa K. Japanese doctors’ attitudes to complementary medicine. Lancet. 1999;354(9191):1735–1736. | ||
Matsumoto K, Zhao Q, Niu Y, et al. Kampo formulations, chotosan, and yokukansan, for dementia therapy: existing clinical and preclinical evidence. J Pharmacol Sci. 2013;122(4):257–269. | ||
Mogami S, Hattori T. Beneficial effects of rikkunshito, a Japanese kampo medicine, on gastrointestinal dysfunction and anorexia in combination with Western drug: a systematic review. Evid Based Complement Alternat Med. 2014;2014:519035. | ||
Tominaga K, Arakawa T. Kampo medicines for gastrointestinal tract disorders: a review of basic science and clinical evidence and their future application. J Gastroenterol. 2013;48(4):452–462. | ||
Uezono Y, Miyano K, Sudo Y, Suzuki M, Shiraishi S, Terawaki K. A review of traditional Japanese medicines and their potential mechanism of action. Curr Pharm Des. 2012;18(31):4839–4853. | ||
Yamakawa J, Motoo Y, Moriya J, et al. Role of Kampo medicine in integrative cancer therapy. Evid Based Complement Alternat Med. 2013;2013:570848. | ||
Liu CL, Tam JC, Sanders AJ, et al. Molecular angiogenic events of a two-herb wound healing formula involving MAPK and Akt signaling pathways in human vascular endothelial cells. Wound Repair Regen. 2013;21(4):579–587. | ||
Monden T, Hosoya T, Nakajima Y, et al. Herbal medicine, Hachimi-jio-gan, and its component cinnamomi cortex activate the peroxisome proliferator-activated receptor alpha in renal cells. Endocrine Journal. 2008;55(3):529–533. | ||
Nakai N, Kawano F, Terada M, Oke Y, Ohira T, Ohira Y. Effects of peroxisome proliferator-activated receptor alpha (PPARalpha) agonists on leucine-induced phosphorylation of translational targets in C2C12 cells. Biochim Biophys Acta. 2008;1780(10):1101–1105. | ||
Sharples AP, Al-Shanti N, Lewis MP, Stewart CE. Reduction of myoblast differentiation following multiple population doublings in mouse C2C12 cells: a model to investigate ageing? J Cell Biochem. 2011;112(12):3773–3785. | ||
Sharples AP, Player DJ, Martin NR, Mudera V, Stewart CE, Lewis MP. Modelling in vivo skeletal muscle ageing in vitro using three-dimensional bioengineered constructs. Aging Cell. 2012;11(6):986–995. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.